Introduction
Nam Et–Phou Louey National Protected Area in northern Lao People's Democratic Republic (hereafter referred to as Lao) has the only confirmed breeding tiger Panthera tigris population remaining in Indochina (Johnson et al., Reference Johnson, Vongkhamheng, Hedemark and Saithongdam2006; Walston et al., Reference Walston, Robinson, Bennett, Breitenmoser, da Fonseca and Goodrich2010). However, the numbers are low (an estimated 7–23) and recovery is dependent on an increase in prey populations and protection from poaching (Johnson et al., Reference Johnson, Vongkhamheng, Hedemark and Saithongdam2006). Although this Protected Area is a landscape of global tiger conservation importance (Dinerstein et al., Reference Dinerstein, Loucks, Heydlauff, Wikramanayake, Bryja and Forrest2006) there has never been a systematic assessment of the status of the ungulate populations. Studies in other tiger landscapes show that prey abundance is an important determinant of tiger density (Karanth & Nichols, Reference Karanth, Nichols, Karanth and Nichols2002; Karanth et al., Reference Karanth, Nichols, Kumar, Link and Hines2004). The observed ratio of approximately one tiger to every 500 deer-sized ungulates allows biologists to estimate the carrying capacity of a given area for tigers (Karanth & Nichols, Reference Karanth, Nichols, Karanth and Nichols2002). This relationship shows that if tiger conservation is to be successful, effective management of prey populations needs to be assured (Karanth & Stith, Reference Karanth, Stith, Seidensticker, Christie and Jackson1999) in addition to tigers being protected from human-caused mortality (Chapron et al., Reference Chapron, Miquelle, Lambert, Goodrich, Legendre and Clobert2008). Thus, lack of reliable data on abundance and distribution of ungulates not only makes it difficult to develop management strategies for long-term conservation of tigers but also hinders the design of monitoring protocols for evaluation of spatial and temporal trends of tiger and prey populations (Williams et al., Reference Williams, Nichols and Conroy2002; Karanth & Nichols, Reference Karanth, Nichols, Tilson and Nyhus2010).
However, obtaining reliable estimates of ungulate abundance is a challenge in tropical forests where ungulate numbers are depressed by hunting, animals are wary of human presence, and visibility is low because of the dense vegetation (Kawanishi, Reference Kawanishi2002; Steinmetz et al., Reference Steinmetz, Chutipong, Seuaturien, Chirngsaard and Khaengkhetkarn2009). In addition, limited access and rugged terrain, such as in Nam Et–Phou Louey National Protected Area, make surveys logistically difficult (Karanth & Nichols, Reference Karanth, Nichols, Karanth and Nichols2002). Line-transect sampling methods (Buckland et al., Reference Buckland, Anderson, Burnham and Laake1993, Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001) have been used extensively in dry tropical forests to estimate ungulate population densities (Karanth & Sunquist, Reference Karanth and Sunquist1992; Srikosamatara, Reference Srikosamatara1993; Varma & Sukumar, Reference Varma and Sukumar1995; Biswas & Sankar, Reference Biswas and Sankar2002) but these methods require numerous sightings of animals to obtain reliable estimates. Thus, where encounter rates are low, as in tropical evergreen forests, these methods do not work well because the number of animals seen is insufficient to fit a reliable detection function and thus population estimates are unreliable (Buckland et al., Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001; Williams et al., Reference Williams, Nichols and Conroy2002). Therefore, the direct observation of animals using distance sampling is often impractical for many locations in South-east Asia where animal abundance is low (Steinmetz et al., Reference Steinmetz, Chutipong, Seuaturien, Chirngsaard and Khaengkhetkarn2009).
To overcome these problems and establish a baseline estimate of ungulate abundance that could be systematically repeated over time to assess change in prey availability for tigers, and evaluate the success of conservation activities, we used a repeated sign-based presence/absence survey (MacKenzie et al., Reference MacKenzie, Nichols, Lachman, Droege, Royle and Langtimm2002), which is conceptually similar to a capture–recapture scheme. An index of animal abundance is generated from the occupancy rate using the Royle/Nichols heterogeneity model in PRESENCE (Royle & Nichols, Reference Royle and Nichols2003; Hines, Reference Hines2006). The model is a likelihood-based approach, which incorporates the heterogeneity of detection probability as a result of variation in animal abundance into the analytical process (Royle & Nichols, Reference Royle and Nichols2003; Royle et al., Reference Royle, Nichols and Kery2005). The model assumes that species that are more abundant have a higher detection probability relative to those that are less abundant.
Study area
The 5,950 km2 Nam Et–Phou Louey National Protected Area lies within the 30,000 km2 Tiger Conservation Landscape 35 (Dinerstein et al., Reference Dinerstein, Loucks, Heydlauff, Wikramanayake, Bryja and Forrest2006) in the northern highlands of Lao along the border with Vietnam (Fig. 1). Altitude across the Protected Area is 400–2,257 m. Climate is monsoonal and temperatures range seasonally from 5 to 30 °C; total annual rainfall is 1,400–1,800 mm (Johnson et al., Reference Johnson, Vongkhamheng, Hedemark and Saithongdam2006). The Protected Area contains a high diversity of carnivores significant to national and international conservation (Johnson et al., Reference Johnson, Vongkhamheng, Hedemark and Saithongdam2006, Reference Johnson, Vongkhamheng and Saithongdam2009).

Fig. 1 The 5,950 km2 Nam Et–Phou Louey National Protected Area in northern Lao, and the 3,000 km2 core zone within the Protected Area. The shaded area on the inset indicates the location of the main map in northern Lao, on the border with Vietnam.
The Protected Area is divided into two zones for management purposes (Fig. 1): a 3,000 km2 core zone where access and harvesting of wildlife are prohibited, surrounded by a 2,950 km2 management zone within which pre-existing villages are allocated land for subsistence. The primary purpose of the core zone is to ensure a safe haven for breeding populations of tigers and their prey. The mixed tropical evergreen and deciduous vegetation in the core zone has the potential to hold sufficient prey and other resources to support up to 150 tigers, including 50 breeding females (Karanth et al., Reference Karanth, Goodrich, Vaidyanathan and Reddy2009). There are 98 villages within and around the management zone, with a mean density of eight people per km2 (Johnson et al., Reference Johnson, Vongkhamheng, Hedemark and Saithongdam2006). They depend largely on forest resources for food and income. In the management zone government regulations limit subsistence hunting to small, highly fecund species such as wild pig Sus spp., bamboo rat Cannomys badius and other rodents, with specified hunting gear and seasons for harvesting (GoL, 2007, 2008). It has been estimated that each household in the Protected Area consumes c. 141 kg of wild meat annually, of which 20% is of pigs and deer (ICEM, 2003).
Methods
We conducted an occupancy survey from January to June 2008 to assess the abundance and distribution of five ungulate species: gaur Bos gaurus, sambar deer Cervus unicolor, serow Capricornis milneedwardsii, wild pig and muntjac Muntiacus spp., in the core zone. A grid-cell of 13 km2 was used as the sampling unit, and the core zone was divided into 289 grid cells (Fig. 2). The biological basis for the cell size was that the largest ungulate home range was expected to be c. 13 km2 or smaller, and there was no variation in seasonal ungulate home range sizes across the site during the survey (Karanth et al., Reference Karanth, Gopalaswamy and Kumar2008). Each grid cell was labelled with a unique ID and consisted of four 3.25 km2 sub-grid cells, which were numbered clockwise 1–4, for data management purposes. Within each sub-grid cell nine equally spaced destination points (points along route to be sampled) were placed at 600 m intervals, for logistical convenience (Fig. 3). Each spatial replicate was a walk of 300 m, which was recorded using the track log function of a global positioning system (GPS). When traversed, the 13 km2 grid cell provided 40 spatial replicates (10 replicates per sub-grid cell). We used 1 : 50,000 topographic maps to traverse each sub-grid cell and navigate the rugged terrain. During the 6-month survey period cells were assumed to be closed to changes in occupancy. Although animals did actually come in and out of the 13 km2 grid cell sampling units these movements were assumed to be random and interpreted as the proportion of site ‘used’ so that the occupancy estimator remained unbiased (Mackenzie & Royle, Reference MacKenzie and Royle2005). All sites were assumed to be heterogeneous in detection probability (Royle & Nichols, Reference Royle and Nichols2003).

Fig. 2 The 3,000 km2 core zone of Nam Et–Phou Louey National Protected Area (Fig. 1) was divided into 13 km2 grid cells. Each cell was given an identification number and divided into four 3.25 km2 sub-grid cells.

Fig. 3 A 13 km2 grid cell was divided into four 3.25 km2 sub-grids, in which nine equally spaced (600 m apart) destination points were demarcated. The survey route (wavy line) deviated from geometrically rigid sampling by as much as 100 m either side of the straight line, and passed through at least five points, including the centre point of each sub-grid cell.
We established nine survey teams of three persons per team, each comprising one university-trained team leader and two village assistants. The village assistants were local hunters with extensive experience at the site and had worked with field surveys in the Protected Area for at least 7 years. The teams were trained in survey techniques and to reliably distinguish tracks and dung of focal ungulate species based on size and shape. Before teams began surveying independently each team was observed while completing a survey of four sub-grids within a grid cell to ensure that each team member could correctly observe and identify ungulate signs, complete data forms precisely, and use navigation tools to move from one point to another.
The teams walked along geometrically rigid sampling paths (Fig. 3), searching thoroughly for signs of prey, including fresh tracks and fresh dung (< 2 weeks old). Direct observations of animals encountered along the survey route were also recorded, noting the position with a GPS as well as the number of individuals and distance from observer. Following Karanth et al. (Reference Karanth, Gopalaswamy and Kumar2008) the teams were allowed to deviate from the path by up to 100 m on either side (Fig. 3), if necessary, to maximize their chances of detecting signs. The survey team needed to pass through at least five sampling points within each sub-grid cell to cover the entire extent of ungulate habitats in the sub-grid cell, recording data for every 300 m replicate, determined with a GPS, by entering 1 when animal sign was detected and 0 otherwise. After a particular sub-grid cell was surveyed the team moved to the adjacent sub-grid cell, and completed all four sub-grids within a 13 km2 grid cell before moving on to the next adjacent 13 km2 grid cell (Fig. 3). The team walked c. 10–12 km each day, taking 2 days to complete the survey of each sub-grid. To avoid biases caused by seasonal migration of ungulates within the core zone, the survey was made from south-west to north-east across the core zone instead of arbitrarily sampling grid cells in a patchy or sporadic manner.
Data were analysed using PRESENCE v. 3.0 beta (Hines, Reference Hines2006), which implements an occupancy model (i.e. the maximum likelihood-based technique) developed by MacKenzie et al. (Reference MacKenzie, Nichols, Lachman, Droege, Royle and Langtimm2002) and Royle & Nichols (Reference Royle and Nichols2003) to generate parameters of probability of habitat occupancy, detection and index of animal abundance. Detection and non-detection of ungulates in each sub-grid cell was used to produce a detection history matrix. This consisted of rows representing the sub-grid cells, and columns representing detection history of animals in each 600-m replicate (sampling occasion) of the sub-grid cell. There were a total of 800 rows of surveyed sub-grid cells, representing 800 sampling units. Applying a single-season heterogeneity model (Royle & Nichols, Reference Royle and Nichols2003) accounted for the heterogeneity in detection probability of a species among sites caused by variation in animal abundance in the survey area. This generated estimates of occupancy rate (Ψ), abundance index (λ) of animal clusters per sub-grid, and an individual detection probability (r).
The population size of each ungulate taxon was estimated by multiplying the number of clusters by the mean cluster (or group) size of the ungulate species encountered. Mean group size (Y) of each prey species was calculated according to the number of animal observations encountered. For gaur, which was never encountered during the course of the field work, we conservatively employed a mean group size of one individual even though a group of tracks of the same age was occasionally found in the field and gaur have been recorded in herds of 2–5 in protected areas in southern Lao (Steinmetz, Reference Steinmetz2004). We assumed that the rarity of sightings of gaur in this study was because of low abundances rather than evasiveness of animals as a result of high poaching pressure during the previous decade (Vongkhamheng, Reference Vongkhamheng2002; Johnson et al., Reference Johnson, Vongkhamheng, Hedemark and Saithongdam2006).
Results
The teams surveyed 200 13-km2 grid cells in 6 months, walking a total distance of 4,617 km and covering 2,600 km2. Estimates of occupancy indicate that smaller ungulate species occupy a higher proportion of the survey area. Values for the muntjac and wild pig were higher than for the larger serow, sambar and gaur (Table 1).
Table 1 Summary of occupancy statistics for prey species of tiger Panthera tigris in Nam Et–Phou Louey National Protected Area (Fig. 1), calculated using the Royle–Nichols heterogeneity model (2003).

1 Number of sites in which a species was detected (without incorporating detection probability)
2 Abundance index (clusters per 3.25 km2 sub-grid)
3 Detection probability for individuals
4 Probability of site occupied
5 Estimated abundance index of clusters (number of animal clusters or groups)
A single-season heterogeneity model generated an abundance index of animal clusters (or groups) per 3.25 km2 sub-grid cell sampling unit for five species of ungulates. Generally, the results show that smaller species were relatively more abundant than larger species (Table 1). The abundance index of clusters per sub-grid ranged from as low as λ = 0.07 for gaur to λ = 4.42 for the muntjac. The spatial distribution of abundance indices for four species (wild pig, serow, sambar and gaur) was mapped (Fig. 4). Applying the mean cluster (or group) size of each prey species (Table 2), multiplied by the number of clusters, resulted in a range of population sizes from c. 58 individuals for gaur to 8,302 for wild pig. By summing the estimated number of individuals per km2 (Table 3) the estimated ungulate population density is 5.29 ± SE 0.30 km−2 in Nam Et–Phou Louey National Protected Area.

Fig. 4 Distribution of abundance (individuals per km2) of larger prey species (gaur Bos gaurus, wild pig Sus spp., serow Capricornis milneedwardsii and sambar Cervus unicolor) of the tiger, calculated by combining values of abundance indices (λ; Table 1) of these species in each 3.25 km2 sub-grid cell.
Table 2 Observed group size, number of groups detected, and mean group sizes of prey species used to estimate the abundance indices of tiger prey in Nam Et–Phou Louey National Protected Area (Fig. 1), and from other sources.
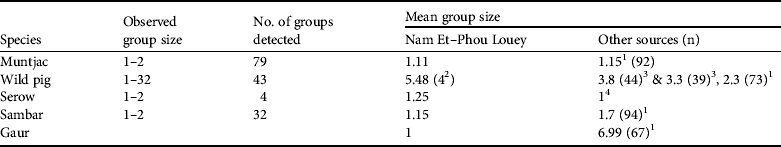
1 Karanth & Sunquist (Reference Karanth and Sunquist1992)
2 Mean group size (Y = 4) used in estimating the abundance index for pigs (Table 3) was compiled by averaging the mean group size (Y = 5.48) in Nam Et–Phou Louey National Protected Area and the data from Ickes et al. (2001; Y = 3.8 and 3.3 in Pasoh Forest Reserve, Malaysia, where pig densities were 27 and 47 km−2)
3 Ickes et al. (Reference Ickes, DeWalt and Appanah2001)
4 Kawanishi & Sunquist (Reference Kawanishi and Sunquist2004)
Table 3 The adjusted abundance index of tiger prey species estimated by multiplying abundance index of clusters by mean cluster size, and estimated density of individuals.

Discussion
The results from this study provide the first systematic assessment of ungulate population status in the forests of Lao using grid-based occupancy surveys, as well as a reliable index of abundance and a distribution map of five ungulate species in the Nam Et–Phou Louey National Protected Area. Assuming a positive relationship between the abundance index and absolute abundance, it is evident that abundance of most ungulate taxa, especially large species, is low throughout this Protected Area. The data show that smaller ungulates such as the muntjac and wild pig are more widely distributed and have higher abundance indices of clusters than those of the larger serow, sambar and gaur.
Because of the rugged terrain, dense ground cover and low prey densities, line-transect distance sampling with direct observation of animals is not a suitable technique to use in this environment. The underlying logic of the alternative grid-based occupancy survey method of Royle & Nichols (Reference Royle and Nichols2003) is that both occupancy and abundance parameters are generated by the incorporation of detection probability parameters into the analytical model, which produces unbiased results (Karanth & Nichols, Reference Karanth, Nichols, Tilson and Nyhus2010). Empirical evidence suggests that the relationship between occupancy and abundance is generally positive (Gaston et al., Reference Gaston, Blackburn, Greenwood, Gregory, Quinn and Lawton2000). The link between occupancy and abundance patterns is that species declining in abundance decline in the number of sites they occupy, whereas species increasing in abundance tend to be increasing in occupancy (Gaston et al., Reference Gaston, Blackburn, Greenwood, Gregory, Quinn and Lawton2000).
Given the small error associated with the estimates, as a result of the high sampling effort, it may be possible to obtain reliable estimates of the abundance index with less effort, using 50% of the grid cells, following a cross-hatch pattern but maintaining the same level of survey effort within each sub-grid cell. The indices of animal abundances obtained in this study will be useful to detect changes in ungulate population size and distribution. This is because the detection probability of animals is a function of their abundance; i.e. species with higher abundance in cells are more likely to be detected than those species with lower abundance (Royle & Nichols, Reference Royle and Nichols2003). Using this occupancy survey approach may allow us to lower both time spent in the field and the overall financial cost as well as the number of people required to conduct the field survey. However, occupancy surveys require well-trained field staff (Vongkhamheng, Reference Vongkhamheng2011).
The results from this survey are useful to the management of the Nam Et–Phou Louey National Protected Area for several reasons. Firstly, the study provides the first reliable estimates of prey abundance that incorporate a detection probability. The results are consistent with indices of prey abundance derived from an earlier study (2003–2004) that used photographic capture–recapture sampling (Johnson et al., Reference Johnson, Vongkhamheng, Hedemark and Saithongdam2006), which showed that abundance of large ungulates (gaur, sambar, serow) was low, whereas abundance of smaller prey (wild pig, muntjac, stump-tailed macaque Macaca arctoides, porcupine Hystrix brachyura and hog badger Arctonyx collaris) was significantly higher, particularly where human density was lower (Johnson et al., Reference Johnson, Vongkhamheng, Hedemark and Saithongdam2006). Secondly, the results illustrate that prey populations are relatively low but that pockets of large ungulates still persist in the regional landscape. Currently, the core zone serves as a source site: the area contains concentrations of ungulates that have the potential to repopulate the larger landscape (Walston et al., Reference Walston, Robinson, Bennett, Breitenmoser, da Fonseca and Goodrich2010). The data suggest that these remaining pockets of large ungulates need to be protected for recovery of both prey and tigers by strictly controlling hunting of ungulates (to maintain and increase their populations), and protecting tigers, particularly breeding females, from illegal poaching. Thirdly, the survey method used will facilitate evaluation of the effectiveness of law enforcement in reducing hunting and recovering prey in the core zone. To facilitate enforcement the core zone is divided into sectors, each of c. 260 km2. In each sector a team of enforcement officers patrol to apprehend poachers and halt any illegal hunting activity. Increases or decreases in animal abundance within each sector will indicate the effectiveness of these patrols. Accurate and timely reporting of problems will allow the teams to adapt their patrol strategies, thereby improving effectiveness.
Ideally, occupancy surveys using presence–absence data for these five ungulate taxa should be repeated every 3 years, to assess the effectiveness of management activities. We assume that increased enforcement and outreach efforts in the Protected Area, together with the reproductive capacity of ungulate populations in the area (presumed to be similar to elsewhere), will allow ungulates to recover within this period. Repeated occupancy surveys in the core zone at regular intervals will allow monitoring of ungulate population trends and allow Protected Area staff to test this assumption. If ungulate populations increase and hunting pressure declines in the core zone we expect to see, firstly, an increase in detection probability as animals become less wary and more easily sighted. We would then expect to see increases in the abundance index, and then the survey effort could be reduced. Once the number of sightings is sufficiently large to calculate a detection function we may be able to incorporate distance sampling, which would generate more reliable estimates of ungulate abundance.
Acknowledgements
We thank the government authorities in Houaphan and Luang Prabang provinces for logistical support to work in Nam Et–Phou Louey National Protected Area, all survey teams and Protected Area staff for help with data collection, all the donors who generously support our study (the Wildlife Conservation Society, Panthera, National Geographic Society, Ocean Park Conservation Foundation of Hong Kong, International Foundation for Science, and a Panthera Kaplan Graduate Award), Dr K. Ullas Karanth and Arjun Gopalaswamy of the Wildlife Conservation Society–India Program for their technical advice on research design and survey protocol, and Dr James Nichols and Dr James Hines of the USGS Patuxent Wildlife Research Center for their advice on data analysis.
Biographical sketches
Chanthavy Vongkhamheng has worked to conserve biodiversity in Lao PDR for more than 18 years and is the founder and director of the Lao Wildlife Conservation Association. Arlyne Johnson works with Foundations of Success, providing technical training for the design, monitoring and evaluation of biodiversity conservation projects. She was the co-director of the Wildlife Conservation Society's programme in Lao PDR in 1998–2011, providing technical supervision for conservation projects, including the development of the Nam Et-Phou Louey National Protected Area as a model for tiger conservation in Indochina. Melvin Sunquist is a Professor Emeritus in the Department of Wildlife Ecology and Conservation at the University of Florida. His research is focused on increasing our understanding of the ecology, behaviour, and conservation of mammalian carnivores, principally felids.








