Introduction
Humans possess strong motivational systems triggered by the rewarding aspects of food. When palatable foods are abundant, constant restraint to avoid overeating is required. There is mounting evidence for a pivotal role of the endocannabinoid system (ECS) in energy balance and homeostatic and non-homeostatic (hedonic) aspects of food intake( Reference Kirkham 1 – Reference Cota, Marsicano and Tschöp 6 ). The ability of the plant Cannabis sativa (and C. indica, in the present paper collectively referred to as Cannabis spp.) and its extracts to stimulate appetite has been documented for many centuries( Reference Abel 7 , Reference Kirkham and Williams 8 ). Numerous anecdotal accounts from recreational cannabis users complement the evidence for the appetite-stimulating properties of plant cannabinoids (CB). Users report persistent hunger when intoxicated, even when previously satiated, and a specific desire for sweet and high-fat foods, a phenomenon known as the ‘munchies’. In research, findings originating from multiple disciplines (medicine, pharmacology, nutrition, sensory science, psychiatry, psychology) illustrate the importance of the ECS in the control of all aspects of food intake and energy balance( Reference Silvestri and Di Marzo 9 – Reference Monteleone 12 ). The last decade prompted a surge of interest in the ECS as a therapeutic target for weight management, the metabolic syndrome, several eating disorders and CVD( Reference Marco, Romero-Zerbo and Viveros 11 , Reference Rosenson 13 – Reference Woods 16 ). Several pharmaceutical companies have heavily invested in development programmes for selective antagonists/inverse agonists of the CB1 receptor. However, soon after the first drug in this class, rimonabant, reached the European market in 2006 (US Food and Drug Administration had already postponed first approval) this development came to an abrupt halt. Although rimonabant was effective in producing weight loss and improving different cardiovascular risk factors, it was also associated with increased rates of depression, anxiety and suicidal ideation( Reference Christensen, Kristensen and Bartels 17 ). The compound was withdrawn in 2008 and many other companies active in the field announced termination of their research programmes( Reference Heal, Gosden and Smith 18 ). Lately, there has been renewed interest in the modulating role of (endo)cannabinoids in hedonic or reward-driven eating and the potential of the ECS as a therapeutic target in the treatment of overeating and possibly also ‘under’-eating. Regarding the latter, the ECS has been linked to unintentional weight loss in elderly individuals( Reference Wernette, White and Zizza 19 ), and CB are clinically applied in the treatment of disease-related loss of appetite( Reference Wilner and Arnold 20 , Reference Glare, Miller and Nikolova 21 ). In the therapeutic field of weight management and metabolic diseases, this renewed interest is fuelled by recent developments in pharmacology, such as the development of partial/neutral antagonists or peripherally restricted compounds to target the ECS( Reference Wargent, Zaibi and Silvestri 22 – Reference Witkamp 24 ). The present review is not intended to fully cover the involvement of the ECS in all aspects of food intake and energy balance. Rather, it expands upon previous reviews by concentrating on the role of the ECS in reward-driven eating and the hedonic aspects of food intake. For its homeostatic role, for example, in controlling metabolic functions such as energy balance and food intake and its actions on peripheral tissues (adipocytes, hepatocytes, gastrointestinal (GI) tract), readers are referred to recent reviews on this topic( Reference Silvestri and Di Marzo 9 , Reference Bermudez-Silva, Viveros and McPartland 25 , Reference Quarta, Mazza and Obici 26 ).
The present review starts with a fundamental perspective on the ECS, introducing the biochemistry of plant-derived and endogenous CB and their role in peripheral and central metabolic regulation. This is followed by a brief discussion of food reward, its key concepts and neurophysiology. Next, the present knowledge on the role of (endo)cannabinoids in normal appetite control is summarised, with a focus on hedonic eating (eating in the absence of any energetic or nutritional need). Then, switching to a clinical perspective, eating disorders where dysfunction of the ECS might be involved are discussed, along with developments in therapeutic applications of CB agents in conditions where appetite either needs to be diminished or stimulated. Finally, the present review identifies some key issues in the translational domain, i.e. the validity of animal models with regard to some aspects of human eating behaviour, and important remaining questions that need to be resolved.
The endocannabinoid system: a versatile and evolutionary well-conserved signalling system
Endocannabinoids and phytocannabinoids
Endocannabinoids are signalling molecules derived from long-chain fatty acids ( ≥ C18), playing important roles in a wide variety of biological processes. By definition, the term endocannabinoid is limited to those compounds displaying significant affinity to the CB receptors CB1 and CB2 ( Reference Pertwee 27 ). These receptors, shown to bind (–)-trans-Δ9-tetrahydrocannabinol (Δ9-THC) from the C. sativa plant, were discovered in the late 1980s(28,29). To date, nine ‘true’ endocannabinoids have been described, including anandamide (N-arachidonoylethanolamine; AEA), its name originating from ‘ananda’ meaning ‘the bliss’ in Sanskrit, and 2-arachidonoylglycerol (2-AG)(30) (Fig. 1).
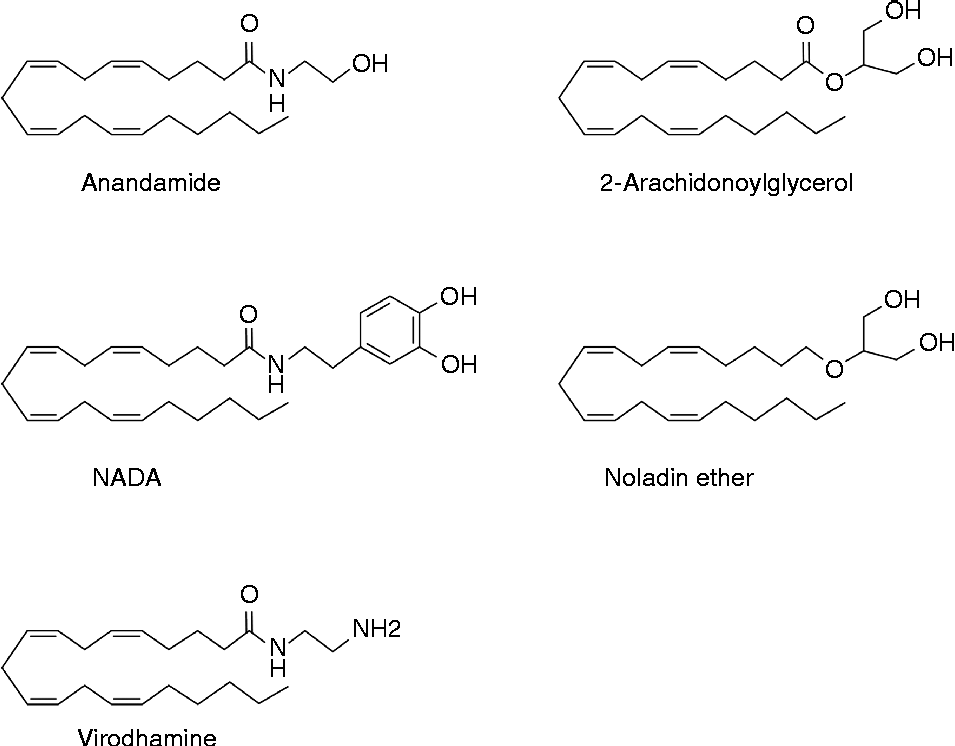
Fig. 1 Chemical structure of some endocannabinoids: anandamide, 2-arachidonoylglycerol, N-arachidonoyldopamine (NADA), noladin ether and virodhamine.
Together with their receptors and enzymes involved in synthesis and breakdown, endocannabinoids constitute the ECS. The ECS is highly pleiotropic and involved in several processes including energy homeostasis and eating behaviour, reproduction, growth and development, learning and memory, as well as anxiety and immune functions( Reference MacCarrone, Gasperi and Catani 31 – Reference Di Marzo and De Petrocellis 33 ). The adjective ‘cannabinoid’ originates from the plant genus Cannabis and predates the discovery of CB receptors by many years, which can lead to some confusion( Reference Pertwee, Howlett and Abood 34 ). The term phytocannabinoids (phyto- used here to distinguish them from endocannabinoids) originally refers to a group of terpenophenolic compounds present in Cannabis spp., of which more than 100 have been found so far( Reference van Bakel, Stout and Cote 35 , Reference Mehmedic, Chandra and Slade 36 ). Three examples of these phytocannabinoids are given in Fig. 2(a–c). The earliest reports on the pharmacological effects of Cannabis spp., including the ability to stimulate appetite, date back to several years BC( Reference Skaper and Di Marzo 37 , Reference Robson 38 ). In general, Cannabis refers to C. sativa cultivars, although there is still some discussion whether the genus Cannabis comprises more than one species, i.e. C. sativa and C. indica ( Reference van Bakel, Stout and Cote 35 , Reference Mehmedic, Chandra and Slade 36 ). Preparations from Cannabis spp. show great variety in absolute and relative phytocannabinoid concentrations, while only a few of these compounds are ligands for CB1 or CB2 receptors. In Cannabis spp., CB are produced as their carboxylic acid derivatives, known as CB acids, which degrade into their neutral counterparts through the action of heat (smoking, vaporising), sunlight, and storage( Reference Verhoeckx, Korthout and Van Meeteren-Kreikamp 39 , Reference Eichler, Spinedi and Unfer-Grauwiler 40 ). In addition to these ‘prototypical’ phytocannabinoids, an increasing number of other plant compounds interacting with the ECS are found (see the Dietary compounds with cannabinoid activity section).
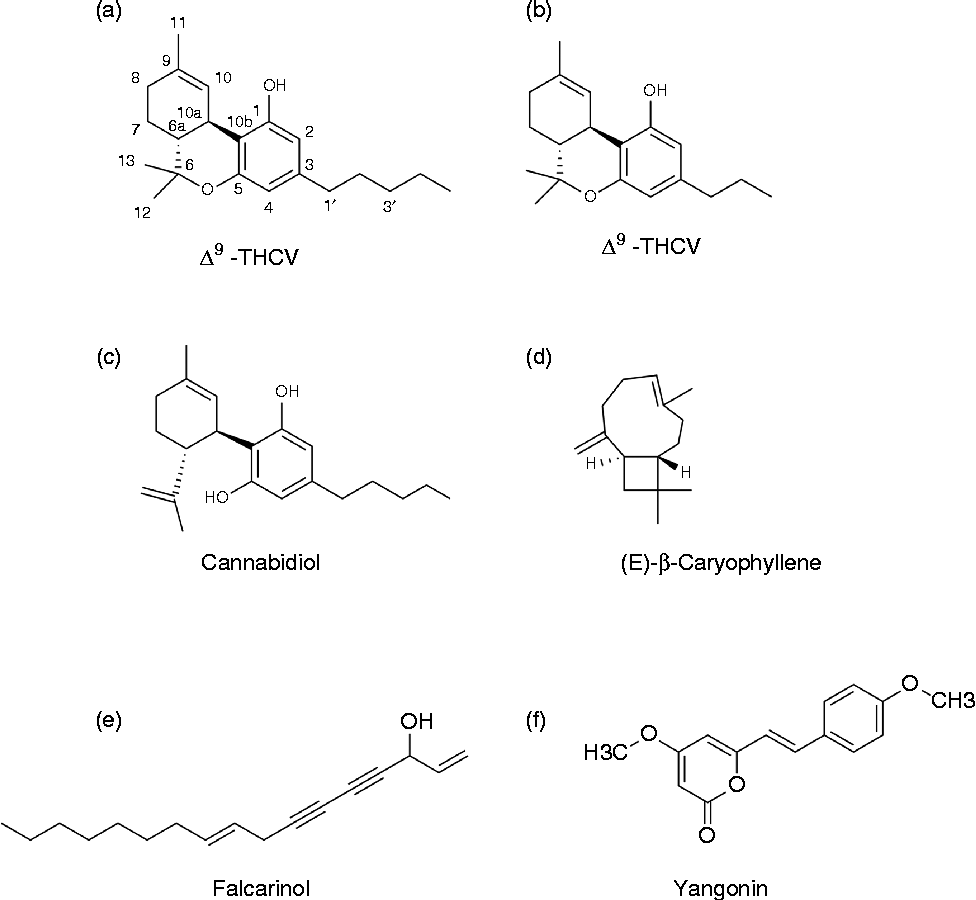
Fig. 2 Examples of some ‘phytocannabinoids’ from Cannabis and other plants. (a) (–)-Trans-Δ9-tetrahydrocannabinol (Δ9-THC), main psychoactive compound present in Cannabis. (b) Δ9-Tetrahydrocannabivarin (Δ9-THCV); cannabinoid (CB)1 antagonist present in Cannabis. (c) Cannabidiol, non-psychoactive compound from Cannabis with diverse pharmacological spectrum, low affinity for CB1 and CB2 receptors, antagonist for G-protein coupled receptor (GPR) 55, agonist for GPR18 and PPAR-γ, etc. (d) (E)-β-caryophyllene, CB2 agonist, widespread in plants. (e) Falcarinol, selective CB1 inverse agonist, covalently binding. Present in different plants, including carrots, celery and Panax ginseng. (f) Yangonin, selective CB1 ligand (over CB2) present in Kava (Piper methysticum). See text for further details and references.
The endocannabinoid system as part of an extensive lipid-based signalling network
More than two decades of research have shown that the ECS is less specific and distinct than originally assumed. Some (if not all) of the ‘true’ endocannabinoids display ‘promiscuous’ behaviour by activating or blocking other receptors besides CB1 or CB2, with potencies that differ little from those that interact with ‘true’ CB receptors( Reference Pertwee 27 , Reference Pertwee, Howlett and Abood 34 , Reference Alexander and Kendall 41 ). Furthermore, endocannabinoids are now known to be part of a large class of structurally related amides, esters and ethers of fatty acids, which exist in a continuous dynamic equilibrium with each other. The vast majority of these molecules belong to the fatty (acid) amides like AEA, although analogues of 2-AG, including 2-oleoylglycerol and 2-linoleoylglycerol, have also been found. Fatty acid amides (Lipid Maps class FA08; http://www.lipidmaps.org) are conjugates of different long-chain fatty acids and amines including ethanolamine, neurotransmitters (serotonin, dopamine), or simple amino acids. These signalling molecules interact with molecular targets that go far beyond the classical CB receptors, and include a wide range of receptors including G-protein-coupled receptor (GPR) 55, GPR18, GPR119, transient receptor potential ankyrin 1 (TRPA1), transient receptor potential channel type V1 (TRPV1), PPAR, as well as several non-receptor targets( Reference Pertwee 27 , Reference Di Marzo, Bisogno and De Petrocellis 32 , Reference Alexander and Kendall 41 , Reference Brown, Cascio and Rotondo 42 ). These receptors and several of their ligands, including fatty acid conjugates with dopamine and glycine, have also been demonstrated in brain tissues of rodents and human subjects, although their relationship with reward circuitry is still unknown( Reference Leishman, Kokesh and Bradshaw 43 – Reference Sylantyev, Jensen and Ross 45 ). Pathways for endocannabinoid synthesis( Reference Tsuboi, Ikematsu and Uyama 46 – Reference Reisenberg, Singh and Williams 48 ) and their metabolism via hydrolysis( Reference Bisogno 49 – Reference Fonseca, Costa and Almada 52 ) or oxygenation( Reference Brown, Cascio and Rotondo 42 , Reference Rouzer and Marnett 53 ) show several crossroads with those of other bioactive lipids. This not only creates several nodes of interaction and regulation, but also results in the formation of ‘hybrid’ structures, including prostamides and other oxidation products, which are often displaying bioactivity themselves( Reference Rouzer and Marnett 53 – Reference Yang, Fredman and Krishnamoorthy 55 ). Therefore, the ECS is increasingly considered part of a versatile network of lipid mediators that serves to fine-tune homeostasis( Reference Silvestri and Di Marzo 9 , Reference DiPatrizio and Piomelli 56 ). However, in relation to food reward and hedonics, the significance of these ‘non-canonical’ endocannabinoid receptors and ligands of the ECS remains to be elucidated. Instead, research in this field has thus far entirely focused on the ‘prototypical’ CB1 receptor. This receptor is presynaptically located at central or peripheral nerve terminals and acts as a modulator of synaptic transmission( Reference Cachope 57 ). Its stimulation leads to the inhibition of adenylate cyclase activity, regulation of different Ca2+ and K+ channels, and causes the stimulation of mitogen-activated protein kinase. In some cases, CB1 receptors signal through Gs proteins( Reference Pertwee 27 , Reference Fonseca, Costa and Almada 52 ). In contrast to the initial view, the distribution of CB1 receptors is not limited to the central nervous system. Their involvement in food intake regulation takes place at different levels, starting from receptors within the GI tract to the regulation of hedonic reward in the brain( Reference Cota 14 , Reference Quarta, Mazza and Obici 26 , Reference MacCarrone, Gasperi and Catani 31 , Reference Cota, Tschop and Horvath 58 ). Their presence in peripheral tissues also provides an explanation for the sustained effects of rimonabant on body weight and the improvement of insulin resistance and blood lipids, in addition to its short-term appetite-decreasing effect. These findings have encouraged the search for so-called ‘peripherally restricted’ CB1 antagonists with fewer central nervous system side effects, of which several are currently under investigation( Reference Pertwee 10 , Reference Silvestri and Di Marzo 23 , Reference Witkamp 24 ). In the brain, CB1 is probably the most abundant GPR( Reference Pertwee 27 ). Herkenham et al. ( Reference Herkenham, Lynn and Little 59 ) published a pioneering study on its distribution in the brain in 1990. More recent reviews include those of Freund et al. ( Reference Freund, Katona and Piomelli 60 ) along with Katona & Freund( Reference Katona and Freund 61 ). Brain CB1 receptors have been linked to both homeostatic and non-homeostatic regulation, with endocannabinoids acting as modulators of orexigenic and anorexigenic neurotransmitters as well as neuropeptides by presynaptic regulation of their release. Anatomically and functionally, the brain ECS shows several connections with other signalling pathways involved in reward, including dopaminergic, opioid and GABA-ergic systems( Reference MacCarrone, Gasperi and Catani 31 , Reference Di Marzo and De Petrocellis 33 , Reference Leishman, Kokesh and Bradshaw 43 , Reference Fattore, Melis and Fadda 62 – Reference Solinas, Goldberg and Piomelli 64 ).
Dietary modulation of endocannabinoid ligands and receptors
Plasma and tissue concentrations of endocannabinoids and related lipid-derived signalling molecules are influenced by several factors, including food intake, dietary pattern and body weight( Reference MacCarrone, Gasperi and Catani 31 , Reference Matias, Gatta-Cherifi and Cota 65 ). In humans, plasma levels show a circadian rhythm( Reference Hanlon, Stuhr and Leproult 66 ). Several studies have found that levels of individual endocannabinoids are higher in the plasma of obese compared with lean individuals( Reference Matias, Gatta-Cherifi and Cota 65 , Reference Matias, Gatta-Cherifi and Tabarin 67 , Reference Matias, Gonthier and Orlando 68 ). Some studies in human subjects report a short-term effect of food intake on endocannabinoid plasma levels. For example, Monteleone et al. ( Reference Monteleone, Piscitelli and Scognamiglio 69 ) showed increased plasma 2-AG levels in a group of eight satiated human subjects who consumed palatable food compared with the consumption of non-palatable food. Remarkably, levels of AEA and other N-acyl ethanolamines measured decreased after consuming both types of food. Previous research has shown that circulating N-acyl ethanolamines in plasma of women correlated well with total and specific (precursor) plasma levels of NEFA, independently of feeding status( Reference Joosten, Balvers and Verhoeckx 70 ). The relevance of endocannabinoid plasma levels remains subject to debate as endocannabinoids are released on demand and rapidly metabolised in tissues. Therefore, their plasma levels will probably not directly reflect dynamic changes in discrete areas in the brain and other tissues. Evidence for changes in CB levels in specific brain areas in response to dietary intervention comes from animal studies. For example, fasting increased levels of AEA and 2-AG in the limbic forebrain and to a lesser extent of 2-AG in the hypothalamus of rats. By contrast, hypothalamic 2-AG declined as animals ate( Reference Kirkham, Williams and Fezza 71 ). In mice, short-term fasting showed increased levels of 2-AG (and sometimes AEA) in areas of the brain involved in the regulation of food intake, whereas feeding reduced these levels( Reference Hanuš, Avraham and Ben-Shushan 72 ). In diet-induced obese mice, increased levels of AEA and 2-AG were reported in the hippocampus, which has also been linked to the hedonic aspects of eating( Reference Massa, Mancini and Schmidt 73 ).
Next to these short-term effects (hours), diet can also affect the relative ratios of individual endocannabinoids and their congeners in the longer term (days). Patterns of individual ligands reflect the local availability of their precursor fatty acids in the phospholipid membranes, which are diet related( Reference Di Marzo, Bisogno and De Petrocellis 32 , Reference Balvers, Verhoeckx and Bijlsma 74 ). For example, studies in rodents and human subjects have shown that increasing the relative proportion of n-3 long-chain PUFA in the diet can lead to a decrease in the formation of the ‘prototypic’ endocannabinoids AEA and 2-AG, which are both derived from the n-6 fatty acid arachidonic acid( Reference MacCarrone, Gasperi and Catani 31 , Reference Balvers, Verhoeckx and Bijlsma 74 – Reference Meijerink, Balvers and Witkamp 76 ). As the individual congeners possess different receptor affinity (and) or intrinsic activity, these shifts in relative concentrations are likely to have biological implications. However, studies to investigate these mixed ligand effects pose large practical and technical challenges and have not been performed so far. Next to modulation at ligand level, diet has also shown to affect the expression of CB1 receptors in hindbrain and forebrain regions( Reference Massa, Mancini and Schmidt 73 ). Interestingly, Rojo et al. ( Reference Rojo, Söderström and Olsson 77 ) showed that a high-fat diet given to rats for 4–12 weeks resulted in an increased functionality of CB1 receptors in the prefrontal cortex, as measured from the stimulation of [3H]GTPγS binding by a CB1 ligand. This was not seen in animals fed with a high-fat diet for longer periods. Furthermore, CB1 receptor densities as well as endocannabinoid hydrolytic enzymes (monoacylglycerol lipase and fatty acid amide hydrolase) remained unchanged. The authors suggested that the apparent disturbance in ECS signalling after consuming a high-fat diet may show an adaptation in the longer term.
For studies in human subjects, quantitative imaging of the CB1 receptor using positron emission tomography offers interesting options( Reference Sanabria-Bohórquez, Hamill and Goffin 78 , Reference Ceccarini, De Hert and Van Winkel 79 ). This technology has recently been introduced in CB research and has been used so far in a small number of applications. In relation to eating behaviour, a study by Gérard et al. ( Reference Gérard, Pieters and Goffin 80 ) showed that the availability of CB1 was increased in cortical and subcortical brain areas of women with anorexia nervosa in comparison with healthy controls. Although the number of patients (n 14) was small, and results should be interpreted with care, similar studies provide interesting opportunities for future research.
Dietary compounds with cannabinoid activity
Remarkably, compounds that can interact with the ECS are also found in plants other than Cannabis spp. For example, fatty acid amides similar to those found in animals are common in plants, in particular in seeds( Reference Coulon, Faure and Salmon 81 – Reference Chapman, Venables and Dian 84 ). However, the amounts consumed, as well as their oral bioavailability, are likely to be low. Therefore, these sources are currently not regarded as relevant for human diets, although local GI effects cannot be excluded. The discovery of AEA in chocolate initially received a great deal of attention( Reference di Tomaso, Beltramo and Piomelli 85 ). The endocannabinoid probably did not originate from the cocoa, and levels were considered not to be of dietary relevance( Reference MacCarrone, Gasperi and Catani 31 ). Interestingly, an increasing number of other plant metabolites with affinity for CB receptors have been discovered in various species. Examples include (E)-β-caryophyllene (present in many different spices and food plants including oregano, cinnamon and black pepper), falcarinol (found in carrots, parsley and celery) and yangonin (present in Kava; Piper methysticum)( Reference Gertsch, Pertwee and Di Marzo 30 , Reference Gertsch 82 , Reference Leonti, Casu and Raduner 86 , Reference Ligresti, Villano and Allarà 87 ). Some examples of structures of these other (non-Cannabis) phytocannabinoids are also given in Fig. 2(d–f). Their dietary relevance is still unclear. It is tempting to speculate on the link between food sensation and reward effects from certain herbs and spices and an interaction with CB1 and/or TRPV1 receptors. The TRPV1 receptor, which has the pepper compound capsaicin as one of its most potent currently known agonists, has been proposed as a candidate CB3 receptor( Reference Pertwee 27 , Reference Pertwee, Howlett and Abood 34 ). Next to the covalent CB1 antagonist falcarinol( Reference Leonti, Casu and Raduner 86 ), one other potent natural CB1 antagonist is known, the phytocannabinoid THCV( Reference Wargent, Zaibi and Silvestri 22 , Reference Pertwee 88 ). This compound has shown interesting properties for weight management and diabetes. Considering the ‘promiscuity’ of the ECS, it does not seem unlikely that other natural compounds acting on the ECS may be found, including some that are relevant to nutrition. Milk (bovine and human) has been reported to contain significant amounts of the endocannabinoid 2-AG, in addition to N-palmitoyl ethanolamine, N-stearoyl ethanolamine and N-oleoyl ethanolamine, the latter being ‘non-cannabinoid’ N-acyl ethanolamine( Reference Fride 89 ). Some rodent studies suggest that there is a critical role for the CB1 receptor in the initiation of milk suckling within the first 24 h of birth( Reference Fride 89 ). More studies are needed to clarify the significance of endocannabinoids in milk or specific milk fractions, either locally within the GI tract or systemically.
Food reward: key concepts and neurophysiology
This section briefly discusses the concept and measurement of food reward in order to provide some context for references in later sections.
It is common knowledge that highly palatable foods can lead people to eat, even when satiated. Thus, eating can be motivated by hedonics, rather than hunger or homeostatic principles. The rewarding properties of food intake follow a typical cyclical time course with appetitive stages (expectation/anticipation), which sometimes lead to a phase of consummation (hedonic experience/evaluation), followed by a satiety phase( Reference Kringelbach, Stein and van Hartevelt 90 ). These phases have been linked to distinct components of reward, including ‘wanting’, ‘liking’ and ‘learning’ (see Fig. 3). Wanting refers to the motivation to obtain foods or the incentive salience of foods (salience as a ‘tag’ that makes a food stimulus attractive, attention grabbing, sought after, and ‘wanted’). Liking is about the affect or emotion, and in the context of foods about the hedonic evaluation or hedonic impact (pleasure, palatability). The learning phase involves associative conditioning and cognitive processes where wanting and liking for the reward are linked over time. Reward processes are regulated in a network of brain regions, including areas in the lateral hypothalamus, brain stem (ventral tegmental area (VTA), parabrachial nucleus (PBN)), mesolimbic system (amygdala, hippocampus, striatum, nucleus accumbens, ventral pallidum) and (pre)frontal cortex( Reference Berridge, Ho and Richard 91 – Reference Finlayson, King and Blundell 94 ). Even though wanting and liking often go together, they are not the same and are both necessary for normal reward. At the neuroanatomical level, wanting and liking have been linked to distinctive networks of so-called wanting and liking ‘hotspots’ in the limbic forebrain (nucleus accumbens, ventral pallidum) and brain stem (VTA, PBN). These hotspots for wanting and liking have (in part) different neurochemistry. Wanting depends on dopaminergic neurotransmission via the medial forebrain bundle. A connected network of hotspots that uses opioid neurotransmission regulates liking and wanting together to enhance food reward, along with other neurochemical signalling systems including endocannabinoids( Reference Kringelbach, Stein and van Hartevelt 90 – Reference Berridge and Kringelbach 92 , Reference Dagher 95 ).

Fig. 3 Food pleasure cycle (redrawn and adapted from Kringelbach et al. ( Reference Kringelbach, Stein and van Hartevelt 90 )). Food rewards, similar to other fundamental rewards, are associated with a cyclical time course. Typically, rewarding occasions include a phase of anticipation (appetitive stage) or ‘wanting’ for a reward, which can lead to a phase of consummation (consummatory stage) or ‘liking’ of the reward, which can have (several) peak levels of pleasure. Depicted here is an increase in appetite during the initial stages of consuming palatable foods, also known as the ‘appetiser effect’(153). Finally, a satiety or learning phase occurs, where one learns and updates predictions for the reward. Note that learning can take place throughout the cycle. (A colour version of this figure can be found online at http://www.journals.cambridge.org/nrr).
Commonly, wanting has been associated with the appetitive stage of food reward, whereas liking is linked to the actual consummation stage. In fact, these terminologies (wanting v. liking; appetitive v. consummation) are not completely synonymous in terms of conceptual meaning( Reference Finlayson, King and Blundell 96 , Reference Havermans 97 ). However, in the present review, for reasons of comprehensibility, appetitive stages of food reward will be mainly described in terms of food ‘wanting’, and consummatory stages in terms of food ‘liking’, and vice versa.
It is important to note that wanting and liking can occur both explicitly and implicitly, that is without conscious awareness. For example, Winkielman et al. ( Reference Winkielman, Berridge and Wilbarger 98 ) showed that subliminally presented happy faces produced no change in self-reported subjective feeling or moods. But it did cause thirsty participants to pour and consume more of a fruit drink afterwards (reflecting wanting). In addition, they also gave higher ratings to the pleasantness and attractiveness of the drink (reflecting liking), with no awareness that they saw the subliminal stimulus. These findings illustrate the need for different procedures to measure explicit wanting and liking on the one hand, and implicit wanting and liking on the other. Several behavioural procedures are available to measure explicit reward components (conscious desire and pleasure), commonly by subjective self-reports and experiments that study goal-directed behaviour( Reference Berridge and Robinson 93 ). Measurement of implicit reward processes such as incentive salience, habits and liking responses requires different methodology compared with assessing explicit reward indices. For desire or wanting, implicit methods may involve operant models; experiments that measure effort or ‘willingness to work’ to obtain a rewarding stimulus. For implicit measurements of (dis)liking, observational methods such as monitoring oro-facial expressions can be used. The respective paradigms used in animal and human studies to measure implicit and explicit wanting and liking components of food reward will be described in more detail where relevant.
Endocannabinoids, appetite control and palatability
Food reward and palatability
Food consumption is rewarding in itself as it replenishes energy and eliminates hunger (an unpleasant sensation); however, everyday experience suggests that humans often eat in the absence of hunger. For Cooper( Reference Cooper 99 ), this fits the distinction between ‘palatability-dependent appetite and depletion- or deprivation-induced feeding and drinking’. He acknowledges that ‘interactions between the two no doubt occur, but we recognize that we may consume food not because we are hungry but because the food is attractive and eating it gives pleasure’. The ECS, with connections to dopaminergic and opioid circuits, appears to be a key player in the modulation of palatability-dependent appetite. It is important to realise that palatability, or pleasantness, is not a fixed function of the sensory properties (taste, aroma, texture, appearance) of a food or beverage. Instead, palatability refers to the hedonic evaluation of the sensory properties and determines food acceptance and preference( Reference Rogers 100 ). The common concept is that only a few taste preferences (sweet) or aversions (bitter) are innately present, but most others are developed throughout life( Reference Lawless 101 ). Examples are numerous where people develop a liking for flavours initially rejected, such as coffee, beer, bitter-tasting vegetables or spicy foods. Taste intensity and its hedonic evaluation display plasticity as well( Reference Bolhuis, Lakemond and de Wijk 102 ). To illustrate this, many processed foods contain high levels of salt and sugar that compromise health. Although this is acknowledged by the food industry, strategies to reduce sugar and salt content are challenged because consumers have developed a preference for salty and sweet-tasting foods and drinks. Hence, they often rate food items with reduced salt and sugar levels as less palatable. In short, palatability is not a sensory property itself, but refers to the hedonic evaluation of qualitative and quantitative (intensity) sensory characteristics of foods and drinks( Reference Rogers 100 , Reference Lawless 101 ). The plasticity of palatability allows modification of palatability-dependent appetite, and is regulated to a substantial degree by the ECS. Still, the underlying mechanisms are far from clear. In the following sections, animal and human studies will be reviewed that illustrate putative central and peripheral mechanisms (neural, biochemical, perceptual and behavioural) of (endo)cannabinoid involvement in food intake and palatability.
Animal studies on the role of (endo)cannabinoids in appetite control and palatability
It has been well documented that CB1 receptor agonists such as THC, AEA and 2-AG stimulate feeding in animals. In contrast, CB receptor inverse agonists such as SR141716A (rimonabant) or AM251 suppress feeding (for a review, see Kirkham & Williams( Reference Kirkham and Williams 103 )). Moreover, CB1-receptor knockout mice are hypophagic and remain leaner overall than wild-type animals( Reference Cota, Marsicano and Tschöp 6 , Reference Ravinet Trillou, Delgorge and Menet 104 , Reference Sanchis-Segura, Cline and Marsicano 105 ). The motivational changes underlying the hyper- and hypophagic effects of the ECS continue to be subject to investigation, but are associated with liking and wanting aspects of reward( Reference Berridge, Ho and Richard 91 ). Stimulation of endocannabinoid activity clearly acts on the appetitive phase of feeding. In these situations, appetitive behaviours are those associated with an animal's tendency to approach foods/drinks, whereas consummatory behaviours refer to the next component, actual ingestion. Appetitive behaviours are also affected by factors other than palatability, such as motivational and emotional factors( Reference Berridge, Ho and Richard 91 , Reference Berridge and Robinson 93 , Reference Berridge 106 ). With regard to the appetitive phase of feeding, several animal studies have shown that both exogenous and endogenous CB1 agonists reduce the latency to feed in pre-satiated or free-feeding animals( Reference Kirkham and Williams 8 , Reference Farrimond, Mercier and Whalley 107 ). ECS effects on appetitive behaviours have also been demonstrated using operant models. These involve so-called progressive ratio paradigms in which experimental animals are required to progressively ‘work harder’ to obtain successive food rewards. CB1 agonists increase the effort an animal is willing to make to obtain food rewards( Reference Guegan, Cutando and Ayuso 108 – Reference Freedland, Poston and Porrino 110 ). In contrast, CB1 inverse agonists (SR141716A, AM4113, AM251) attenuate instrumental responses to rewarding foods and liquids( Reference Gallate, Saharov and Mallet 111 – Reference Salamone, McLaughlin and Sink 114 ). There is tentative evidence suggesting that CB-induced modifications in feeding behaviour may be differential with regard to macronutrient content, taste and texture of the ingested foods. For example, Thornton-Jones et al. ( Reference Thornton-Jones, Kennett and Vickers 115 ) tested the effects of the CB1 antagonist rimonabant on both motivational state and palatability in rats that consumed a highly palatable fat emulsion (10 % Intralipid) or a 10 % sucrose solution. Via a microstructure analysis of licking behaviour, changes in motivational state and hedonic impact (palatability) could be distinguished. The effects of the CB1 antagonist were compared with the effects of behavioural manipulations: pre-feeding which reduces the motivation to feed, adding quinine (bitter) to the lipid solution, or changing the sucrose concentrations. In general, the effects of rimonabant on the ingestion of the fat solution were greater compared with the sucrose solution, suggesting an interaction between CB1 modulation and macronutrients, taste and texture. In addition, comparisons between the effects observed after drug administration with the diverse behavioural manipulations indicated that the hypophagic effects were mostly explained by changes in the motivation to feed, and not by the changed hedonic impact of the ingesta( Reference Thornton-Jones, Kennett and Vickers 115 ). The latter conclusion, however, has been challenged by other studies that do find support for CB actions selectively increasing appetitive behaviours and intake of highly palatable foods and solutions, whereas no effect was found with standard isoenergetic chow (compared with highly palatable pellets) or neutral or mildly aversive solutions (water, low-concentration quinine solutions)( Reference Guegan, Cutando and Ayuso 108 , Reference DiPatrizio and Simansky 116 , Reference Shinohara, Inui and Yamamoto 117 ).
There is growing evidence that CB affect consummatory components of ingestion (as distinguished from appetitive components), such as palatability and orosensory reward. Endocannabinoid stimulation appears to enhance palatability more for rewarding types of foods (sweet and fatty foods) than for bland foods (normal laboratory chow), whereas reducing endocannabinoid tone diminishes perceived palatability (see also Farrimond et al. ( Reference Farrimond, Mercier and Whalley 107 )). Earlier works from Koch & Matthews( Reference Koch and Matthews 118 ), as well as Williams and colleagues( Reference Williams and Kirkham 119 , Reference Williams, Rogers and Kirkham 120 ) investigated the effects of systemic administration of relatively low doses of THC (for example, 0·5, 1·0 and 2·5 mg/kg; intraperitoneally) on food intake in rats receiving different types of rewarding foods. The results showed that stimulation of CB receptors evoked an increase in intake of more palatable foods compared with normal rat chow in the first 1–4 h after drug administration. Interestingly, of the different types of palatable chow, those high in only fat increased intake more than chow that was high in both fat and sugar. Still, palatability of sweet (sugar) foods/drinks appears to be affected by CB. For example, Higgs et al. ( Reference Higgs, Williams and Kirkham 121 ) used microstructural analysis of sucrose drinking in rats to examine the effects on palatability of THC, the endocannabinoids AEA and 2-AG, and of the CB1 inverse agonist rimonabant. Free-feeding rats (hence, not deprived of foods) were trained to consume a 10 % sucrose solution and their licking behaviour (rate, number of licking bouts, duration per bout, cumulative lick curves across fixed time bins) was closely monitored and compared with reference standards to distinguish between licking patterns seen when manipulations affect orosensory reward variables v. satiation. The results were overall supportive for a CB-induced increase in palatability for both exogenous and endogenous agonists, whereas the antagonist seemed to reduce the palatability of the sucrose solution( Reference Higgs, Williams and Kirkham 121 ). Another direct measure of palatability is the taste reactivity (TR) test developed by Grill & Norgren( Reference Grill and Norgren 122 ). The TR test measures palatability of a flavoured solution by monitoring mimetic responses of an animal. The solution is infused directly into the animal's oral cavity via an implanted intra-oral cannula( Reference Berridge 123 ). By doing this, the TR test is able to measure consummatory responses in the absence of appetitive behaviour. Palatable solutions, such as sucrose, elicit a characteristic set of hedonic oro-facial responses, involving tongue protrusions and licking of the mouth/lips; whereas aversive solutions, such as quinine, elicit rejection responses, such as gaping, chin rubs and paw threading( Reference Grill and Norgren 122 , Reference Berridge 123 ). Jarrett et al. ( Reference Jarrett, Limebeer and Parker 124 , Reference Jarrett, Scantlebury and Parker 125 ) performed several experiments with rats to test the effects of both CB1 agonists and antagonists on palatability using the TR test. Their findings showed that low doses of THC (0·5 mg/kg intraperitoneally) enhanced the palatability of sucrose solutions regardless of their concentration. Therefore, the effect of THC was not differentially affected by the baseline palatability of the infused solution. This effect appears to be mediated by the CB1 receptor, because it was reversed by pre-treatment with rimonabant( Reference Jarrett, Scantlebury and Parker 125 ). Intriguingly, further findings from Jarrett et al. ( Reference Jarrett, Scantlebury and Parker 125 ) suggest that the effect of CB ligands on palatability of foods is not restricted to positive hedonic properties of taste, but also involves modulation of aversive (bitter) tastes. The authors demonstrated that THC could reduce rejection of a highly unpalatable quinine solution, whereas pre-treatment with CB1 antagonists/inverse agonists increased the rejection of unpalatable tastants( Reference Jarrett, Scantlebury and Parker 125 ). There are other reports, however, where amplification of the positive hedonic impact of endocannabinoids such as AEA to intra-oral sucrose is confirmed, but no changes in ‘disliking’ reactions to bitter quinine were found( Reference Mahler, Smith and Berridge 126 ).
As for the neurophysiological mechanisms by which endocannabinoids exert their actions on regulation of food intake and palatability, most of the attention has gone to the central nervous system. In the brain, areas in the nucleus accumbens shell have been identified as an endocannabinoid hotspot for sensory pleasure( Reference Berridge, Ho and Richard 91 , Reference Mahler, Smith and Berridge 126 – Reference Fulton 128 ). Interestingly, there is emerging evidence that taste function can also be modulated at the peripheral level by hormones and other neuromodulatory factors that act on receptors present in the peripheral gustatory system. Sweet-sensitive taste cells in the peripheral taste system not only express sweet taste receptors (T1R2/T1R3) but CB (CB1) and leptin (Ob-Rb) receptors as well. This suggests that sweet taste sensitivity could be altered peripherally by both anorexigenic (for example, leptin) and orexigenic factors (for example, CB)( Reference Jyotaki, Shigemura and Ninomiya 129 ). Support for peripheral alteration of sweet taste sensitivity by endocannabinoids comes from a study in rats, which showed that administration of AEA and 2-AG increased gustatory nerve responses and mimetic responses to sweeteners in a concentration-dependent manner without affecting responses to salty, sour, bitter and umami (savoury) compounds( Reference Yoshida, Ohkuri and Jyotaki 130 ). To summarise, the ECS modulates food palatability via both central and peripheral pathways in a way that enables sophisticated fine-tuning of palatability.
Studies in human subjects: marijuana ‘munchies’ v. controlled laboratory studies
Numerous anecdotal and descriptive accounts indicate that marijuana (mostly THC and possibly other CB present in C. sativa) stimulates ingestive behaviour and enhances the appreciation of food in humans (for example, Abel( Reference Abel 7 ), Tart( Reference Tart 131 ) and Haines & Green( Reference Haines and Green 132 )). This phenomenon is known as the ‘munchies’ in popular culture. It has also been well established that marijuana induces a craving for sweet or fatty foods( Reference Cooper 99 , Reference Mahler, Smith and Berridge 126 ), although findings from controlled laboratory studies in human subjects are limited and less compelling. Reported data are highly variable and clearly depend on methodological conditions, such as social setting (subjects alone or in groups), different dosing schedules and routes of administration resulting in variable plasma drug levels and subjective levels of becoming ‘high’, as well as on the energy status of the participants (drug administration under fed or fasted conditions)( Reference Mattes, Engelman and Shaw 133 ). Taking these factors into consideration, controlled laboratory studies in human subjects on the effects of marijuana administration can provide overall verification of the anecdotal accounts of marijuana-induced increases in food intake( Reference Mattes, Engelman and Shaw 133 – Reference Foltin, Fischman and Byrne 135 ). In addition, findings from these studies suggest that marijuana specifically increases appetite for sweet foods (for example, cakes and candy bars). This preference for sweet foods appears to depend on sensory properties rather than macronutrient or energy content alone and as such reflects palatability factors( Reference Foltin, Fischman and Byrne 135 ). Surprisingly, only one study investigated whether the reported taste-enhancing properties of marijuana reflect true shifts in gustatory functions( Reference Mattes, Shaw and Engelman 136 ). In a double-blind, placebo-controlled study, the effects of acute marijuana administration on taste intensity and hedonic responses for sweet, sour, salty and bitter food stimuli were monitored at baseline, as well as 2, 4 and 6 h post-dosing. Findings on taste responses were negative, suggesting that self-reported shifts in taste-responsiveness and hedonics could be a consequence of the effects of marijuana intoxication on memory and higher cognitive processes, instead of alterations of sensory systems( Reference Mattes, Shaw and Engelman 136 ).
Regarding body weight, studies in healthy volunteers and in obese patients suggest that THC-induced increases in body weight do not solely rely on increased energy intake( Reference Mattes, Engelman and Shaw 133 , Reference Mattes, Shaw and Engelman 136 , Reference Le Foll, Trigo and Sharkey 137 ). An illustrative finding concerns that of body-weight change due to marijuana administration in healthy volunteers. Foltin et al. ( Reference Foltin, Fischman and Byrne 135 ) exposed their volunteers to 3 d periods of active marijuana administration alternated with 3 d placebo periods, whilst subjects lived for 2 weeks in a residential laboratory. They observed that body weight increased an average of 3 kg over a 3 d marijuana period and subsequently decreased by nearly 3 kg over a 3 d placebo period. These rather dramatic changes in body weight could not be accounted for by the changes in energy intake as a function of marijuana and placebo administration. Possible explanations for the extra weight gain/loss include marijuana-induced increased fluid retention, reduced physical activity and increases in sleeping time, and hypothermia resulting in decreased rest metabolism( Reference Foltin, Fischman and Byrne 135 ). In chronic cannabis users, however, there is little evidence for consistent and maintained weight gain( Reference Tart 131 ). On the contrary, it has recently been reported that the prevalence of obesity is paradoxically much lower in cannabis users as compared with non-users( Reference Le Foll, Trigo and Sharkey 137 ). It is not known why acute THC produces hyperphagic effects and weight gain, whereas chronic administration of THC does not result in increased body weight. Explanations proposed include that THC, which is a partial agonist of the CB1 receptor, might functionally act as an antagonist of endocannabinoids such as 2-AG and AEA, resulting in a relative reduction of endogenous ECS tone. Additionally, chronic THC administration may cause down-regulation and desensitisation of CB1 receptors, resulting in adaptive down-regulation of endocannabinoid signalling( Reference Le Foll, Trigo and Sharkey 137 ). Taken together, the previous study indicates weight gain following marijuana administration to be a temporary phenomenon, and this constitutes an interesting avenue for further investigations regarding the role of (endo)cannabinoids in food intake control in humans.
Endocannabinoid dysregulation and food intake
Eating disorders and obesity
The relevance of the ECS for the central mechanisms that drive humans to eat and often over-consume is further strengthened by the notion that visceral obesity is associated with hyperactivity of the ECS. Data from both animal and human studies have shown that the development of obesity co-occurs with increased endocannabinoid levels and CB1 receptor expression( Reference Matias, Gatta-Cherifi and Cota 65 , Reference Matias, Gonthier and Orlando 68 ). A detailed discussion of this notion is beyond the scope of the present review. For more details, see other review papers on this topic( Reference Kirkham and Tucci 2 , Reference Silvestri and Di Marzo 9 , Reference Matias and Di Marzo 138 , Reference Cervino, Vicennati and Pasquali 139 ).
Reports concerning the involvement of the ECS and eating disorders such as anorexia nervosa, bulimia nervosa or binge eating disorder are still limited. Interestingly, recent publications point out a possible link between alterations in the physiology of endocannabinoids and other central and peripheral appetite modulators like leptin and ghrelin in patients with eating disorders, thus going beyond the homeostatic control of food intake( Reference Marco, Romero-Zerbo and Viveros 11 , Reference Monteleone, Piscitelli and Scognamiglio 69 , Reference Monteleone, Bifulco and Di Filippo 140 , Reference Monteleone, Castaldo and Maj 141 ). It is becoming more evident that the ECS is deregulated in eating disorders, which is not solely linked to homeostatic control, but also in the rewarding aspects of aberrant (eating) behaviours occurring in anorexia nervosa, bulimia nervosa and binge eating disorder. For example, abnormal eating behaviours like self-starvation (in anorexia nervosa) or binge-eating episodes (in bulimia nervosa and binge eating disorder) seem to become rewarding in themselves and this may be modulated by endocannabinoid deregulation( Reference Marco, Romero-Zerbo and Viveros 11 , Reference Monteleone, Piscitelli and Scognamiglio 69 , Reference Cervino, Vicennati and Pasquali 139 ). Although less specific to eating behaviours, lower levels of CB1 receptor mRNA in female eating disorder patients have been associated with wrist cutting as impulsive self-injurious behaviour, which may have some rewarding or addictive properties( Reference Schroeder, Eberlein and de Zwaan 142 ).
Therapeutic modulation of the endocannabinoid system in disease-related anorexia and involuntary weight loss
A second line of evidence on the appetitive properties of THC comes from clinical investigations. Certain diseases, for example advanced stages of cancer or AIDS, are accompanied by wasting syndromes (cachexia), anhedonia and loss of appetite. In these situations, the primary interest in the appetitive properties of THC is in its potential to stabilise and increase body weight. Disease-related anorexia often presents a major medical problem and is associated with decreased quality of life, and increased morbidity and mortality. Malnutrition and food anhedonia are also an issue in elderly, especially in frail subpopulations suffering from chronic disease or dementia syndromes( Reference Serra-Prat, Mans and Palomera 143 ). At present, a number of approved medical applications of CB agonists including dronabinol (synthetic THC and main compound of the schedule 3 drug Marinol®) and nabilone (THC analogue and main ingredient of the schedule 2 drug Cesamet®) are available as appetite stimulants. A number of studies show that CB are effective in treating loss of appetite and to reverse weight loss in seriously ill patients( Reference Wilner and Arnold 20 , Reference Wargent, Zaibi and Silvestri 22 , Reference Volicer, Stelly and Morris 144 – Reference Dejesus, Rodwick and Bowers 148 ). Yet, they do not discuss how CB improve appetite and food enjoyment, meaning that the underlying behavioural, physiological and psychological mechanisms are still largely unknown. In part, these mechanisms could be similar to those active in the regulation of normal appetite and food reward value, i.e. CB-induced alterations in chemosensory perception, and both appetitive (food wanting) and consummatory (palatability, food liking) components of ingestion. Treatments like chemotherapy are well known for causing temporary disturbances in taste and smell functions( Reference Gamper, Zabernigg and Wintner 149 , Reference Schiffman 150 ). There is tentative evidence that THC palliates altered chemosensory perception in cancer patients( Reference Brisbois, de Kock and Watanabe 145 ). However, appetite may also improve because CB relieve disease-related discomfort that negatively affects appetite, such as nausea, pain, anxiety and depression( Reference Glare, Miller and Nikolova 21 , Reference Beal, Olson and Laubenstein 147 ).
Translational issues and future prospects
Translational issues
So far, knowledge of the involvement of the ECS in modulating appetite, food intake and food reward is predominantly based on animal work. This can be explained by ethical and legal restrictions connected to pharmacological interventions with CB compounds and/or invasive techniques in human subjects. One might consider, for example, studying morphological changes or alterations in neurochemistry in the brain areas important for food hedonics. This is obviously one of the questions that can better or only be investigated in animals. In addition, animal studies have the advantage of a distinct level of experimental control necessary to draw causal conclusions and to control for a range of confounding variables. Nonetheless, it is important to keep in mind the complexities of interspecies scaling and differences in pharmacokinetics. Furthermore, although many aspects of human appetite and eating behaviour can be effectively modelled in animals, some clearly cannot (see also Kim( Reference Kim 151 )). This issue has particular relevance when considering hedonic hunger or reward-driven eating. For example, with respect to odours serving as anticipatory cues signalling food reward value, one can argue that studies in rodents can elucidate the basic mechanisms of endocannabinoid involvement in modulation of food cue reactivity. That might be true, but translation to the human situation remains doubtful, if only because in rodents the sense of smell is far better developed and serves many functions related to food detection, localisation and orienting towards foods. These are no longer, or in a rudimentary form, present in humans( Reference Keller and Vosshall 152 ). In addition, concepts like food enjoyment and reward in humans relate to complex cognitive and emotional appraisal processes that simply cannot be mimicked in animal models.
Another reason why scientific evaluation of CB and medicinal cannabis applications in humans are still in their infancy has to do with what Robson( Reference Robson 38 ) called the ‘pariah status of cannabis’. This presents profound methodological challenges reflected in the shortcomings in many of the clinical trials and almost all of the experimental studies conducted in human subjects during the last decades. Studies tend to be underpowered, with suboptimal designs, and are restricted to a limited number of CB compounds licensed for use in humans. Often, these compounds have unsatisfactory profiles or contain herbal material of variable composition and irregular bioavailability( Reference Robson 38 ). Since 2005, when Robson( Reference Robson 38 ) reviewed the clinical research, there has been a temporary expansion in human studies of CB, mainly fuelled by and co-occurring with the rise and fall of the CB1-selective reverse agonist rimonabant. Therefore, some clinical work has been performed in patients with a focus on food intake and weight status, but despite the urgency and topicality, studies in healthy human volunteers addressing the role of the ECS in modulating food reward are virtually non-existent.
Future prospects
As the present review illustrates, the ECS appears to be closely involved in the physiology underlying ‘hedonic’ eating, or eating motivated by pleasure and reward. Current knowledge relies heavily on preclinical work. Building on what we have learned from animal work, several domains can be identified where novel information can be gained on the modulation by endocannabinoids of food liking and wanting. For one, enhancing endocannabinoid activity apparently stimulates the intake of especially sweet and high-fat foods and increases the palatability of these tastes( Reference Shinohara, Inui and Yamamoto 117 , Reference Jarrett, Limebeer and Parker 124 , Reference Jyotaki, Shigemura and Ninomiya 129 , Reference Yoshida, Ohkuri and Jyotaki 130 ). The sensory and psychophysical processes underlying these alterations have not yet been adequately characterised in humans. Furthermore, in studying the role of CB in human food reward and appetite control, it is necessary to go beyond measuring effects on food intake. Intake is the end result of a complex and dynamic cycle of responding to signals of hunger and satiety, along with hedonic cues. This requires a microstructure approach on appetite control( Reference Higgs, Williams and Kirkham 121 , Reference Yeomans 153 , Reference Yeomans, Blundell and Leshem 154 ) that distinguishes between different aspects like latency to eat, eating rate, intake/volume, as well as changes in hunger and satiety ratings during and in between meals.
The focus in research seems to have been on central pathways, on how the ECS modulates brain neurochemistry in the areas important for food hedonics (‘hedonic hotspots’), as well as its expression in experienced reward and eventually in feeding behaviour( Reference Kirkham 1 , Reference Berridge, Ho and Richard 91 ). For translational research in human subjects, modern neuroimaging techniques like positron emission tomography( Reference Sanabria-Bohórquez, Hamill and Goffin 78 , Reference Ceccarini, De Hert and Van Winkel 79 ) and pharmacological MRI( Reference Jenkins 155 ) open up promising avenues to investigate the neurophysiological correlates (brain functions) of CB modulation of food liking and wanting. But apart from central pathways, future research should also focus on conjoint peripheral and metabolic factors involved in endocannabinoid modulation of appetite and food reward. Recent evidence suggests that the stomach and upper intestines produce endocannabinoids that act as neuromodulators in concert with the major gut peptides (for example, ghrelin, cholecystokinin( Reference Ameloot, Janssen and Scarpellini 156 , Reference Storr and Sharkey 157 )). This implies that endocannabinoids interact with hormones released from the GI tract to communicate with the brain about the current energy status and food reward and palatability simultaneously( Reference Monteleone, Piscitelli and Scognamiglio 69 , Reference Dickson, Egecioglu and Landgren 158 , Reference Perelló and Zigman 159 ). One of the underlying mechanisms might involve alterations of cephalic phase responses (CPR). CPR are induced by the sensory properties of food, such as the sight, smell and taste of food, and include a cascade of pre-absorptive physiological responses that prepare the GI tract for the optimal processing of ingested foods( Reference Smeets, Erkner and De Graaf 160 ). CPR involve changes in plasma levels of NEFA( Reference Joosten, de Graaf and Rietman 161 ) and probably in endocannabinoid levels( Reference Joosten, Balvers and Verhoeckx 70 ). This potential direct link between palatable food-related sensory cues and (alterations in) circulating NEFA and endocannabinoid plasma levels still has to be established.
Conclusions
It has become increasingly clear that the ECS is critically involved in modulating food reward. The ECS is a neurochemical signalling system consisting of CB receptors and their endogenous ligands. Endocannabinoid activity stimulates appetite by increasing palatability and promotes the deposition of energy as fat in adipose tissue. Food reward comprises two components: one appetitive (orienting towards food), the other consummatory (hedonic evaluation of food during consumption), also referred to as ‘wanting’ and ‘liking’, respectively. In the present review an overview of current insights was presented on how (endo)cannabinoids moderate food reward, predominantly based on animal work. With regard to ‘liking’, administration of CB agonists enhances the hedonic value of foods, probably by amplifying the palatability of foods, especially for sweet and fatty tastes. Cannabinoid antagonists result in a diminished hedonic value of foods, and therefore reduce intake. The modulation of ‘wanting’ by endocannabinoid activity has been demonstrated by using free field studies or operant models (progressive ratio paradigms in which experimental animals are required to progressively ‘work harder’ to obtain successive food rewards). In humans, the physiology underlying hedonic eating, or eating motivated by pleasure and reward, is not fully understood, nor is it clear whether the ECS is involved in similar ways as described in animal literature. At present, there are a number of approved medical applications of CB agonists to stimulate disease-related loss of appetite in wasting syndromes. In addition, clinical experience with the (failed) CB1 reverse agonist rimonabant provided some support for reduction in appetite and intake in humans, but there are many remaining questions regarding the relative importance and subtle regulation by the ECS of normal appetite regulation and food reward in humans. There is a pressing need for translational research on the regulation by endocannabinoids of food reward in humans, and several domains where novel information can and should be gained were identified. The applied potential of increasing our knowledge on how endocannabinoids modulate reward-driven eating in humans will guide improvement of existing or development of new strategies to guide hedonic eating. The ultimate goal might be to either decrease the sensitivity to reward signals connected to ‘unhealthy’ foods, or to increase the hedonic value of ‘healthy’ or nutrient-dense foods and diets. This contributes to solving two major problems with huge public health, societal and economic impacts: overeating and obesity on one side of the spectrum, and malnutrition and loss of appetite/food enjoyment in the sick and elderly on the other.
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial or non-profit sectors. G. J. and R. F. W. conducted a review of the literature and co-drafted the manuscript. Both authors made a critical review of the draft. We thank Claire Aucella for proofreading the manuscript.
There are no conflicts of interest.





