Introduction
Milk is primarily composed of milk protein, fat, lactose, vitamins and minerals, which are important nutrient sources for neonates. Maternal nutrients are considered as building blocks for milk synthesis. In humans, breast-feeding decreases the risk of neonatal acute illnesses, diarrhoea and overweight/obesity (breast-feeding for more than 6 months)(Reference Pattison, Kraschnewski and Lehman1). However, in pigs, sufficient and quality colostrum (first 24 h) supplies are important for neonates to gain higher weaning weight and have better growth performance later in life(Reference Theil, Lauridsen and Quesnel2). Calves fed adequate colostrum during the first week of their life exhibit significantly enhanced metabolic and immunological status(Reference Rauprich, Hammon and Blum3). Thus, understanding nutritional strategies to regulate milk synthesis and its underlying mechanism is important for human beings and other mammals.
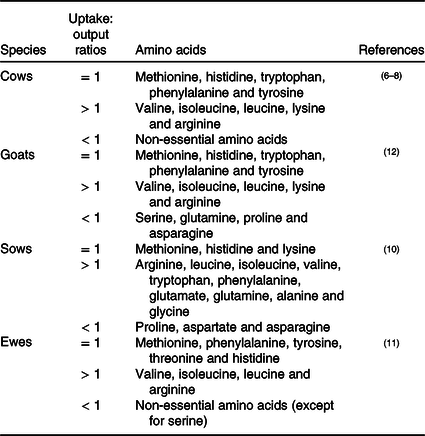
Amino acids are not only basic components of proteins, but also act as functional regulators in a variety of biological processes. The functions of amino acids have been extensively studied in the gut, liver, muscle and adipose tissue, especially in the field of protein(Reference Wu4) and fat metabolism(Reference Zhang, Zeng and Ren5). In the mammary gland, amino acid uptake from blood is almost equal to milk output based on a nitrogen basis in dairy cows(Reference Omphalius, Lapierre and Guinard-Flament6–Reference Haque, Guinard-Flament and Lamberton8), goats(Reference Safayi and Nielsen9), sows(Reference Trottier, Shipley and Easter10) and ewes(Reference Davis, Bickerstaffe and Hart11). However, the destinies of different amino acids in mammary cells are different(Reference Omphalius, Lapierre and Guinard-Flament6–Reference Haque, Guinard-Flament and Lamberton8,Reference Trottier, Shipley and Easter10–Reference Mepham and Linzell12) . The mammary amino acid uptake:output ratios could be larger (for example, valine, isoleucine, leucine and arginine), equal (for example, methionine and histidine) or less than 1 (for example, asparagine and proline) (mammary amino acid uptake:output ratios in cows, goats, sows and ewes are shown in Table 1)(Reference Omphalius, Lapierre and Guinard-Flament6–Reference Haque, Guinard-Flament and Lamberton8,Reference Trottier, Shipley and Easter10–Reference Mepham and Linzell12) . Those amino acids with uptake:output ratios greater than 1 can be metabolised to CO2, urea, polyamine or simply other non-essential amino acids(Reference Mepham13). In addition, various amino acids (especially branched-amino acids, methionine and arginine) are involved in the regulation of milk synthesis. A variety of signalling molecules have been proposed to cooperate with amino acids to regulate biological functions in the mammary gland. Signalling pathways regulating mammary epithelial cell proliferation and differentiation have been well characterised previously(Reference Hennighausen and Robinson14). However, the underlying mechanisms and signalling pathways by which amino acids regulate milk and fat synthesis were largely unknown until recently. The aim of the present review is to describe how amino acids are transported, sensed and transduced in the mammary gland, as well as their functions in the regulation of milk synthesis.
Table 1. Mammary amino acid uptake:output ratios in different mammals
Branched-chain amino acids
Transportation and metabolism of branched-chain amino acids in the mammary gland
The plasma membrane transport system L is the most critical amino acid transporter system for branched-chain amino acids (BCAA) in mammary cells(Reference Jackson, Bryson and Wang15). Transporters from the L system have been well characterised to regulate cell growth and proliferation by directly transporting branched or aromatic amino acids into the cytoplasm in a range of cell lines(Reference Luo, Coon and Su16–Reference Kurayama, Ito and Nishibori18). These transporters are heterodimeric proteins, which comprise of a catalytic subunit (l-type amino acid transporter 1 (LAT1), encoded by SLC7A5, or l-type amino acid transporter 2 (LAT2), encoded by SLC7A6) and a glycoprotein 4F2 heavy chain (4F2hc). Both LAT1 and LAT2 are highly expressed in the mammary tissues(Reference Jackson, Bryson and Wang15), but which subtype is predominately expressed in the mammary gland seems to be different among species(Reference Matsumoto, Nakamura and Nakamura19–Reference Chen, Zhang and Deng21). In rats, the gene expression of LAT1 in the mammary gland is greater than that of LAT2 during the lactation period(Reference Matsumoto, Nakamura and Nakamura19). The gene expression of LAT2, but not LAT1, is significantly increased with the progression of lactation in sows(Reference Chen, Zhang and Deng21). In bovine mammary glands, more research has been focused on the effects of LAT1(Reference Lin, Duan and Lv20,Reference Duan, Lin and Lv22) . Depletion of LAT1 in the bovine mammary gland dephosphorylates and inhibits the activity of mTORC1 (mammalian target of rapamycin complex 1), thereby blunting cell viability and β-casein synthesis(Reference Lin, Duan and Lv20). Additionally, the inhibition of mTORC1 can be rescued by re-expressing LAT1(Reference Lin, Duan and Lv20). However, whether LAT2 also plays an important role in the bovine mammary gland has not been determined and remains to be studied.
BCAA catabolism in mammary tissue is similar to that in other tissues (such as muscle, liver and intestine)(Reference Li, Knabe and Kim23). Leucine, isoleucine and valine share a number of BCAA catabolic enzymes, such as branched-chain aminotransferase (BCAT) and branched-chain α-keto acid dehydrogenase (BCKD)(Reference Wohlt, Clark and Derrig24). In the porcine mammary gland, the protein levels of BCAT are higher than those in the small intestine, skeletal muscle and liver(Reference Li, Knabe and Kim23), which indicates that BCAA are actively metabolised in the mammary gland. Mammary BCAA catabolism primarily produces glutamine and aspartate(Reference Li, Knabe and Kim23). Notably, as BCAA share the same catabolic enzymes, excessive supplementation of either BCAA might affect the metabolism of other BCAA.
Potential signalling pathway of branched-chain amino acids in the mammary gland
Leucine
In the mammary gland, leucine regulates various biological processes, such as cell proliferation and milk synthesis (αs-casein, β-casein and κ-casein) (as shown in Table 2). Numerous studies have demonstrated that mTOR functions as a critical regulator of these processes. Until recently, it was not clear of how leucine regulates mTOR until recently (Fig. 1). Before scientists started studying the effect of leucine on mTOR signalling in mammary cells, most pioneering studies were conducted in human embryonic kidney 293 (HEK 293) cells, which is a classical cell line for research investigating the cellular signalling pathway. In HEK 293 cells, it has been demonstrated that leucine regulates the mTOR signalling pathway primarily through mTOR complex 1 (mTORC1) which consists of regulatory-associated protein of mTOR (Raptor), mammalian lethal with SEC13 protein 8 (mLST8), 40 kDa proline-rich protein kinase B (Akt) substrate (PRAS40) and DEP domain-containing mTOR-interacting protein (DEPTOR). After being absorbed into the cytosol, leucine beings to regulate mTORC1 activation by first dephosphorylating Sestrin2(Reference Saxton, Knockenhauer and Wolfson25,Reference Kimball, Gordon and Moyer26) . Dephosphorylated Sestrin2 induces the dissociation of GTPase-activating protein activity toward Rags 2 (GATOR2), which further inhibits the function of GTPase-activating protein activity toward Rags 1 (GATOR1, a negative regulator of RagA/B)(Reference Wolfson, Chantranupong and Saxton27). Finally, activated RagA/B promotes the translocation of mTORC1 to lysosomes for further activation(Reference Wolfson, Chantranupong and Saxton27).
Table 2. Effects of branched-chain amino acids (BCAA) on mammary gland function and its potential signalling pathways
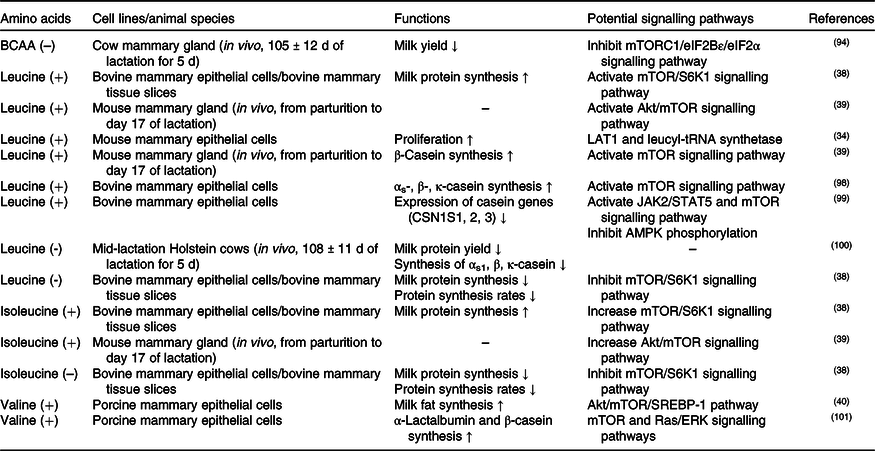
mTORC1, mammalian target of rapamycin complex 1; eIF, eukaryotic initiation factor; mTOR, mammalian target of rapamycin; S6K1, S6 kinase 1; Akt, protein kinase B; LAT1, l-type amino acid transporter 1; CSN1S1, 2, 3, casein αs1, 2, 3; JAK2, Janus kinase 2; STAT5, signal transducers and activators of transcription 5; AMPK, AMP-activated protein kinase; SREBP-1, sterol regulatory element-binding protein 1; ERK, extracellular signal-regulated kinase.

Fig. 1. Branched-chain amino acid (BCAA) and mammalian target of rapamycin complex 1 (mTORC1) signalling networks in the mammary gland. Note: l-type amino acid transporter 1/4F2 heavy chain (LAT1/4F2hc) and l-type amino acid transporter 2 (LAT2)/4F2hc derived from transporter system L are highly expressed and play a dominant role in BCAA transportation in the mammary gland. All three BCAA activate mTORC1 pathways in mammary glands. Leucine induces dephosphorylation of Sestrin2 and further promotes mTORC1 activation through GTPase-activating protein activity toward Rags (GATOR) 2, GATOR1 and RagA/B. In addition, GCG12, SH3-domain binding protein 4 (SH3BP4) and leucyl-tRNA synthetase (LeuRS) are crucial regulators in leucine-related mTORC1 activation. Extracellular valine activates G-protein-coupled receptors (GPRC) T1R1/T1R3, increases phospholipase Cβ (PLCβ) activity and further enhances an influx of intracellular Ca2+. Increased Ca2+ regulates the mTORC1 signalling pathway through extracellular signal-regulated kinase 1/2–tuberous sclerosis complex 1/2–Rheb (ERK1/2–TSC1/2–Rheb) signalling. Intracellular isoleucine activates mTORC1 through an unknown mechanism. In the mammary gland, activated mTORC1 not only increases milk protein synthesis but also milk fat synthesis through lipin 1 (Lpn1)–sterol regulatory element-binding protein 1c (SREBP-1c) pathways. ER, endoplasmic reticulum. Please refer to the main text for details.
Recent advances also strongly suggest that the leucine-regulated mTOR signalling pathway is also conserved in the mammary gland. In bovine mammary glands, overexpression of Sestrin2 depresses mTORC1 activity and synthesis of casein, indicating that Sestrin2 plays a major physical role in mammary gland cells(Reference Luo, Zheng and Zhao28). Danio rerio SH3-domain binding protein 4 (SH3BP4) has been previously reported to abrogate mTORC1 activation by hydrolysing GTP to GDP of RagB in HEK 293 cells(Reference Kim, Stone and Hwang29). In the mammary gland, SH3BP4 is also proposed to play a vital role between Sestrin 2 and Rag GTPase(Reference Luo, Zheng and Zhao28). Interestingly, not only leucine but also other essential amino acids and non-essential amino acids can also regulate mTORC1 through Sestrin2 in the mammary gland(Reference Luo, Zhao and Zhang30), which is inconsistent with observations in HEK 293 cell lines and warrants further investigation.
In the mammary gland, the other potential leucine-mediated mTORC1 signalling pathway is through guanine nucleotide-binding protein subunit γ-12 (GNG12) and leucyl-tRNA synthetase (LeuRS). GNG12 regulates mTORC1 via interaction with Regulator(Reference Luo, Zhao and Dai31), which affects the translocation of mTORC1 to lysosomal membranes(Reference Sancak, Bar-Peled and Zoncu32). LeuRS acts as a vital intracellular leucine sensor that can directly bind to Rag GTPase and activate mTORC1(Reference Han, Jeong and Park33). In mouse mammary cells, LeuRS activates the mTOR signalling pathway and increases cell proliferation(Reference McGuckin, Manjarin and Peterson34). In bovine mammary cells, GNG12 enhances cell growth and milk protein synthesis by activating the mTORC1 signalling pathway(Reference Luo, Zhao and Dai31).
Valine and isoleucine
In addition to l-leucine, l-isoleucine and l-valine are also proposed to regulate milk synthesis. Dietary supplementation of l-isoleucine and l-valine during the whole lactation period enhances milk synthesis in sows and supports increased weaning weight of their litters(Reference Richert, Goodband and Tokach35,Reference Richert, Tokach and Goodband36) . Similarly, l-isoleucine and l-valine deficiency during the mid-lactation period inhibits milk synthesis in dairy cows(Reference Haque, Rulquin and Lemosquet37). Furthermore, recent advances indicate that both l-isoleucine and l-valine can activate the mTOR signalling pathway and have the potential to enhance milk protein synthesis(Reference Appuhamy, Knoebel and Nayananjalie38–Reference Che, Xu and Gao40). Additionally, l-valine enhances fatty acid synthesis through activation of the mTOR/sterol regulatory element-binding protein 1 (SREBP-1) pathway(Reference Che, Xu and Gao40). SREBP-1 is a transcription factor and is proposed to be an important regulator of mammary gland fat synthesis in sheep(Reference Carcangiu, Mura and Daga41), cows(Reference Ma and Corl42) and mice(Reference Rudolph, McManaman and Phang43). Lipin 1, a phosphatidic acid phosphatase, is a critical link between mTOR and SREBP-1(Reference Peterson, Sengupta and Harris44). When mTORC1 is activated, it phosphorylates lipin 1, which releases SREBP-1 and activates SREBP-1-regulated lipogenetic gene expression(Reference Peterson, Sengupta and Harris44). As l-valine regulates lipogenesis through activation of mTORC1, this finding strongly suggests that l-leucine and l-isoleucine might also participate in lipogenesis progression. The isotope tracing experiment showed that the carbon from l-leucine can incorporate into milk fat on goat mammary explants, which provides direct evidence that l-leucine is involved in milk fat production(Reference Roets, Massart-Leën and Peeters45).
Recently, mTORC1 stimulation was observed before amino acid absorption into the cytostome(Reference Nelson, Chandrashekar and Hoon46–Reference Liu, Wang and Li48). T1R1/T1R3, a G-protein-coupled receptor (GPCR) in the cell membrane, is an important player in this process(Reference Nelson, Chandrashekar and Hoon46). It has been demonstrated that amino acids regulate milk protein synthesis through T1R1/T1R3 in the mouse mammary gland(Reference Wang, Liu and Wu47,Reference Liu, Wang and Li48) . Activation of the G-protein-coupled receptors (GPRC) T1R1/T1R3 increases phospholipase Cβ (PLCβ) activity and further enhances an influx of intracellular Ca2+(Reference Wauson, Zaganjor and Lee49,Reference Wauson, Zaganjor and Cobb50) . Extracellular signal-regulated kinase (ERK) 1 and 2 (ERK1/2), which are increased with Ca2+ concentration, regulate the activation of mTORC1 directly via the phosphorylation of Raptor (regulatory-associated protein of mTOR)(Reference Carriere, Romeo and Acosta-Jaquez51). The other possible pathway by which ERK1/2 activates mTORC1 signalling is through the inactivation of tuberous sclerosis complex 2 (TSC2), which is an inhibitor of mTORC1(Reference Rolfe, McLeod and Pratt52). As T1R1/T1R3 can be widely activated by l-amino acids(Reference Nelson, Chandrashekar and Hoon46), it is supposed that all of the BCAA (leucine, valine and isoleucine) could activate mTORC1 through this signalling pathway in the mammary gland. However, in bovine mammary glands, valine, not isoleucine and leucine, regulates the mTOR signalling pathway through the membrane GPRC receptor T1R1/T1R3(Reference Zhou, Zhou and Peng53), which might be due to insufficient supplementation with isoleucine and leucine. Future experiments are warranted to verify these results in the mammary cells of other species.
Methionine
Transportation system of methionine in the mammary gland
In the mammary gland, three amino acid transporter systems are involved in methionine transportation, namely systems A, ASC and L(Reference Verma and Kansal54). System A consists of Na-coupled neutral amino acid transporter 1 (SNAT1; detected in pigs) and Na-coupled neutral amino acid transporter 2 (SNAT2; detected in rats and cows)(Reference Shennan and Boyd55). System ASC primarily contains Na-dependent alanine cotransport 1 (ASCT1) (detected in humans, mice, cows and pigs) and Na-dependent alanine cotransport 2 (ASCT2) (detected in rats and cows)(Reference Shennan and Boyd55). System L is composed of two heteromeric Na+-independent transporters LAT1/4F2hc (detected in humans, rats, mice, cows) and LAT2/4F2hc (detected in rats and cows)(Reference Chillaron, Roca and Valencia56). In bovine mammary glands, SNAT2 inhibition strongly prevents the activation of mTORC1 caused by decreased methionine transportation(Reference Qi, Meng and Jin57). The functional evaluation of the importance of specific methionine transporters is still insufficient and warrants further research to demonstrate which transporter system may play a dominant role in the mammary gland.
Potential signalling pathway of methionine in the mammary gland
The effects of methionine on milk synthesis in the mammary gland have been demonstrated for many years (Table 3). It has been shown through meta-analyses that methionine is one of the first two limiting amino acids in dairy cows(Reference Schwab, Satter and Clay58,Reference Rulquin, Pisulewski and Vérité59) and is an important limiting amino acid in lactating sows(Reference Dourmad, Etienne and Valancogne60,61) . Intriguingly, the effect of methionine on milk fat synthesis is not linked to the use of methionine carbon in fatty acid synthesis since its ratio of mammary uptake to milk output is always at 1 in dairy cows(Reference Lapierre, Lobley and Doepel62). Advanced research in HEK 293 cells has demonstrated that when methionine is deficient, the cellular methyl donor S-adenosylmethionine (SAM) level will be reduced, which further increases the association of SAMTOR (SAM sensor) with GATOR2 and inhibits mTORC1 signalling(Reference Gu, Orozco and Saxton63). However, to the best of our knowledge, whether SAMTOR also acts as a conserved SAM sensor in the mammary gland has not been determined and merits further research.
Table 3. Effects of methionine on mammary gland function and its potential signalling pathways
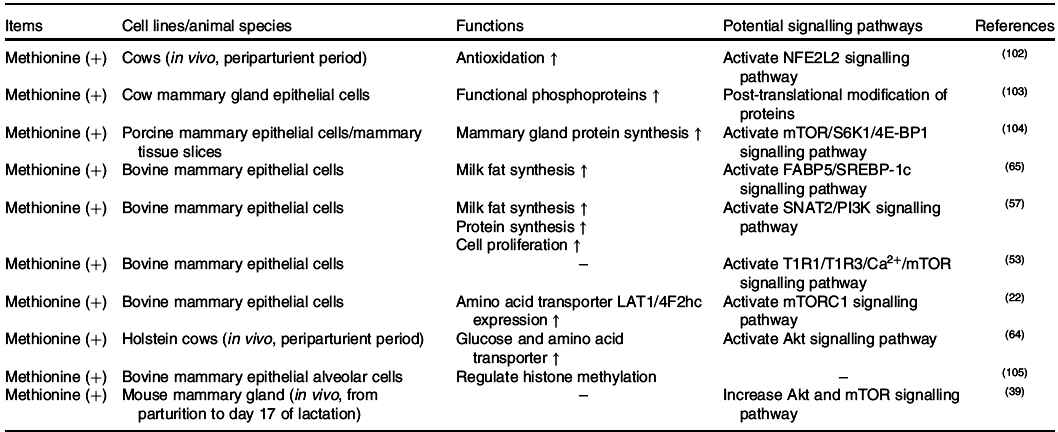
NFE2L2, nuclear factor erythroid 2-like 2; mTOR, mammalian target of rapamycin; S6K1, S6 kinase 1; 4E-BP1, eukaryotic initiation factor 4E-binding protein 1; FABP5, fatty acid-binding protein 5; SREBP-1c, sterol regulatory element-binding protein 1c; SNAT2, Na-coupled neutral amino acid transporter 2; PI3K, inositol 1,4,5-trisphosphate 3-kinase; mTORC1, mammalian target of rapamycin complex 1; Akt, protein kinase B.
Two other potential methionine-regulated mTORC1 signalling pathways have been verified in the mammary gland (Fig. 2). One possible approach is the inositol 1,4,5-trisphosphate 3-kinase (PI3K)/Akt signalling pathway. Activated PI3K/Akt/mTORC1 significantly stimulates milk protein synthesis(Reference Qi, Meng and Jin57,Reference Ma, Batistel and Xu64) . Furthermore, methionine also plays a crucial role in milk fat synthesis. Fatty acid-binding protein 5 (FABP5) is a crucial regulator that activates SREBP-1c for milk fatty synthesis(Reference Li, Yu and Zhou65), which can be partly activated by PI3K(Reference Lv, Wang and Zhang66,Reference Li, Li and Wang67) . All of this information indicates that methionine may regulate milk lipid synthesis through the PI3K/Akt/FABP5/SREBP-1c signalling pathway. The other novel and crucial signalling pathway is the T1R1/T1R3 signalling pathway. Similar to isoleucine and valine, methionine also increases the influx of intracellular Ca2+ and regulates the effects of mTORC1 through T1R1/T1R3 in the mammary gland(Reference Zhou, Zhou and Peng53).

Fig. 2. Methionine and mammalian target of rapamycin complex 1 (mTORC1) signalling networks in the mammary gland. Note: sodium-coupled neutral amino acid transporter 1 (SNAT1) and SNAT2 originate from transporter system A and are crucial methionine transporters in the mammary gland. Intracellular methionine increases cellular S-adenosylmethionine (SAM) levels, which decreases the association of SAMTOR (SAM sensor) with GTPase-activating protein activity toward Rags 2 (GATOR2) and inhibits the mTORC1 signalling pathway. In addition, intracellular methionine regulates mTORC1 through the inositol 1,4,5-trisphosphate 3-kinase/protein kinase B/Rheb (PI3K/Akt/Rheb) signalling pathway. Extracellular methionine activates the G-protein-coupled receptors (GPCR) T1R1/T1R3, increases phospholipase Cβ (PLCβ) activity and further enhances the influx of intracellular Ca2+. Increased Ca2+ regulates the mTORC1 signalling pathway through extracellular signal-regulated kinase 1/2–tuberous sclerosis complex 1/2–Rheb (ERK1/2–TSC1/2–Rheb) signalling. Activated mTORC1 increases milk protein synthesis and regulates milk fat synthesis through sterol regulatory element-binding protein 1 (SREBP-1) and fatty acid-binding protein 5 (FABP5). ER, endoplasmic reticulum. Please refer to the main text for details.
PI3K/Akt was previously demonstrated to be regulated by hormones, but not amino acids, in cell models. One possible cause of this discrepancy is that an unknown link between methionine and PI3K exists in the mammary gland. As IGF-1/PI3K/Akt/mTOR is the canonical pathway in cells(Reference Latres, Amini and Amini68,Reference Stitt, Drujan and Clarke69) , the other possible cause is that dietary methionine deficiency might indirectly inhibit PI3K activation via decreased IGF-1 secretion(Reference Miller, Buehner and Chang70–Reference Stubbs, Wheelhouse and Lomax72).
Arginine and lysine
Transportation system of arginine and lysine in the mammary gland
Arginine and lysine are both cationic amino acids and have the same amino acid transporter systems in the mammary gland(Reference Broer73). Four cationic amino acid transporter (CAT) systems have been identified in the mammary gland as follows: (1) y+ system: CAT-1 (detected in humans, cows, pigs and rats) and CAT-2 (detected in pigs); (2) y+L system: y+LAT1 (detected in pigs and cows) and y+LAT2 (detected in pigs); (3) b0,+ system: b0,+AT (detected in pigs); and (4) B0,+ system: ATB0,+ (detected in pigs, humans, rats)(Reference Laspiur, Burton and Weber74–Reference Shennan, McNeillie and Jamieson77). Among all transporters, CAT-1 seems to play a central role in arginine uptake in the mammary gland. In mammary MCF-7 cells, when 50 % cellular CAT-1 was knocked down, arginine uptake was inhibited by 35–40 %(Reference Abdelmagid, Rickard and McDonald78,Reference Too, Abdelmagid, Patel, Preedy and Rajendram79) . Furthermore, blocking ATB0,+ also inhibits arginine uptake in the MCF-7 cell line(Reference Karunakaran, Ramachandran and Coothankandaswamy80). Recently, ATB0,+ has also been considered as the most crucial lysine transporter in the mammary gland. In bovine mammary gland epithelial cells, lysine activates the mTOR signalling pathway, which is inhibited by blockade of the lysine transporter ATB0,+(Reference Lin, Li and Zou81). In the sow mammary gland, the transportation of lysine is partly inhibited by excessive arginine supplementation(Reference Hurley, Wang and Bryson82). This evidence indicates that arginine and lysine have the same critical transporter system (CAT-1 and ATB0,+) in the mammary gland.
Potential signalling pathway of arginine in the mammary gland
Positive effects of arginine on placental growth and fetal survival and growth have been demonstrated in pigs, rats, mice and sheep(Reference Wu, Bazer and Satterfield83), whereas its functions in the mammary gland were not determined until recently (Table. 4). In the mammary gland, arginine catabolism produces proline, ornithine, urea, glutamate, glutamine, CO2 and polyamines (putrescine, spermidine and spermine)(Reference O’Quinn, Knabe and Wu84). In sows, supplementation with 0·5 or 1·0 % l-Arg-HCl activates the milk synthesis and increases the litter weight of sucking piglets(Reference Cui, Guo and Gao85). Similar to other amino acids, arginine also regulates milk protein synthesis through mTORC1(Reference Ma, Hu and Bannai86,Reference Wang, Xu and Wang87) . The central regulator linking arginine to the mTORC1 signalling pathway is cellular arginine sensor for mTORC1 (CASTOR1)(Reference Chantranupong, Scaria and Saxton88,Reference Saxton, Chantranupong and Knockenhauer89) . Sufficient arginine dissociates GATOR2 from CASTOR1 and further activates the mTOR signalling pathway(Reference Chantranupong, Scaria and Saxton88,Reference Saxton, Chantranupong and Knockenhauer89) (Fig. 3). The other crucial function of arginine in the mammary gland is primarily achieved through its metabolite NO(Reference Kim and Wu90). Briefly, NO increases the mammary blood vessel density and diameter, which might enhance the transportation of nutrients to the mammary gland and support milk synthesis(Reference Holanda, Marcolla and Guimarães91).
Table 4. Effects of arginine on mammary gland function and its potential signalling pathways
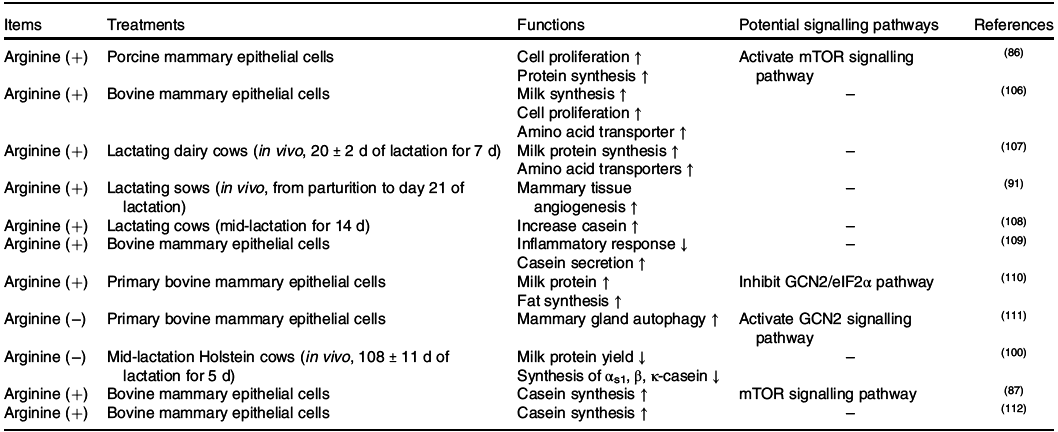
mTOR, mammalian target of rapamycin; GCN2, general control non-derepressible 2; eIF, eukaryotic initiation factor.

Fig. 3. Lysine and arginine regulate the mammalian target of rapamycin complex 1 (mTORC1) signalling network in the mammary gland. Note: cationic amino acid transporter-1 (CAT-1) and ATB0,+ are critical cationic amino acid transporters for arginine and lysine transportation in the mammary gland. The intracellular arginine regulator mTORC1 acts through the cellular arginine sensor for mTORC1–GTPase-activating protein activity toward Rags 2–GTPase-activating protein activity toward Rags 1–RagA/B (CASTOR1–GATOR2–GATOR1–RagA/B) signalling pathway, whereas extracellular lysine regulates mTORC1 through the G-protein-coupled receptor (GPCR) GPCR6A. As a Gαi/Gαq receptor, GPCR6A can activate milk protein synthesis through the GPRC6A–inositol 1,4,5-trisphosphate 3-kinase–protein kinase B–tuberous sclerosis complex 1/2–Rheb (GPRC6A–PI3K–Akt–TSC1/2–Rheb) and GPRC6A–extracellular signal-regulated kinase 1/2 (ERK1/2)–TSC1/2–Rheb pathways. Dashed lines represent potential signalling pathways that have not been verified in the mammary gland. FABP5, fatty acid-binding protein 5; SREBP-1, sterol regulatory element-binding protein 1. Please refer to the main text for details.
Potential signalling pathway of lysine in the mammary gland
Similar to methionine, lysine is one of the first two limiting amino acids both in dairy cows(Reference Rulquin, Pisulewski and Vérité59,92) and sows(Reference Dourmad, Etienne and Valancogne60,61) . In the bovine mammary gland, the use of lysine is dose dependent as it can be the first limiting amino acid. In case of low supply, lysine is mainly utilised in milk protein synthesis with the ratio of lysine uptake to lysine output close to 1(Reference Haque, Guinard-Flament and Lamberton8). However, when sufficient amount of lysine is provided through the diet, it can also be used to either synthesise non-essential amino acids(Reference Lapierre, Lobley and Doepel62,Reference Lapierre, Doepel and Milne93) or be oxidised into CO2 as BCAA(Reference Lin, Li and Zou81). When lysine is deficient, milk protein synthesis is inhibited(Reference Doelman, Kim and Carson94), with a decrease in mTORC1 activity in dairy cows(Reference Dong, Zhou and Saremi95). However, the function of lysine in the mammary gland has largely not been determined. Recent advances have found that lysine increases milk fat synthesis through the GPRC6A/PI3K/FABP5 signalling pathway(Reference Li, Li and Wang67). GPRC6A is a G protein-coupled receptor that is specific for cationic amino acid sensing(Reference Clemmensen, Smajilovic and Wellendorph96). As a Gαi/Gαq receptor, GPRC6A has the potential to regulate cellular cAMP levels and activate the MAPK signalling pathway(Reference Husted, Trauelsen and Rudenko97). Thus, both GPRC6A/PI3K/Akt/mTOR and GPRC6A/ERK/mTOR can be the potential signalling pathways for lysine to regulate milk protein and fat synthesis in the mammary gland.
Conclusion
Amino acids play crucial roles in the synthesis of milk protein and fat in the mammary gland. The dominant amino acid transposers (BCAA, methionine, lysine and arginine) of the mammary gland are summarised in the present review. In addition, our review has focused on a number of canonical and novel signalling molecules involved in amino acid signalling pathway in the mammary gland. Remarkably, mTORC1 acts as the central node of the amino acid-regulated signalling pathway and can be activated intracellularly and extracellularly (through a G-protein-coupled receptor (GPCR)). Currently, the amino acid signalling pathway in the mammary gland still warrants further investigation. Achieving a better understanding of the amino acid signalling pathway might help us to optimise the amino acid profiles in maternal diets for human beings and other mammals in the future.
Acknowledgements
The present review was supported by the National Natural Science Foundation of the Peopleʼs Republic of China (no. 31802067 and 31872364) and the Natural Science Foundation of Guangdong Province (no. 2018A030310201).
S. Z. initiated the idea, the scope, and the outline of this review paper. Z. W., J. H., M. T., H. S. and F. C. studied and analysed all of the publications cited in this paper and were involved in manuscript preparation. W. G. conducted the final editing and proofreading. All authors read and approved the final manuscript.
The authors declare that they have no competing interests.









