Introduction
Dietary guidelines are important policy reference standards(1,2) . They provide advice on the foods and dietary patterns that can assist in the prevention of obesity and diet-related chronic diseases while providing the nutrients required for optimal health(1,Reference Mozaffarian and Forouhi3) . Conducting an evidence review is an important step in the dietary guideline development process(1,2) . According to the FAO, the evidence review should include a review of country-specific evidence (for example, population-level data on food availability, food access, dietary intake, prevalence of diet-related diseases) and a review of the best available national and international evidence on relationships between diet and health(1,2) . Nutrient-based, food-based or dietary patterns research can be used to investigate these relationships(Reference Cespedes and Hu4-Reference Jacobs, Tapsell and Temple6). Nutrient-based research examines individual nutrients as the exposure, whereas food-based research focuses on particular foods or food groups(Reference Mozaffarian and Forouhi3,Reference Jacobs, Tapsell and Temple6,Reference Tapsell, Neale and Satija7) . Rather than examining nutrients in isolation, a food-based approach considers the interactions between the nutrients and other components that foods contain(Reference Mozaffarian and Forouhi3,Reference Jacobs, Tapsell and Temple6,Reference Tapsell, Neale and Satija7) . Dietary patterns research examines whole dietary patterns as the exposure. This approach takes into account the interactions between foods that are frequently consumed and the combinations of nutrients those foods contain(Reference Cespedes and Hu4,Reference Reedy, Subar and George8,Reference Jacobs and Tapsell9) .
Relationships between dietary exposures and health outcomes
As the discipline of nutrition science has evolved, nutrient-based, food-based and dietary patterns research has identified relationships between dietary exposures and particular health outcomes(Reference Mozaffarian and Forouhi3,Reference Mozaffarian, Rosenberg and Uauy10) . For example, in the early 1900s, evidence from nutrient-based research was critical in understanding deficiency diseases(Reference Jacobs, Tapsell and Temple6,Reference Mozaffarian, Rosenberg and Uauy10) . This new knowledge informed strategies to reduce the prevalence of such diseases(Reference Mozaffarian, Rosenberg and Uauy10). Since the 1980s, evidence from food-based and dietary patterns research has demonstrated the importance of foods and dietary patterns (rather than individual nutrients) in the development of obesity and chronic diseases including CVD, type 2 diabetes and some types of cancer(Reference Mozaffarian, Rosenberg and Uauy10-Reference Schulze, Martínez-González and Fung12). The food synergy theory suggests that relationships between dietary patterns and health outcomes can be explained by complex interactions between the foods that are consumed and the nutrients those foods contain, that extend beyond the sum of each individual nutrient(Reference Jacobs, Tapsell and Temple6,Reference Jacobs and Tapsell9) . For these reasons, dietary guidelines aiming to reduce the prevalence of obesity and diet-related chronic diseases and promote nutritional adequacy should be informed by evidence from food-based and dietary patterns research, combined with relevant evidence from nutrient-based research(Reference Mozaffarian and Forouhi3,Reference Tapsell, Neale and Satija7) .
Study designs for exploring relationships between dietary exposures and health outcomes
The study designs that are most suitable for exploring relationships between diet and health can vary according to the exposure and outcome of interest(11,Reference Bero, Norris and Lawrence13,Reference Satija, Yu and Willett14) . For example, randomised controlled trials (RCTs) can provide evidence on causal relationships between dietary exposures and short-term health outcomes, including chronic disease risk factors such as blood pressure, blood lipids and body weight(Reference Mozaffarian and Forouhi3,Reference Satija, Yu and Willett14) . However, it is often not feasible to conduct RCTs with food or dietary pattern exposures and long-term health outcomes such as chronic disease incidence and mortality(Reference Jacobs, Tapsell and Temple6,Reference Tapsell, Neale and Satija7,Reference Satija, Yu and Willett14) . Compliance with food and dietary pattern interventions can be difficult, particularly for studies that are conducted over long periods of time, and the costs associated with conducting such studies can be prohibitive(Reference Jacobs, Tapsell and Temple6,Reference Tapsell, Neale and Satija7,Reference Satija, Yu and Willett14) . For these reasons, evidence on relationships between foods and dietary patterns and long-term health outcomes comes primarily from prospective cohort studies that allow longer follow-up times, and do not require compliance with particular diets(Reference Jacobs, Tapsell and Temple6,11,Reference Schulze, Martínez-González and Fung12) . The major disadvantage of observational studies compared with RCTs is the risk of bias associated with confounding(Reference Schulze, Martínez-González and Fung12,Reference Satija, Yu and Willett14) . However, the risk of bias associated with prospective cohort studies is lower than other observational study designs(Reference Satija, Yu and Willett14). Compared with retrospective studies and cross-sectional studies, prospective cohort studies are less prone to recall bias and selection bias and their prospective nature allows temporal relationships between exposures and outcomes to be established(Reference Satija, Yu and Willett14).
Systematic review methods
It is increasingly expected that dietary guidelines are underpinned by systematic reviews of high-quality scientific literature(Reference Bero, Norris and Lawrence13,Reference Blake, Durao and Naude15,Reference Zeraatkar, Johnston and Guyatt16) . According to the WHO and the Cochrane Collaboration (Cochrane), the first step in conducting a systematic review is to develop a research question structured in the form of a PICO statement that reflects the Population, Intervention (or exposure), Comparator, and Outcome of interest(17,18) . The research question guides development of the search strategy and inclusion criteria. Once the final set of included studies has been identified, data are extracted and the risk of bias associated with each individual study is assessed using a standardised tool(17,18) . Data from included studies can be synthesised qualitatively or quantitatively. A narrative synthesis provides a qualitative description of the data(17). If included studies are comparable, a meta-analysis can be conducted(17,18) . The body of evidence for each outcome is then assessed, and evidence statements are produced(17,19,20) . Both the WHO and Cochrane state that the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach should be used to assess the quality (or certainty) of the evidence(17,18,20) . Using this method, evidence from RCTs is initially considered ‘high quality’, whereas evidence from observational studies is initially considered ‘low quality’(20,Reference Balshem, Helfand and Schunemann21) . Additional criteria are then applied sequentially. Evidence can be downgraded based on risk of bias, inconsistency, indirectness, imprecision or publication bias. It can be upgraded if the effect size is large, a dose–response gradient is observed, or if all suspected confounders would exert an effect opposite to that observed(20,Reference Balshem, Helfand and Schunemann21) .
Use of systematic reviews in dietary guideline development
Dietary guidelines can be informed by original systematic reviews (conducted for the purpose of dietary guideline development), or existing systematic reviews that were conducted for other purposes(Reference Blake, Durao and Naude15,22) . According to a recent study by Blake et al. (Reference Blake, Durao and Naude15), existing systematic reviews were used to inform dietary guidelines in Canada, Chile, India, New Zealand, Norway, South Africa, Sweden and the UK, and a combination of existing and original systematic reviews were used in Australia, the USA and Germany(Reference Blake, Durao and Naude15). In some countries, evidence review methods have recently been revised. For example, to inform Canada’s Dietary Guidelines (published in 2019), an Evidence Review Cycle model was created(23,24) . The purpose of this model was to make the process of identifying relevant sources of evidence, including existing systematic reviews, more systematic and transparent(23,Reference Colapinto, Ellis and Faloon-Drew25) . With continuous use, the model is intended to enable identification of new evidence that can be used to inform future iterations of the guidelines(23). In the USA, systematic review methods were assessed as part of a broader consensus study conducted by the National Academies of Sciences to inform the process used to develop the 2020 Dietary Guidelines for Americans(26,27) . The committee concluded that dietary guidelines should continue to be underpinned by systematic reviews, but that the original systematic reviews conducted by the National Evidence Library should be independently reviewed(27). The importance of using the most appropriate evidence assessment methods was also highlighted(27).
Systematic reviews conducted to inform the Australian Dietary Guidelines
The current Australian Dietary Guidelines (ADG) provide population-level recommendations to promote health and reduce chronic disease risk(28). The ADG were published in 2013 and were the first in Australia to be informed by original systematic reviews(Reference Allman-Farinelli, Byron and Collins29,30) .
In 2009, the Dietitians Association of Australia (DAA) was commissioned by the National Health and Medical Research Council (NHMRC) to conduct the evidence review that would inform the 2013 ADG(28,30,Reference Williams, Allman-Farrinelli and Collins31) . A combination of systematic reviews and narrative reviews were conducted to answer research questions that were determined by the Dietary Guidelines Working Committee(28,30) . The systematic reviews were conducted in accordance with NHMRC guidelines(19,28,32,33) . Searches were conducted to identify literature published between 2002 and 2009. This start date was selected because the evidence that informed the previous iteration of the guidelines (published in 2003) was published before 2002, and these systematic reviews were intended to provide an update(30). Some additional sources of evidence published between 2009 and 2013 were included in the final dietary guidelines report, but did not contribute to the evidence statements that were produced(28,30) .
For the systematic reviews with food or nutrient exposures, primary studies were eligible for inclusion as well as existing systematic reviews and meta-analyses(30). Cross-sectional studies were excluded, as was grey literature(30,Reference Williams, Allman-Farrinelli and Collins31) . Primary studies that were already included in existing reviews and meta-analyses were excluded to avoid duplication(28,Reference Williams, Allman-Farrinelli and Collins31) . For the systematic reviews with dietary pattern exposures, only existing systematic reviews and meta-analyses were eligible for inclusion(28,30) . The risk of bias associated with the studies and reviews included in each systematic review was assessed using the American Dietetic Association quality assessment checklist(Reference Williams, Allman-Farrinelli and Collins31,Reference American Dietetic34) . A minimum of five high-quality studies was needed for an evidence statement to be produced(28,Reference Williams, Allman-Farrinelli and Collins31) .
The NHMRC grading system was used to assess the body of evidence for each outcome and produce evidence statements(19,30) . This system consists of five components: evidence base, consistency, clinical impact, generalisability, and applicability. For each component, a rating of ‘excellent’, ‘good’, ‘satisfactory’ or ‘poor’ was awarded. Under the evidence base component, the quantity, level and quality of evidence were considered(19,Reference Williams, Allman-Farrinelli and Collins31) . The quantity of evidence reflected the number, size and statistical power of included studies. The level of evidence was assessed using the NHMRC evidence hierarchy for intervention studies(19,28) . In this hierarchy, a systematic review of RCTs provides the highest level of evidence (level I), followed by an RCT (level II), a pseudo-RCT (level III-1), a comparative study with concurrent controls, i.e. non-randomised experimental trial, cohort study, case–control study, or interrupted time series with a control group (level III-2), a comparative study without concurrent controls (level III-3), and a case series (level IV). As part of the risk of bias assessment, the quality of included studies and reviews was classified as positive, neutral or negative. In most cases, only ‘positive’ or ‘neutral’ studies and reviews contributed to evidence statements(30).
The ratings for each of the five components of the NHMRC grading system were used to calculate an overall rating: grade A, B, C or D(19,30) . A rating of ‘excellent’ was required for the evidence base and consistency components for grade A, ‘excellent’ or ‘good’ for grade B, and ‘good’ or ‘satisfactory’ for grade C(19,Reference Williams, Allman-Farrinelli and Collins31) . According to the NHMRC guidelines, to achieve a rating of ‘excellent’, the evidence base should include well-designed level I or level II studies(19). However, to account for the challenges associated with conducting RCTs with dietary pattern or food exposures and long-term health outcomes, evidence from prospective cohort studies alone was rated as ‘excellent’ on some occasions(30).
Since the ADG were published, the dietary guideline development process in Australia has been compared with other countries in terms of evidence review methods(Reference Blake, Durao and Naude15), attempts to incorporate environmental sustainability(Reference Gonzalez Fischer and Garnett35) and the use of dietary pattern modelling to inform food guides(Reference Davis, Esslinger and Elvidge Munene36). However, a detailed analysis of the types of evidence included in the original systematic reviews has not been conducted. The primary aim of the present study was to analyse the systematic reviews conducted to inform the 2013 ADG according to dietary exposure. The secondary aim was to analyse the reviews by health outcome, and design of included studies.
This type of analysis is important, because the inclusion of particular studies in a systematic review reflects the research question that was asked (in terms of exposure and outcome), the study designs that were considered most suitable for answering that question, and the availability of relevant evidence(Reference Tapsell, Neale and Satija7,Reference Bero, Norris and Lawrence13) . More broadly, the research questions that are asked (or not asked) as part of the evidence review process, and the methods used to identify and assess the quality of the available evidence, can influence the extent to which evidence from nutrient-based, food-based and dietary patterns research is translated into dietary guidelines(Reference Tapsell, Neale and Satija7,Reference Bero, Norris and Lawrence13) . Decisions about the research questions that are asked can be influenced by many factors, including the public health nutrition concerns that the dietary guidelines are intended to address, and the current state of knowledge about relationships between particular dietary exposures and health outcomes(1,2) . Decisions about the methods used to review the evidence can also be influenced by a range of factors, including the availability of time and financial resources(1,2) . By analysing the evidence that was included in the systematic reviews that were conducted to inform the ADG, the present study provides further insight into the systematic review methods that were used and the evidence that was available. The results of the present study can be used to inform systematic review methods used in future dietary guideline development.
Methods
To identify the systematic reviews that were conducted to inform the 2013 ADG, the dietary guidelines report(28) was used as a starting point. The content of this report was assessed and key references were retrieved. To ensure that the most recent and relevant information had been obtained, the FAO food-based dietary guidelines database(37), the Australian NHMRC website(38) and the Australian Government’s Eat for Health website(39) were hand searched.
The systematic reviews were published in a NHMRC report in 2011(30). This ‘evidence report’ contained the data used in this analysis. Additional information on systematic review methods (for example, search strategy, inclusion criteria, risk of bias assessment methods, NHMRC grading system) was obtained from the dietary guidelines report(28), the DAA process manual(Reference Williams, Allman-Farrinelli and Collins31) and relevant NHMRC guidelines(19,32,33) . The methodological details described in these documents informed the methods used to analyse the systematic reviews.
Only systematic reviews with evidence statements were eligible for inclusion in this analysis. In some cases, a single research question led to the production of more than one evidence statement based on the framing of the research question and the availability of evidence(30). In order to standardise the unit of analysis, an individual systematic review was defined based on the outcome of the evidence statement produced, rather than the research question that was asked. For example, a broadly framed research question about dietary patterns and health that led to the production of two evidence statements with different outcomes was counted as two systematic reviews.
Systematic reviews were assessed using the eligibility criteria in Table 1. In line with the aims of the present study, systematic reviews with dietary intake as the exposure and health as the outcome were eligible for inclusion. For the purpose of the present study, systematic reviews with the following exposures were not considered to be relevant measures of dietary intake and were therefore excluded: determinants of dietary intake, eating behaviours and meal patterns, breast-feeding and infant feeding practices, non-diet lifestyle behaviours such as physical activity, and food production and processing methods. Systematic reviews with non-health outcomes (for example, environmental outcomes, food safety outcomes), and those with dietary intake as the outcome rather than the exposure were also excluded. Data on exposure, outcome and design of included studies were extracted from the evidence statements of included systematic reviews.
Table 1. Eligibility criteria for systematic reviews
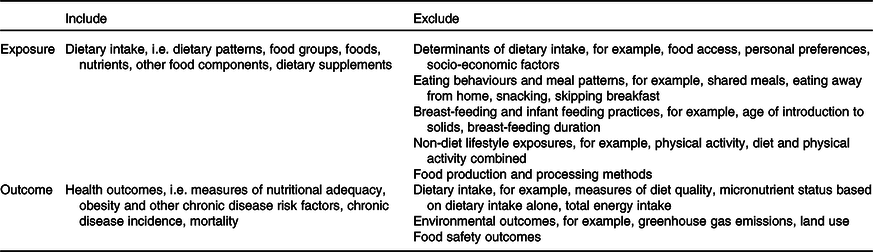
Dietary exposures were coded as dietary patterns, foods (including individual foods and food groups), nutrients, or other food components (for example, artificial sweeteners). This classification system was adapted from a taxonomy developed by Stok et al. (Reference Stok, Renner and Allan40). If the exposure could not be easily classified based on the information provided by systematic review authors, the more inclusive code was used. For example, if it was unclear whether the exposure was saturated fat, or foods high in saturated fat, the exposure was coded at ‘foods’ rather than ‘nutrients’.
Health outcomes were coded as nutritional adequacy (for example, growth, micronutrient status), chronic disease risk factors (for example, blood pressure, blood lipids, body weight), or chronic disease morbidity and/or mortality (for example, chronic disease incidence, cause-specific mortality, all-cause mortality). This classification system was intended to reflect the purpose of the ADG, i.e. to promote health and reduce chronic disease risk(28). Chronic disease risk factors were separated from chronic disease morbidity and mortality because risk factors can be considered health outcomes in themselves(Reference Satija, Yu and Willett14). If an evidence statement included multiple outcomes that would be categorised differently, the more inclusive code was used. For example, if one evidence statement included blood pressure and CVD, the outcome was coded as ‘chronic disease morbidity and/or mortality’ rather than ‘chronic disease risk factors’.
The studies included in each systematic review were categorised as RCTs, pseudo-RCTs or non-randomised experimental trials, cohort or nested case–control studies, case–control studies or cross-sectional studies. These categories were adapted from existing study design classification systems(Reference Satija, Yu and Willett14,19) . Systematic reviews could include studies from more than one study design category, i.e. the categories were not mutually exclusive. Each study design category included primary studies as well as systematic reviews, meta-analyses and pooled analyses. For example, a systematic review that included individual RCTs, individual cohort studies and a meta-analysis of cohort studies was coded as ‘RCTs’ and ‘cohort or nested case–control studies’. Primary studies were grouped with existing systematic reviews, meta-analyses and pooled analyses because we were interested in the design of the primary studies that underpinned each evidence statement. If the design of an included study was not clearly stated by systematic review authors, the study was retrieved and the study design was identified.
Descriptive statistics were used to describe systematic reviews according to dietary exposure, health outcome and design of included studies. Data analysis was conducted using Stata 15 (StataCorp LLP).
Results
A total of 184 systematic reviews with evidence statements were produced to inform the ADG. Of these, forty-one systematic reviews were excluded from this analysis based on exposure and/or outcome. The remaining 143 systematic reviews provided evidence on relationships between dietary exposures and health outcomes and were included.
Foods were the most common exposure (86·7 % of reviews), followed by nutrients (10·5 %) and dietary patterns (2·8 %) (Table 2). None of the reviews had other food components as the exposure. Examples of the food exposures that were assessed include fruit, fruit and vegetables, red meat, fats and oils, dairy foods, and milk. Examples of nutrient exposures include sodium, MUFA and total fat. The only dietary patterns that were assessed were Mediterranean dietary patterns, and dietary patterns that aligned with existing dietary guidelines (measured using diet quality scores).
Table 2. Characteristics of systematic reviews conducted to inform the 2013 Australian Dietary Guidelines (n 143 systematic reviews)
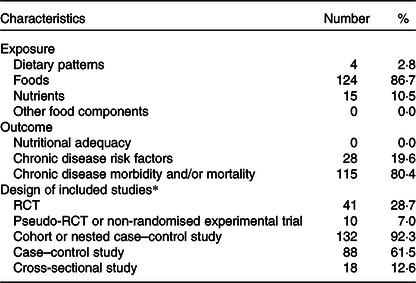
RCT, randomised controlled trial.
* Study design categories were not mutually exclusive, so frequencies add up to more than 100 %. While individual cross-sectional studies were not eligible for inclusion in the systematic reviews that were conducted, evidence from cross-sectional studies was included as some of the existing systematic reviews incorporated cross-sectional studies.
Chronic disease morbidity and/or mortality was the most common outcome (80·4 %), followed by chronic disease risk factors (19·6 %). None of the reviews had nutritional adequacy as the outcome. Examples of the chronic disease morbidity and mortality outcomes that were assessed include risk of colorectal cancer, mortality from CVD and total mortality. Examples of chronic disease risk factors include weight gain, blood pressure and HDL-cholesterol.
Most reviews included evidence from cohort or nested case–control studies (92·3 %). Many included evidence from case–control studies (61·5 %). A smaller proportion included evidence from RCTs (28·7 %). Many reviews included studies from more than one study design category. The study design categories were not mutually exclusive, so the frequencies add up to more than 100 %. Many reviews included existing systematic reviews and meta-analyses in addition to primary studies. While individual cross-sectional studies were not eligible for inclusion in the systematic reviews that were conducted(Reference Williams, Allman-Farrinelli and Collins31), evidence from cross-sectional studies was included as some of the existing systematic reviews incorporated cross-sectional studies.
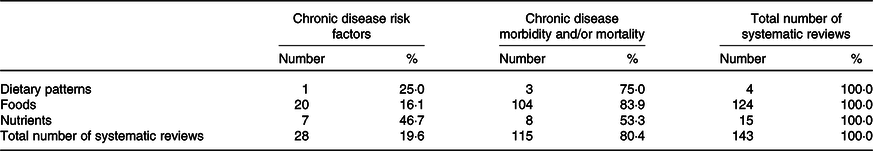
Overall, 104 of the 143 systematic reviews assessed relationships between foods and chronic disease morbidity and/or mortality (Table 3). Of the four reviews with dietary pattern exposures, the most common outcome was chronic disease morbidity and/or mortality (75·0 %). Of the 124 reviews with food exposures, the most common outcome was also chronic disease morbidity and/or mortality (83·9 %). Of the fifteen reviews with nutrient exposures, approximately half had chronic disease risk factors as outcomes (46·7 %) and half had chronic disease morbidity and/or mortality outcomes (53·3 %).
Table 3. Systematic reviews conducted to inform the 2013 Australian Dietary Guidelines classified according to exposure and outcome (n 143 systematic reviews)
Of the four reviews with dietary pattern exposures, 50·0 % included at least one RCT and 100·0 % included at least one cohort or nested case–control study (Table 4). Of the 124 reviews with food exposures, 21·8 % included at least one RCT and 96·0 % included at least one cohort or nested case–control study. Of the fifteen reviews with nutrient exposures, 80·0 % included at least one RCT and 60·0 % included at least one cohort or nested case–control study. Of the twenty-eight reviews with chronic disease risk factors as outcomes, 75·0 % included at least one RCT and 71·4 % included at least one cohort or nested case–control study (Table 5). Of the 115 reviews with chronic disease morbidity and/or mortality outcomes, 17·4 % included at least one RCT and 97·4 % included at least one cohort or nested case–control study.
Table 4. Systematic reviews conducted to inform the 2013 Australian Dietary Guidelines classified according to exposure and study design* (n 143 systematic reviews)
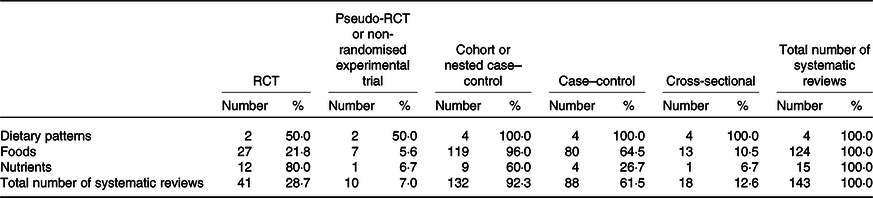
RCT, randomised controlled trial.
* Study design categories were not mutually exclusive, so frequencies add up to more than 100 %. While individual cross-sectional studies were not eligible for inclusion in the systematic reviews that were conducted, evidence from cross-sectional studies was included as some of the existing systematic reviews incorporated cross-sectional studies.
Table 5. Systematic reviews conducted to inform the 2013 Australian Dietary Guidelines classified according to outcome and study design* (n 143 systematic reviews)

RCT, randomised controlled trial.
* Study design categories were not mutually exclusive, so frequencies add up to more than 100 %. While individual cross-sectional studies were not eligible for inclusion in the systematic reviews that were conducted, evidence from cross-sectional studies was included as some of the existing systematic reviews incorporated cross-sectional studies.
Discussion
The aims of the present study were to analyse the systematic reviews conducted to inform the 2013 ADG according to dietary exposure, health outcome, and design of included studies. Most systematic reviews synthesised evidence from food-based research and a small proportion synthesised evidence from nutrient-based or dietary patterns research. Chronic disease morbidity and/or mortality was the most common outcome, followed by chronic disease risk factors. Most systematic reviews included evidence from cohort and nested case–control studies, many included evidence from case–control studies, and a smaller proportion included evidence from RCTs.
The framing of the research questions that are asked can influence the types of evidence included in systematic reviews(Reference Tapsell, Neale and Satija7,Reference Bero, Norris and Lawrence13) . In Australia, most of the research questions developed by the Dietary Guidelines Working Committee defined foods as the exposure of interest(28,30) . Therefore, it is not surprising that most systematic reviews synthesised evidence from food-based research (Table 2). In contrast, many of the research questions developed by the Dietary Guidelines Advisory Committee in the USA defined dietary patterns as the exposure of interest(22). This may be a reflection of the time at which the evidence reviews were conducted. Dietary patterns research first appeared in the literature in the 1980s, and the body of evidence has increased exponentially in the last decade(Reference Borges, Rinaldi and Conde41,Reference Kumanyika, Afshin and Arimond42) . In the USA, dietary guidelines are updated every 5 years(22). A combination of existing and original systematic reviews underpinned the 2015–2020 Dietary Guidelines for Americans(22,43) . This included a series of systematic reviews on dietary patterns and body weight, CVD and type 2 diabetes, which synthesised evidence published between 1980 and 2013(44). In contrast, the systematic reviews conducted to inform the 2013 ADG were completed in 2009 and included evidence published between 2002 and 2009(30). In developing future iterations of the ADG, there is an opportunity to review the latest evidence from dietary patterns research.
Most systematic reviews included evidence from cohort and nested case–control studies, many included evidence from case–control studies, and a smaller proportion included evidence from RCTs (Table 2). Compared with reviews with food or dietary pattern exposures, a larger proportion of reviews with nutrient exposures included evidence from RCTs (Table 4). Compared with reviews with chronic disease morbidity and/or mortality as outcomes, a larger proportion of reviews with chronic disease risk factors as outcomes included evidence from RCTs (Table 5). These results may be a reflection of the challenges associated with conducting RCTs with food or dietary pattern exposures and long-term health outcomes(Reference Jacobs, Tapsell and Temple6,Reference Tapsell, Neale and Satija7,Reference Satija, Yu and Willett14) . In dietary guideline development, the totality of evidence needs to be assessed(Reference Mozaffarian and Forouhi3,Reference Tapsell, Neale and Satija7,Reference Bero, Norris and Lawrence13) . However, identifying the most suitable study designs for answering particular research questions from the outset can strengthen the systematic review process(Reference Bero, Norris and Lawrence13). This information can guide decisions about the study designs that are eligible for inclusion and the methods that are most suitable for assessing the quality of the body of evidence(Reference Bero, Norris and Lawrence13).
Evidence assessment methods can influence the types of evidence that are used (or not used) to inform dietary guidelines(Reference Tapsell, Neale and Satija7,Reference Bero, Norris and Lawrence13) . The methods used by systematic review authors to assess the evidence tended to rate evidence from RCTs more highly than evidence from cohort studies, regardless of the exposure and outcome(19,30) . However, in some cases, the ‘evidence base’ component of the NHMRC grading system was adapted to better reflect the exposure and outcome(30). Due to the limitations associated with observational research, it has been argued that dietary guidelines should reflect evidence from RCTs rather than evidence from cohort studies(Reference Ioannidis45). This argument suggests that evidence assessment methods similar to GRADE, that rate RCT more highly than cohort studies regardless of the research question, are appropriate. A counter argument is that evidence assessment methods that consider the most suitable study designs for answering particular research questions, and seek to integrate evidence from multiple study designs may be more appropriate(11,Reference Bero, Norris and Lawrence13,Reference Katz, Karlsen and Chung46) . For example, an evidence assessment method developed by the World Cancer Research Fund is built on the premise that evidence from observational studies can be used to infer causality(11). This method has been used to assess the latest evidence on the relationships between foods, dietary patterns and cancer(11). It has also been used to inform dietary guideline development in Norway(Reference Blake, Durao and Naude15). In developing future iterations of the ADG, depending on the research questions that are asked, use of alternative evidence assessment methods may need to be considered.
The present study provides a detailed analysis of the types of evidence included in the systematic reviews conducted to inform the ADG. Although many research questions were addressed using systematic reviews(28,30) , this analysis focused on systematic reviews on diet and health, and only systematic reviews with evidence statements were assessed. Therefore, our analysis did not capture all the research questions that were asked. Coding was based primarily on the information provided by systematic review authors. However, data collection and analysis were conducted in a consistent and systematic way. Some additional sources of evidence published after the systematic reviews were conducted were included in the final dietary guidelines report(28,30) , but as these sources of evidence did not contribute to the evidence statements they were not included in this analysis. In line with NHMRC guidelines, grade D evidence was not used to inform the ADG(19,28) . Evidence statements that were underpinned by grade D evidence were still eligible for inclusion in this analysis because our aim was to analyse the systematic reviews that were conducted. Translation of evidence into dietary guidelines can be influenced by factors beyond the results of the evidence review, including politics and conflicts of interest(Reference Gonzalez Fischer and Garnett35,Reference Nestle47) . However, analysis of the evidence translation process was beyond the scope of the present study.
Conclusion
Dietary guidelines provide advice on healthy eating and are intended to inform policies that promote public health. They should be underpinned by systematic reviews that synthesise the best available evidence on relationships between diet and health, including evidence from nutrient-based, food-based and dietary patterns research. Systematic review methods can influence the types of evidence that are used to inform dietary guidelines. Most of the systematic reviews conducted to inform the 2013 ADG synthesised evidence from food-based research. In developing future iterations of the ADG, there is an opportunity to review the latest evidence from dietary patterns research.
Acknowledgements
K. W. is supported by an Australian Government Research Training Program Scholarship. S. A. M. is supported by an NHMRC Career Development Fellowship Level 2 (ID1104636). The Australian Government and the NHMRC had no role in the design, analysis or writing of the present study.
All authors contributed to the design of the study. K. W. collected and analysed the data with S. A. M. and M. A. L. providing support. K. W. prepared the initial draft of the manuscript. All authors contributed to the final version of the manuscript.
K. W. and S. A. M. have no conflicts of interest. M. A. L. was a member of the NHMRC Working Committee for the review of the Australian Dietary Guidelines (2008–2013).








