Introduction
Two main causes of death and morbidity in the world are CVD and diabetes. The metabolic syndrome (MetS) is a cluster of important risk factors related to these diseases, including central obesity, dyslipidaemia, elevated fasting plasma glucose and hypertension. Having the MetS increases the risk of developing CVD and diabetes by two and five times, respectively( Reference Alberti, Eckel and Grundy 1 ). Genetics, physical activity and diet are known key factors to make an impact on the status of this syndrome. The National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) and the American Heart Association have recommended diet-based plans as one principal approach to prevent metabolic disorders predisposing to CVD( 2 , Reference Sonnenberg, Pencina and Kimokoti 3 ).
Diet is composed of interacting and inter-correlated nutrients, making it challenging to identify the influence of each nutrient independently( Reference Hu 4 ). Moreover, metabolic disorders including the MetS have been shown to have an association with food and dietary patterns rather than nutrients, with a few exceptions such as vitamin D( Reference Forouhi and Sattar 5 – Reference Fung, Steffen and Zhou 7 ). Researchers have been successful in reducing the risk of MetS development when using an overall diet modification approach in interventional trials( Reference de Lorgeril, Salen and Martin 8 ). Therefore, exploring dietary patterns with regards to the MetS may be a beneficial way to understand the impact of diet on the MetS( Reference Hu 4 ).
Dietary patterns are determined based on two main approaches including the a posteriori and a priori methods( Reference Panagiotakos, Pitsavos and Chrysohoou 9 ). In the a posteriori approach, derived data are applied in multivariate statistical approaches to explain the inter-correlation of food or food groups. This approach includes cluster analysis, common factor analysis and principal component analysis as well as new approaches such as reduced ranked regression and partial least squares regression( Reference Hoffmann, Schulze and Schienkiewitz 10 ). In the second main analytical approach, the a priori, existing food/food group knowledge, guidelines and recommendations, and healthy known dietary patterns are used to develop indices( Reference Davenport, Roderick and Elliott 11 , Reference Garriguet 12 ).
Researchers have evaluated the association between dietary patterns and the MetS using different study designs including randomised controlled trials (RCT) and population-based studies( Reference Kastorini, Milionis and Esposito 13 ). Kastorini et al. ( Reference Kastorini, Milionis and Esposito 13 ) have conducted a meta-analysis to evaluate the association of the MetS and Mediterranean diet using both population-based studies and clinical trials published up to 2010. The updated systematic review by Esposito et al. ( Reference Esposito, Kastorini and Panagiotakos 14 ) in 2013 confirms their previous results (Kastorini et al. ( Reference Kastorini, Milionis and Esposito 13 )). Further, Calton et al. ( Reference Calton, James and Pannu 15 ) conducted a review on the literature from 2000 to 2012 including prospective studies and RCT to evaluate the beneficial dietary patterns that have a protective role on MetS status with emphasis on the contribution of these patterns in the Asia–Pacific region. They have also concluded the beneficial effects of the Mediterranean diet, Nordic diet and Dietary Approaches to Stop Hypertension (DASH) diet and the need for further RCT to investigate their effect for the future. High-standard RCT are the most valuable evidence for inferring causality. However, we chose to focus on population-based studies where subjects are consuming their usual self-selected diets for two reasons. First, this allows investigation of ‘real-world’ populations with different characteristics( Reference Meyskens and Szabo 16 , Reference Willett 17 ). Second, population-based studies help in understanding the dietary patterns that are prevalent among a population and their association with the MetS. This focus would contribute to understanding the real-life dietary practices of populations upon which dietary recommendations can be structured( Reference Satija, Yu, Willett and Hu 18 ).
The association between the MetS and dietary patterns, however, has not been recently evaluated using population-based studies. Furthermore, the methodologies to extract the dietary patterns among populations have not been categorised and compared within this context. Therefore, the objective of this research study is to perform a scoping review of the most recent evidence on the association between dietary patterns and the MetS using population-based studies. A secondary aim was to identify commonly used methodological approaches for investigating dietary patterns and justifications behind them among population-based studies in developed and developing countries.
Methodology
For the purpose of the present review, a scoping literature review was conducted using the framework provided by Arksey & O’Malley( Reference Arksey and O’Malley 19 ). A scoping review, a tool to understand the available knowledge of a field( Reference Sanou, O’Reilly and Ngnie-Teta 20 ), includes the following steps: identifying the studies based on the research question; selecting studies and charting the data; and, finally, summarising the results( Reference Arksey and O’Malley 19 ). We conducted the review based on the following research question: what is the relationship between dietary patterns and the MetS among populations comparing two common methods of dietary pattern approaches including the a priori and the data-driven approaches?
The inclusion criteria considered for this review study were: (1) published full-text articles between 2005 and 2014 (inclusive); (2) studies that investigated the association between MetS status and dietary patterns (using a priori-defined and/or data-driven analytical approaches); (3) population-based studies including cohorts and cross-sectional-designed studies with a sample size of more than 300 individuals; and (4) English-language journals. Exclusion criteria were: (1) studies that have used uncommon dietary pattern analytical approaches (for example, reduced ranked regression and partial least square); (2) studies that have focused on a special migrated ethnicity to another country; and (3) review articles.
The search was conducted using three electronic databases including MEDLINE, EMBASE and hand searching in Google Scholar. As well, the bibliographies of relevant articles were evaluated. Initially, the combination of keywords/subject headings of ‘metabolic syndrome’, ‘metabolic syndrome X’, ‘insulin resistance syndrome’ with general terms of the topic including ‘diet’ and ‘dietary pattern’ or specific dietary pattern methods including the following terms: ‘factor analysis’, ‘cluster analysis’, ‘reduced rank regression’, ‘partial-least square regression’, ‘dietary index’, ‘Mediterranean diet’, ‘Healthy Eating Index’, ‘DASH/Dietary Approaches to Stop Hypertension’, ‘Dietary Guidelines for American Index’ and ‘vegetarian diet’ were searched. The identified studies were evaluated in terms of inclusion and exclusion criteria by the three authors independently (Z. H., H. V. and S. W.). In case of disagreement, discussion led to consensus among the authors. The selection procedure was done starting from evaluating the title, abstract and full text. The subject headings/keywords used to conduct the search in MEDLINE and EMBASE are included in the Supplementary document.
Results
Characteristics of studies
From a total of 4660 records screened (2560 records from MEDLINE and EMBASE and 2100 records from hand searching in Google Scholar), ninety-eight full-text articles were assessed for eligibility. A total of thirty-nine studies, published from 2005 to 2014, met the inclusion criteria and were included in the present review. Seven of these epidemiological studies had longitudinal( Reference Duffey, Steffen and Van Horn 21 – Reference Pimenta, Toledo and Rodriguez-Diez 27 ) design and the remaining (n 32) had a cross-sectional design. The studies were conducted in twenty-three different countries including Algeria( Reference Thanopoulou, Karamanos and Angelico 28 ), Australia( Reference Ambrosini, Huang and Mori 29 ), Bulgaria( Reference Thanopoulou, Karamanos and Angelico 28 ), China( Reference He, Li and Lai 30 ), Egypt( Reference Thanopoulou, Karamanos and Angelico 28 ), Finland( Reference Kouki, Schwab and Lakka 31 ), France( Reference Kesse-Guyot, Ahluwalia and Lassale 25 ), Germany (n 2)( Reference Barbaresko, Siegert and Koch 32 , Reference Heidemann, Scheidt-Nave and Richter 33 ), Greece (n 4)( Reference Thanopoulou, Karamanos and Angelico 28 , Reference Panagiotakos, Pitsavos and Skoumas 34 – Reference Gouveri, Tzavara and Drakopanagiotakis 36 ), Guatemala( Reference Gregory, McCullough and Ramirez-Zea 37 ), Iran (n 3)( Reference Esmaillzadeh, Kimiagar and Mehrabi 38 – Reference Saneei, Fallahi and Barak 40 ), Italy (n 3)( Reference Thanopoulou, Karamanos and Angelico 28 , Reference Buscemi, Sprini and Grosso 41 , Reference Leite and Nicolosi 42 ), Japan( Reference Akter, Nanri and Pham 43 ), Korea (n 4)( Reference Hong, Song and Lee 44 – Reference Kim and Jo 47 ), Lebanon( Reference Naja, Nasreddine and Itani 48 ), Mexico( Reference Denova-Gutiérrez, Castañón and Talavera 49 ), Portugal( Reference Fonseca, Gaio and Lopes 50 ), Samoa( Reference DiBello, McGarvey and Kraft 51 ), Serbia-Montenegro( Reference Thanopoulou, Karamanos and Angelico 28 ), Spain( Reference Álvarez León, Henriquez and Serra-Majem 52 ), Sweden( Reference Berg, Lappas and Strandhagen 53 ), Taiwan( Reference Shang, Shu and Wang 26 ) and the USA (n 10)( Reference Sonnenberg, Pencina and Kimokoti 3 , Reference Duffey, Steffen and Van Horn 21 – Reference Rumawas, Meigs and Dwyer 24 , Reference Deshmukh-Taskar, O’Neil and Nicklas 54 – Reference Pan and Pratt 58 ). The studies enrolled from 323( Reference Naja, Nasreddine and Itani 48 ) to over 93 209( Reference Shang, Shu and Wang 26 ) participants.
Study populations
General adult populations over the age of 18 years were mainly studied. In addition, the middle-aged and/or elderly population, which included individuals over 45 years of age, was the focus of four studies( Reference Lutsey, Steffen and Stevens 23 , Reference He, Li and Lai 30 , Reference Esmaillzadeh, Kimiagar and Mehrabi 38 , Reference Leite and Nicolosi 42 ). Only two studies( Reference Ambrosini, Huang and Mori 29 , Reference Pan and Pratt 58 ) evaluated the association of the MetS and dietary patterns among adolescents. Five studies included only women( Reference Sonnenberg, Pencina and Kimokoti 3 , Reference Kimokoti, Gona and Zhu 22 , Reference Esmaillzadeh, Kimiagar and Mehrabi 38 , Reference Saneei, Fallahi and Barak 40 , Reference Cho, Kim and Cho 45 ), one study included only men( Reference Yang, Farioli and Korre 55 ) and the remaining included both sexes as their participants.
Measurement of the metabolic syndrome
The NCEP-ATP III criteria( 2 ) or its adjusted or modified versions were mainly used to evaluate the MetS. However, some studies used the joint 2005 International Diabetes Federation (IDF) and American Heart Association criteria( Reference Alberti, Eckel and Grundy 1 ) and other studies used the IDF criteria( Reference Alberti, Zimmet and Shaw 59 ) to define the MetS in the population. Among the adolescent population, the MetS was evaluated using the age-adjusted NCEP-ATP III criteria by Pan & Pratt( Reference Pan and Pratt 58 ) and cluster analysis of MetS components was used by Ambrosini et al. ( Reference Ambrosini, Huang and Mori 29 ).
Determining dietary patterns
The dietary patterns were assessed using both a posteriori (i.e. factor analysis and cluster analysis) and a priori analytical approach (index-based) methods.
Dietary patterns based on a posteriori analytical methods
Cluster analysis and factor analysis were the most common a posteriori methods used to determine dietary patterns among different populations. Cluster analysis was used in six articles summarised in Table 1. In each of the studies two to five clusters were extracted. Each of the six studies had clusters representative of a healthy and an unhealthy dietary pattern. The findings of four studies( Reference Sonnenberg, Pencina and Kimokoti 3 , Reference Duffey, Steffen and Van Horn 21 , Reference Leite and Nicolosi 42 , Reference Berg, Lappas and Strandhagen 53 ) indicated a higher risk of the MetS among the populations within the unhealthier clusters compared with the healthier clusters. Seventeen studies used the factor analysis method to obtain the patterns prevalent among their study population and to examine the patterns’ association with MetS prevalence or incidence indicated in Table 2. These studies have found two to six dietary patterns for their study population. The dietary patterns extracted among their study populations included ‘Western/unhealthy’ (n 13)( Reference Lutsey, Steffen and Stevens 23 , Reference Ambrosini, Huang and Mori 29 , Reference He, Li and Lai 30 , Reference Heidemann, Scheidt-Nave and Richter 33 , Reference Panagiotakos, Pitsavos and Skoumas 34 , Reference Esmaillzadeh, Kimiagar and Mehrabi 38 , Reference Buscemi, Sprini and Grosso 41 , Reference Akter, Nanri and Pham 43 , Reference Cho, Kim and Cho 45 , Reference Naja, Nasreddine and Itani 48 , Reference Denova-Gutiérrez, Castañón and Talavera 49 , Reference DiBello, McGarvey and Kraft 51 , Reference Deshmukh-Taskar, O’Neil and Nicklas 54 ), ‘healthy’ (n 10)( Reference Lutsey, Steffen and Stevens 23 , Reference Ambrosini, Huang and Mori 29 , Reference Heidemann, Scheidt-Nave and Richter 33 , Reference Panagiotakos, Pitsavos and Skoumas 34 , Reference Esmaillzadeh, Kimiagar and Mehrabi 38 , Reference Buscemi, Sprini and Grosso 41 , Reference Akter, Nanri and Pham 43 , Reference Cho, Kim and Cho 45 , Reference Denova-Gutiérrez, Castañón and Talavera 49 , Reference Deshmukh-Taskar, O’Neil and Nicklas 54 ) and ‘traditional’ (n 6)( Reference He, Li and Lai 30 , Reference Barbaresko, Siegert and Koch 32 , Reference Esmaillzadeh, Kimiagar and Mehrabi 38 , Reference Cho, Kim and Cho 45 , Reference Naja, Nasreddine and Itani 48 , Reference DiBello, McGarvey and Kraft 51 ) dietary patterns. The dietary patterns observed in three of the studies were unclear( Reference Hong, Song and Lee 44 , Reference Kim and Jo 47 , Reference Fonseca, Gaio and Lopes 50 ); therefore, they were not included in this classification (summarised at the bottom of Table 2). Based on these studies, the ‘healthy’ patterns were characterised by high intakes of vegetables, fruits, legumes, whole grains and fish. The unhealthy or the so-called ‘Western’ dietary pattern mainly constituted red/processed meat, refined grains, sweets, sugar-sweetened beverages and processed food, which resembled an unhealthy diet. The ‘traditional’ dietary patterns were specific for each country, for example, Lebanese, Korean or German ‘traditional’ dietary pattern. Further, ten studies, which had found a ‘Western/unhealthy’ dietary pattern among their populations, also found a direct association between this type of dietary pattern and MetS risk( Reference Lutsey, Steffen and Stevens 23 , Reference Ambrosini, Huang and Mori 29 , Reference He, Li and Lai 30 , Reference Heidemann, Scheidt-Nave and Richter 33 , Reference Panagiotakos, Pitsavos and Skoumas 34 , Reference Esmaillzadeh, Kimiagar and Mehrabi 38 , Reference Akter, Nanri and Pham 43 , Reference Naja, Nasreddine and Itani 48 , Reference Denova-Gutiérrez, Castañón and Talavera 49 , Reference DiBello, McGarvey and Kraft 51 ). Four out of ten studies that found a ‘healthy’ dietary pattern among their population indicated an inverse association between the healthy dietary patterns and risk of the MetS( Reference Panagiotakos, Pitsavos and Skoumas 34 , Reference Esmaillzadeh, Kimiagar and Mehrabi 38 , Reference Cho, Kim and Cho 45 , Reference Deshmukh-Taskar, O’Neil and Nicklas 54 ). Regarding the third most common pattern, the ‘traditional’ dietary pattern, mixed results showing both inverse( Reference He, Li and Lai 30 , Reference DiBello, McGarvey and Kraft 51 ) and direct( Reference He, Li and Lai 30 , Reference Barbaresko, Siegert and Koch 32 ) association between this type of diet and the MetS were obtained. Other dietary patterns similar to the ‘Western’ dietary pattern were found by the researchers, named as ‘high glycaemic index and high-fat’( Reference Panagiotakos, Pitsavos and Skoumas 34 ), ‘modern’( Reference DiBello, McGarvey and Kraft 51 ), ‘processed foods’( Reference Heidemann, Scheidt-Nave and Richter 33 ) and ‘fast food/desserts’( Reference Naja, Nasreddine and Itani 48 ) dietary patterns.
Table 1 Summary of population-based studies that have investigated the relationship between dietary patterns, derived using the cluster analysis method from 2005 to 2014

MetS, metabolic syndrome; T, total population size; M, male population size; F, female population size; PA, physical activity; FUP, follow-up period; EI, energy intake.
Table 2 Summary of population-based studiesthat have investigated the relationship between dietary patterns, derived using the factor analysis method from 2005 to 2014

MetS, metabolic syndrome; T, total population size; M, male population size; F, female population size; PA, physical activity; EI, energy intake; FUP, follow-up period; SES, socio-economic status.
In the aforementioned studies the analyses were adjusted for common potential confounders including age, sex, socio-economic status, energy intake, physical activity, BMI, smoking status, self-reported history or family history of chronic diseases and medication usage.
Dietary patterns based on a priori analytical methods
Sixteen studies( Reference Rumawas, Meigs and Dwyer 24 – Reference Thanopoulou, Karamanos and Angelico 28 , Reference Kouki, Schwab and Lakka 31 , Reference Tzima, Pitsavos and Panagiotakos 35 – Reference Gregory, McCullough and Ramirez-Zea 37 , Reference Hosseini-Esfahani, Jessri and Mirmiran 39 , Reference Saneei, Fallahi and Barak 40 , Reference Álvarez León, Henriquez and Serra-Majem 52 , Reference Yang, Farioli and Korre 55 – Reference Pan and Pratt 58 ) indicated in Table 3 used the a priori scoring method to investigate the relationship of the MetS and dietary patterns. These studies used different scoring methods to obtain an overall diet score for each individual in the population. The following score-based methods and indices were used: Alternative Healthy Eating Index-2006( Reference Pimenta, Toledo and Rodriguez-Diez 27 ), DASH( Reference Pimenta, Toledo and Rodriguez-Diez 27 , Reference Saneei, Fallahi and Barak 40 ), Dietary Guidelines for Americans Index( Reference Pimenta, Toledo and Rodriguez-Diez 27 , Reference Hosseini-Esfahani, Jessri and Mirmiran 39 , Reference Fogli-Cawley, Dwyer and Saltzman 56 ), Dietary Inflammatory Index( Reference Pimenta, Toledo and Rodriguez-Diez 27 ), Diet Quality Index-International( Reference Pimenta, Toledo and Rodriguez-Diez 27 , Reference Gregory, McCullough and Ramirez-Zea 37 ), Healthy Eating Index-1995( Reference Pan and Pratt 58 ), MedDiet score( Reference Tzima, Pitsavos and Panagiotakos 35 , Reference Gouveri, Tzavara and Drakopanagiotakis 36 ), Mediterranean Adequacy Index( Reference Pimenta, Toledo and Rodriguez-Diez 27 ), Mediterranean Diet Quality Index( Reference Pimenta, Toledo and Rodriguez-Diez 27 ), Mediterranean Diet Score( Reference Kesse-Guyot, Ahluwalia and Lassale 25 , Reference Pimenta, Toledo and Rodriguez-Diez 27 ), Mediterranean Food Pattern( Reference Pimenta, Toledo and Rodriguez-Diez 27 ), Mediterranean Score( Reference Kesse-Guyot, Ahluwalia and Lassale 25 ), Mediterranean-Style Dietary Pattern Score( Reference Kesse-Guyot, Ahluwalia and Lassale 25 ), Modified Mediterranean Diet Score( Reference Pimenta, Toledo and Rodriguez-Diez 27 , Reference Yang, Farioli and Korre 55 ), Pro-Vegetarian Diet( Reference Pimenta, Toledo and Rodriguez-Diez 27 ), Recommended Food Score( Reference Gregory, McCullough and Ramirez-Zea 37 ), Not Recommended Food Score( Reference Gregory, McCullough and Ramirez-Zea 37 ), Total Mediterranean score( Reference Álvarez León, Henriquez and Serra-Majem 52 ), Traditional Mediterranean diet score( Reference Thanopoulou, Karamanos and Angelico 28 ) and other vegetarian dietary patterns( Reference Shang, Shu and Wang 26 , Reference Rizzo, Sabate and Jaceldo-Siegl 57 ).
Table 3 Summary of population-based studies that have investigated the relationship between a priori dietary patterns and the metabolic syndrome from 2005 to 2014
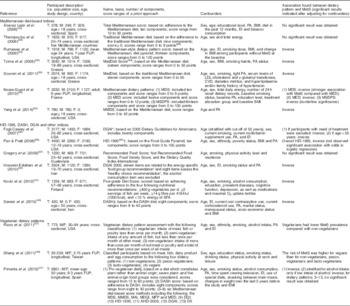
MetS, metabolic syndrome; T, total population size; M, male population size; F, female population size; PA, physical activity; EI, energy intake; FUP, follow-up period; MDS, Mediterranean Diet Score; MED, Mediterranean Score; MSDPS, Mediterranean Style Dietary Pattern Score; MMDS, Modified Mediterranean Diet Score; HEI-1995, Healthy Eating Index-1995; DASH, Dietary Approaches to Stop Hypertension; DGAI, Dietary Guidelines for Americans Index; MAI, Mediterranean Adequacy Index; MDQI, Mediterranean Diet Quality Index; MFP, Mediterranean Food Pattern; DQI, Diet Quality Index-International; AHEI-2006, Alternative Healthy Eating Index-2006; DII, Dietary Inflammatory Index.
* Another dietary index developed to assess the dietary pattern of individual in epidemiological studies is the Dietary Guidelines for Americans Index. This dietary index is developed based on the adherence to the Dietary Guidelines for Americans. The purpose of the Dietary Guidelines for Americans is to reduce the risk of chronic conditions such as CVD. This index includes twenty categories within two main components. The first component is related to the recommendations regarding intake of energy-specific food groups, which includes eleven categories. These items include fruits; five vegetable subgroups; a variety of vegetables; grains; milk and milk products; meat and legumes; and discretionary energy. The second component is the healthy choice or nutrient intake recommendations that include nine categories. The items are as follows: fibre intake; percentage of grains that are whole grain; Na intake; alcohol consumption; five recommendations related to fat and cholesterol intake, including, total fat and saturated fat as a percentage of energy, low-fat milk and meat choices, trans-fat intake and cholesterol intake( Reference Fogli-Cawley, Dwyer and Saltzman 56 ).
† One dietary index developed based on the adherence to the Food Guide Pyramid is the Healthy Eating Index. This index includes the sum of scores from the following ten components: grains, vegetables, fruits, milk, meat or meat alternatives, total fat intake, saturated fat, cholesterol, Na and diet variety. The scores range from 0 to 100, with higher scores indicating a better diet quality( Reference Kennedy, Ohls and Carlson 85 ).
‡ The Dietary Approaches to Stop Hypertension (DASH) score is based on the adherence to the DASH diet plan developed to control specific chronic conditions including hypertension. The scoring method to evaluate the adherence to DASH was based on the consumption of eight foods as adequate or inadequate foods. The eight components were as follows: high intake of fruits; vegetables; nuts and legumes; low-fat dairy products; whole grains and low intake of Na, sweetened beverages, and red and processed meats. The sum of the scores ranged from 8 to 40 points, with the higher scores indicating a greater adherence. The DASH diet is mainly investigated in randomised controlled studies( Reference Saneei, Hashemipour and Kelishadi 86 , Reference Al-Solaiman, Jesri and Mountford 87 ).
§ The Pro-Vegetarian Diet is a diet based on plants rather than food derived from animals. Seven plant- and five animal-origin food groups were considered. The score of this diet ranges from 12 to 60, higher scores indicating better adherence to this diet. The results showed an inverse association between Pro-Vegetarian Diet scores and MetS incidence( Reference Pimenta, Toledo and Rodriguez-Diez 27 ).
The studies that used the Healthy Eating Index-1995, Dietary Guidelines for Americans Index or indices developed based on the adherence to a vegetarian diet for evaluating their populations’ diet quality obtained mixed results, indicating a negative association or no significant association between these diets and risk of the MetS (Table 3). However, the two studies using the DASH diet score indicated the higher the DASH score the lower the risk of the MetS was for their population( Reference Pimenta, Toledo and Rodriguez-Diez 27 , Reference Saneei, Fallahi and Barak 40 ).
Adherence to the Mediterranean diet or pyramid has been evaluated by eight studies( Reference Rumawas, Meigs and Dwyer 24 , Reference Kesse-Guyot, Ahluwalia and Lassale 25 , Reference Pimenta, Toledo and Rodriguez-Diez 27 , Reference Thanopoulou, Karamanos and Angelico 28 , Reference Tzima, Pitsavos and Panagiotakos 35 , Reference Gouveri, Tzavara and Drakopanagiotakis 36 , Reference Álvarez León, Henriquez and Serra-Majem 52 , Reference Yang, Farioli and Korre 55 ). In these studies, the Mediterranean dietary pattern consisted of high intakes of fruits, vegetables, cereals, legumes, fish, nuts, olives and a high ratio of monounsaturated to saturated fats, moderate to low intake of alcohol/wine, dairy products and meat. Five of these studies found that the higher the adherences to the Mediterranean diet the lower the risk of the MetS would be( Reference Rumawas, Meigs and Dwyer 24 , Reference Kesse-Guyot, Ahluwalia and Lassale 25 , Reference Tzima, Pitsavos and Panagiotakos 35 , Reference Gouveri, Tzavara and Drakopanagiotakis 36 , Reference Yang, Farioli and Korre 55 ). The remaining studies did not find any significant results( Reference Pimenta, Toledo and Rodriguez-Diez 27 , Reference Thanopoulou, Karamanos and Angelico 28 , Reference Álvarez León, Henriquez and Serra-Majem 52 ).
Discussion
Based on the search strategy of the present review, thirty-nine population-based studies were identified that investigated the association of the MetS and dietary patterns. Findings of the present review showed that population-based studies have been conducted in different countries especially in European and North American countries, and on adult populations of both sexes. These studies tended to use the factor analysis method and also the a priori dietary approaches, such as the Mediterranean dietary pattern. Based on this review of population-based studies, the Mediterranean diet and the ‘Western’ dietary pattern seemed to be the two extremes of the MetS and diet association continuum.
The relationship between MetS components and CVD is established from the early stages of life and remains until adulthood. Therefore, it is essential to identify and investigate the presence of the MetS in children and adolescents( Reference Rosenberg, Moran and Sinaiko 60 ). Based on the findings of this review study a gap exists in the investigation of the MetS among adolescents and children. This gap is a consequence of recommendations by authoritative bodies to investigate the MetS only among individuals above the age of 10 years; as well, there remains an inconsistency in MetS definitions used by researchers working with children and adolescents( Reference Ford, Ajani and Mokdad 61 – Reference Jolliffe and Janssen 63 ). The methodological approach in determining the dietary pattern of a population, whether a priori or a posteriori, was chosen based on the research question. However, researchers did not explain the reason behind the choice of methodological approach in a posteriori methods. Those who used the a priori methods indicated that assessing adherence to the specific diet through its relevant index was the reasoning behind their choice.
The a posteriori analytical methods
Among the aforementioned possible a posteriori approaches, factor analysis and cluster analysis were the most common methods used in the epidemiological studies included in this review and similarly indicated by Newby & Tucker( Reference Newby and Tucker 64 ). In factor analysis, the dietary patterns are determined by statistically evaluating the correlation between the entering variables, which generate discrete factors from similar input variables( Reference Oddy, Herbison and Jacoby 65 ), while cluster analysis is a method in which individuals with similar dietary characteristics are aggregated into one categorical cluster( Reference Hu 4 ). These methods extract the actual dietary patterns of the populations. However, the disadvantage of these two commonly used methods is their subjectivity and that they do not account for the disease risk( Reference Hoffmann, Schulze and Schienkiewitz 10 ). An approach that derives dietary patterns based on disease risk is the reduced ranked regression method, which has been introduced to nutritional epidemiology by Hoffmann et al. ( Reference Hoffmann, Schulze and Schienkiewitz 10 ). This method is mainly based on the scientific evidence of disease-specific response variables, which may be the components of a disease or nutrients related to a disease( Reference Hoffmann, Schulze and Schienkiewitz 10 ). To our knowledge, three studies( Reference Barbaresko, Siegert and Koch 32 , Reference Yeh, Chang and Pan 66 , Reference Liu, Nettleton and Bertoni 67 ) have used the reduced ranked regression method, which focuses on nutrients or MetS components as dependent variables to investigate the association between the MetS and diet. Therefore, based on the aim, to have real-world dietary patterns or dietary patterns related to a specific disease risk, researchers have to choose one of these empirical analytical methods( Reference Hoffmann, Schulze and Schienkiewitz 10 ). Researchers should consider potential limitations when applying a posteriori methods including the limited reproducibility due to several decision-making points and the limited data available regarding the validity of this approach in epidemiological studies( Reference Moeller, Reedy and Millen 68 ).
In nearly all studies, which have used factor analysis or cluster analysis, both a ‘Western/unhealthy’ and a ‘healthy/prudent’ dietary pattern were observed to be prevalent among their study populations. As for the ‘Western/unhealthy’ dietary pattern and similar dietary patterns such as ‘energy dense’, ‘fast energy’, ‘empty calorie’ and ‘modern’, these patterns were shown to have a direct association with MetS status in most of the studies. Even though in some of the studies indicated in Tables 1 and 2, researchers adjusted for BMI, weight change, energy intake, physical activity and smoking status as potential confounders, the association remained singificant. This suggests that the effect of the ‘Western/unhealthy’ dietary pattern on MetS status is beyond the effect of anthropometric and other lifestyle factors as similarly indicated by Calton et al. ( Reference Calton, James and Pannu 15 ). Previous evidence suggests that high intakes of refined grains, sugar, saturated fats and low intake of fruits and vegetables increase the risk of the MetS by increasing inflammation( Reference Giugliano, Ceriello and Esposito 69 ). This can be one explanation for the associations observed among dietary patterns and the MetS. Further, findings of only four of these studies have indicated an inverse association between the ‘healthy’ dietary pattern and the MetS.
The a priori analytical methods
The overall aim of the a priori approach is to compare and classify the population into categories based on their adherence to recommendations or well-known healthy diets( Reference Panagiotakos 70 ). This score-based method is used to indicate the characteristics of the overall diet( Reference Davenport, Roderick and Elliott 11 , Reference Garriguet 12 ). While this method is more reproducible compared with the a posteriori methods( Reference Moeller, Reedy and Millen 68 ), the disadvantage of using recommendations to develop an index of diet quality is that the index score is the sum of the points allocated to each of the components of the index. The inter-correlation of the score components with one another may have not been proven. Hence, a total score is not representative of the overall effect of the diet( Reference Hoffmann, Schulze and Schienkiewitz 10 ). Studies included in this review that have used the Healthy Eating Index-1995, Dietary Guidelines for Americans Index and the vegetarian dietary patterns observed mixed results, which indicate the need for further research.
In addition to the recommendation-based indices, dietary patterns such as the Mediterranean diet, initially identified via a posteriori methods, have been used to develop indices with the aim of evaluating the adherence to these healthy-known dietary patterns( Reference Panagiotakos 70 , Reference Bountziouka, Tzavelas and Polychronopoulos 71 ). Eight studies included here (Table 3) have used different indices developed based on the Mediterranean diet. Five of these studies have indicated the higher the adherence to this diet the lower risk of the MetS. This may be evidence to the reproducibility and validity of this diet as a beneficial diet for preventing the MetS. Similar to the present results, the preventative impact of the Mediterranean diet on the MetS has been proven in interventional studies( Reference Jones, Park and Lee 72 ). As well, a systematic review study conducted on observational and RCT studies has concluded that the Mediterranean diet reduces the risk of the MetS( Reference Kastorini, Milionis and Esposito 13 ). This nutrient-dense diet not only targets weight loss, but also reduces the levels of inflammatory biomarkers and atherogenic lipoproteins due to its high phytonutrient and beneficial fatty acid contents( Reference Andersen and Fernandez 73 ). More studies have observed the protective effect of the Mediterranean diet (five out of eight) compared with studies that evaluated the ‘prudent/healthy’ dietary pattern (four out of ten). This may be due to the emphasis on higher intakes of nuts, olive/olive oil, monounsaturated:saturated ratio and moderate intakes of alcohol/wine in the Mediterranean diet compared with the ‘prudent/healthy’ dietary pattern. A similar association between DASH and the MetS was observed in two studies( Reference Pimenta, Toledo and Rodriguez-Diez 27 , Reference Saneei, Fallahi and Barak 40 ). An RCT study has conclusively demonstrated that the DASH diet has an improving impact on MetS status compared with not only a normal control diet but also a weight-reducing diet( Reference Azadbakht, Mirmiran and Esmaillzadeh 74 ). Although the Mediterranean diet seems to have a higher fat content compared with the DASH diet( Reference Calton, James and Pannu 15 ), the promising results from the included population-based studies indicate that both diets contribute to a lower risk of MetS prevalence and incidence.
The Mediterranean and the ‘Western’ dietary patterns
Based on this review of the population-based studies, the Mediterranean diet and the ‘Western’ dietary pattern seem to be the two most common extracted dietary patterns having a significant association with the MetS. The different effect of the two diets on the MetS reflects the opposite impact of their components on the MetS. Based on these studies, the Mediterranean diet was defined as a diet high in whole grains, fruits and vegetables, fish, legumes, nuts, monounsaturated fats and olive oil and moderate to low intake of meat, dairy products and alcohol. However, the ‘Western’ dietary pattern is characterised by high intakes of red/processed meat, fast food, refined grains/cereals, sugar-sweetened beverages, eggs, sweets/desserts and low intake of fruit and vegetables, and dairy products. While the Mediterranean diet consists of high intakes of fibre and whole grains, the ‘Western’ dietary pattern constitutes of high intakes of refined grains. The effect of whole grains and high fibre on the waist circumference component of the MetS has been observed in epidemiological studies. The inverse association between the MetS and whole grains intake may be due to the impact of whole grain consumption on the components of the MetS such as HDL-cholesterol( Reference Jacobs and Gallaher 75 ). On the other hand, intake of refined grains contributes to a high glycaemic index, which could increase the risk of the MetS. Findings from a study conducted among older adults indicated an inverse and direct association for whole and refined grains, respectively( Reference Sahyoun, Jacques and Zhang 76 ).
The Mediterranean diet has a moderate to low intake of red meat, while in the ‘Western’ dietary pattern a high intake of red/processed meats is observed. The association of MetS status and meat has been evaluated in a few studies( Reference Lutsey, Steffen and Stevens 23 , Reference Azadbakht and Esmaillzadeh 77 , Reference Damião, Castro and Cardoso 78 ). Regarding red meat, most studies have yielded a direct association between the MetS and meat intake. Results of the Atherosclerosis Risk in Communities Study indicated that meat products such as hamburgers, hotdogs and processed meats increase the risk of the MetS( Reference Lutsey, Steffen and Stevens 23 ). The increasing risk of the MetS with higher meat consumption could be due to the high saturated fat content of meat and its association with MetS components such as blood pressure and abdominal obesity( Reference Lutsey, Steffen and Stevens 23 ).
The Mediterranean diet is rich in fruits and vegetables while the ‘Western’ dietary pattern is deficient in these foods. The relationship between vegetables and fruit intake and the MetS has been assessed in a few studies. A cross-sectional study( Reference Esmaillzadeh, Kimiagar and Mehrabi 79 ) among adult women in Tehran revealed an inverse association. However, in the Atherosclerosis Risk in Communities Study( Reference Lutsey, Steffen and Stevens 23 ) and a cross-sectional study( Reference Setayeshgar, Whiting and Vatanparast 80 ) based on Canadian Health Measures Survey Cycle 1 data, no significant association was observed between fruit and vegetable intake and MetS status. The expected reducing impact of fruit and vegetables on the MetS is due to these foods optimising the effect on MetS components such as blood pressure( Reference Lutsey, Steffen and Stevens 23 , Reference Djousse, Padilla and Nelson 81 ) or fasting plasma glucose( Reference Calton, James and Pannu 15 ). Further research is required in this area to reveal the impact of this food group on the MetS, which may have been obscured due to including foods such as potatoes within the vegetables group.
Based on this review study, twelve studies( Reference Sonnenberg, Pencina and Kimokoti 3 , Reference Duffey, Steffen and Van Horn 21 – Reference Lutsey, Steffen and Stevens 23 , Reference Ambrosini, Huang and Mori 29 , Reference Heidemann, Scheidt-Nave and Richter 33 , Reference Panagiotakos, Pitsavos and Skoumas 34 , Reference Buscemi, Sprini and Grosso 41 , Reference Denova-Gutiérrez, Castañón and Talavera 49-51 , Reference Buscemi, Sprini and Grosso 54 ) have found a ‘Western/unhealthy’ dietary pattern prevalent in Westernised countries of the world. None of these studies indicated a high intake of alcohol as a characterising factor of the ‘Western/unhealthy’ dietary pattern. However, one of these studies( Reference Deshmukh-Taskar, O’Neil and Nicklas 54 ) has considered alcohol consumption as a potential confounding factor in the statistical analysis. Four studies among Western populations( Reference Sonnenberg, Pencina and Kimokoti 3 , Reference Kimokoti, Gona and Zhu 22 , Reference Panagiotakos, Pitsavos and Skoumas 34 , Reference Fonseca, Gaio and Lopes 50 ) have extracted a separate dietary pattern which is characterised by high intakes of alcohol. Two of these studies have indicated a direct association between an alcohol dietary pattern and the MetS( Reference Panagiotakos, Pitsavos and Skoumas 34 , Reference Fonseca, Gaio and Lopes 50 ).
The other components of the Mediterranean diet including olive/olive oil, moderate alcohol/red wine, nuts, legumes and fish also contribute to a high MUFA and PUFA and antioxidant profile, which results in lowering the risk for chronic inflammatory conditions such as the MetS( Reference Babio, Bullo and Salas-Salvado 82 ). For example, virgin olive oil has a high monounsaturated fat and polyphenol profile, which has an optimal effect on blood lipids, hypertension and insulin sensitivity. All these effects contribute to a lower risk of the MetS( Reference Babio, Bullo and Salas-Salvado 82 ).
The overall impact of the Mediterranean diet on the MetS is related to the impact on each of the MetS components, the anti-inflammatory effect and the impact on insulin resistance which is known to have a significant role in the development of the MetS( Reference Babio, Bullo and Salas-Salvado 82 ). The complexity of this syndrome with multiple components requires a diet that prevents and/or controls the risk such as the Mediterranean diet that affects all the components of the MetS in an optimising direction( Reference Djousse, Padilla and Nelson 81 ). However, the components of the ‘Western’ diet provoke the MetS components. Thus, in view of the beneficial effects of the Mediterranean diet on the MetS, promoting this dietary pattern or its most beneficial components relevant to different populations’ cultural practices may be an effective preventive strategy.
There are limitations to be considered due to the nature of a review study. First, only English-language studies have been included. However, the included articles are from a variety of countries where the official language is not necessarily English. In addition, studies published between 2005 and 2014 have been included in this review, while there are earlier review papers that include studies published before 2005.
Conclusion
The high worldwide burden of CVD and diabetes as the most common cause of mortality and morbidity has led many researchers across the globe to investigate the link between the MetS and diet as a modifiable factor. Findings of studies from twenty-three countries indicate that ‘Western’, Mediterranean, ‘healthy’ and ‘traditional’ dietary patterns are common diets among adult populations across the globe. Using different MetS criteria, the studies included in this review concluded an association between these dietary patterns and MetS status during adulthood. Since no unified definition of the MetS exists for adolescence, it creates challenges in investigating the association between dietary patterns and the MetS in this age group.
Findings of the present review suggest that the methods used to determine the dietary pattern of a population should be chosen based on the purpose of research. As the MetS consists of many components, investigation of a dietary pattern is beneficial in understanding the overall effect of diet rather than individual nutrients or food items on the MetS. Our scoping review revealed support for diets based on the Mediterranean diet and for the avoidance of the ‘Western’ diet in preventing the MetS, based on population-based studies which are in agreement with RCT. Promoting the Mediterranean components among populations where the Western dietary pattern is prevalent could be considered.
Acknowledgements
We would like to acknowledge the help of Vicky Duncan, Librarian, University of Saskatchewan, for helping in determining the outline of the search procedure.
Z. H. is a PhD student supported by the Dairy Research Cluster Initiative (Dairy Farmers of Canada, Agriculture and Agri-Food Canada, the Canadian Dairy Network and the Canadian Dairy Commission) (grant no. AIP-CL04). The abovementioned funder had no role in the design, analysis or writing of this article.
Z. H., S. J. W. and H. V. planned and were actively involved in organising the review as well as drafting the manuscript.
There are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S095442241600007X





