Introduction
Natural habitats are disappearing or degrading at global scales and at an unprecedented rate as a result of the combined effects of current climatic processes and changes in land use during the Anthropocene (Fahrig, Reference Fahrig1997; Balmer & Erhardt, Reference Balmer and Erhardt2000; Davidson, Reference Davidson2014; Hu et al., Reference Hu, Niu, Chen, Li and Zhang2017). One of the main driving factors is the expansion of intensive forms of agricultural land use, which has led to the reduction and sometimes even complete disappearance of various native habitats across Europe (O'Connor & Shrubb, Reference O'Connor and Shrubb1986; Potter, Reference Potter, Pain and Pienkowski1997). These declines are especially severe amongst grassland breeding animals that are considered to be sensitive to environmental changes, as evidenced by recent steep declines of steppe species (Fuller, Reference Fuller2000; Massa & La Mantia, Reference Massa and La Mantia2010; Ward et al., Reference Ward, Semel and Herkert2010; Guerrero et al., Reference Guerrero, Morales, Oñate, Geiger, Berendse, de Snoo and Tscharntke2012). As a consequence of the loss of grassland habitats, birds that traditionally bred in open natural habitats now increasingly breed on arable land and in agricultural areas (Galbraith, Reference Galbraith1987; Böhning-Gaese & Bauer, Reference Böhning-Gaese and Bauer1996; Brady & Flather, Reference Brady and Flather1998).
However, agricultural landscapes can lead to mal-assessment in habitat choice as they appear suitable to prospective breeders but the conditions they offer often result in inferior reproductive success (Székely, Reference Székely1992), and thus they may be ecological traps (Schlaepfer et al., Reference Schlaepfer, Runge and Sherman2002; Robertson & Hutto, Reference Robertson and Hutto2006; Pärt et al., Reference Pärt, Arlt and Villard2007; Gilroy et al., Reference Gilroy, Anderson, Vickery, Grice and Sutherland2011; Hollander et al., Reference Hollander, Titeux, Holveck and Van Dyck2017). Additionally, the intensification of agricultural practices can affect the nesting success of ground-breeding birds in numerous ways, including direct loss of nests, chicks and/or adults through mowing, cultivation by agricultural machinery, use of pesticides, irrigation and/or drainage (Berg et al., Reference Berg, Lindberg and Källebrink1992; Wilson et al., Reference Wilson, Whittingham and Bradbury2005; Kentie et al., Reference Kentie, Hooijmeijer, Trimbos, Groen and Piersma2013). There are numerous examples of the negative affects of agriculture on ground-breeding birds, including local extinctions of flagship species such as the great bustard Otis tarda and grey partridge Perdix perdix (Donald et al., Reference Donald, Green and Heath2001; Arroyo, et al., Reference Arroyo, García and Bretagnolle2002; De Leo et al., Reference De Leo, Focardi, Gatto and Cattadori2004; Alonso & Palacín, Reference Alonso López and Palacín2010; Potts, Reference Potts2012; Gooch et al., Reference Gooch, Ashbrook, Taylor and Székely2015). These pressures on farmland birds have intensified as a result of global climate change, which has amplified predation rates in human-modified habitats. Specifically, environmental changes have boosted the populations of mesopredators, which have further reduced the nest or offspring survival rates of ground-breeding birds (Roodbergen et al., Reference Roodbergen, van der Werf and Hötker2012; Kentie et al., Reference Kentie, Both, Hooijmeijer and Piersma2015; Kubelka et al., Reference Kubelka, Šálek, Tomkovich, Végvári, Freckleton and Székely2018; Brzeziński et al., Reference Brzeziński, Żmihorski, Nieoczym, Wilniewczyc and Zalewski2020). To mitigate these negative effects, targeted conservation actions are needed (Arroyo et al., Reference Arroyo, García and Bretagnolle2002; Schekkerman et al., Reference Schekkerman, Teunissen and Oosterveld2008; Zámečník et al., Reference Zámeĉník, Kubelka and Šálek2018).
Here we report the results of a 10-year conservation effort focused on the collared pratincole Glareola pratincola, which is affected by habitat alterations and has undergone population declines across many parts of Europe (Yuri et al., Reference Yuri, Lokhman, Fedosov, Mikhail, Keller, Herrando, Voríšek, Franch, Kipson and Foppen2020). The collared pratincole is a ground-nesting shorebird that used to breed in loose colonies on alkaline grasslands close to wetlands in Central Europe (Cramp & Simmons, Reference Cramp and Simmons1983), and a large inland breeding population existed in Hungary until the mid 1900s (Aradi, Reference Aradi1979; Kiss et al., Reference Kiss, Molnár, Monoki, Kapocsi, Kovács and Aradi2018). Collared pratincoles feed on flying insects including dragonflies, flies and beetles of various sizes. They scrape their nests into livestock hoofprints or into the bare ground (Beretzk, Reference Beretzk1954; Cramp & Simmons, Reference Cramp and Simmons1983). The collared pratincole is categorized as Least Concern on the IUCN Red List but the global population is declining (BirdLife International, 2021). It has been difficult to assess population changes because of the high dispersal propensity and semi-nomadic strategy of the species, leading to large fluctuations in breeding densities (Yuri et al., Reference Yuri, Lokhman, Fedosov, Mikhail, Keller, Herrando, Voríšek, Franch, Kipson and Foppen2020). Collared pratincoles are now breeding on agricultural land in Europe (Calvo & Alberto, Reference Calvo and Alberto1990; Calvo, Reference Calvo1994; Calvo & Furness, Reference Calvo and Furness1995; Lebedeva, Reference Lebedeva1998; Kiss et al., Reference Kiss, Monoki and Végvári2017; Yuri et al., Reference Yuri, Lokhman, Fedosov, Mikhail, Keller, Herrando, Voríšek, Franch, Kipson and Foppen2020), and most breeding attempts now occur on arable farmland even in some of the coastal breeding populations (Vincent-Martin, Reference Vincent-Martin2007; Nardelli et al., Reference Nardelli, Andreotti, Bianchi, Brambilla, Brecciaroli and Celada2008; Kiss et al., Reference Kiss, Monoki and Végvári2017). During the past decade, the Hungarian population has fluctuated between 22 and 65 pairs, at two major breeding sites (the Nagykunság and Kiskunság regions). These sites are the last remaining regular breeding locations of collared pratincoles in the Carpathian Basin (Kiss et al., Reference Kiss, Molnár, Monoki, Kapocsi, Kovács and Aradi2018).
We have four objectives: firstly, to quantify nesting success of the collared pratincole and investigate the ecological variables that could predict nesting success, including habitat type, timing of breeding, proximity to open water and breeding density; secondly, to compare nest survival rates between different agricultural habitats; thirdly, to investigate the effects of conservation measures on nest survival; and finally, to investigate potential associations between predator control and nest survival.
Study area
We carried out data collection and conservation activities in the Nagykunság region in eastern Hungary (Fig. 1). The climate is eastern continental, characterized by dry and warm periods during the breeding season interspersed with short, heavy rainfall of 20–100 mm/h (Hungarian Meteorological Service, 2021). We focused on the southern part of Nagykunság, where the landscape is dominated by cultivated lands, primarily rice fields (Fig. 1). Because of the requirements of rice cultivation, each year > 1,500 ha of farmland is flooded, providing important habitats for breeding and migrating shorebirds (Kiss et al., Reference Kiss, Monoki and Végvári2017). In Nagykunság, the estimated number of collared pratincoles fluctuated between 13 and 56 breeding pairs during 2012–2021 (mean 32.4 ± SE 5.3; data from the database of the Hortobágy National Park Directorate).
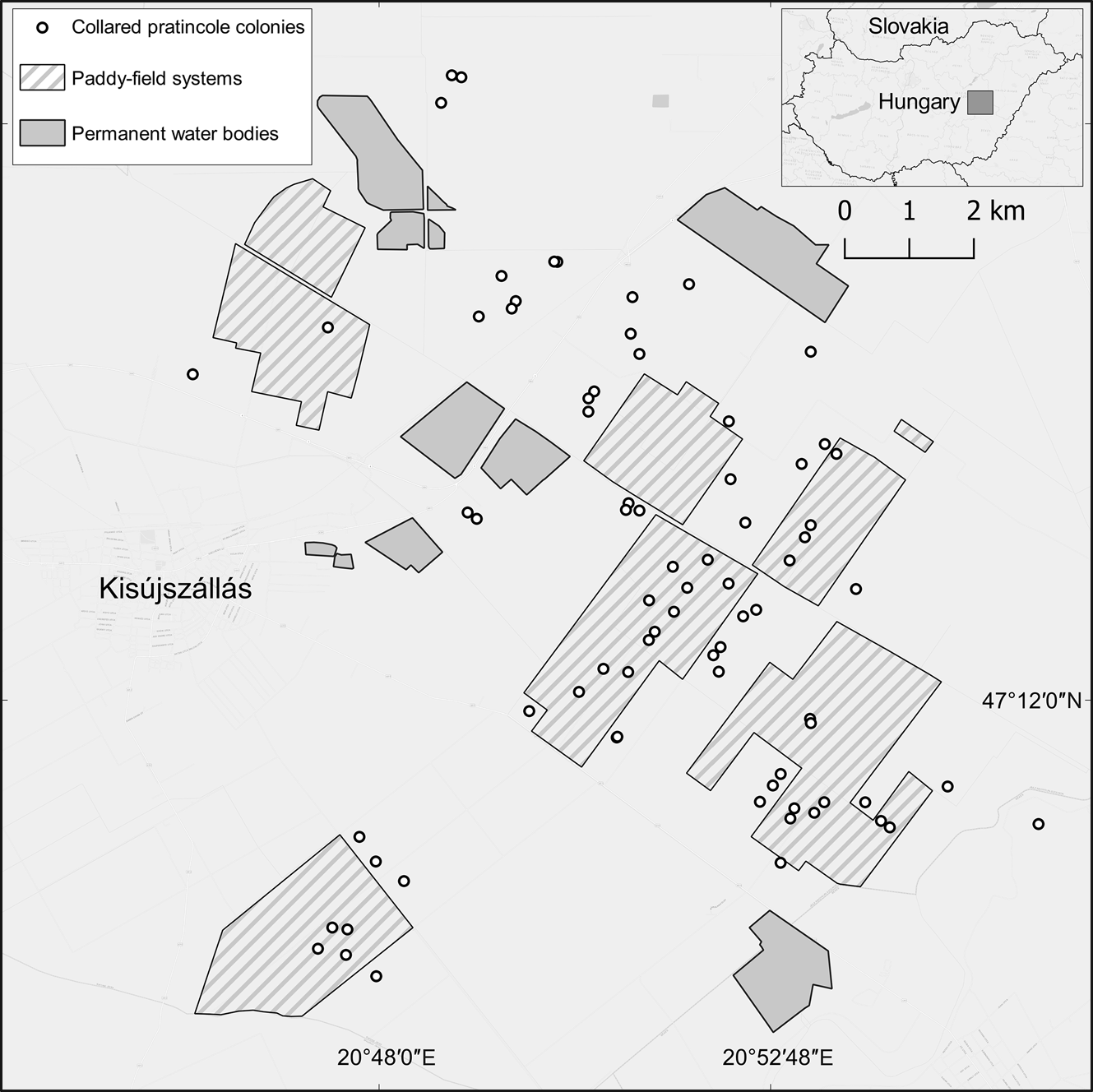
Fig. 1 The c. 12,500-ha study area in eastern Hungary.
Methods
Data collection
Starting in 2012, we collected data on breeding sites, including nest-site location, nesting success and behaviour. We recorded the data using a handheld digital assistant and then transferred the data to ArcMap 10.1 (Esri, Redlands, USA). We also collected observations in croplands used by collared pratincoles and in shallow wetlands. Using nest points and various agricultural variables we created a map of nest points and shapefiles of arable lands and shallow water bodies.
We prepared maps of nest locations and polygons of croplands and water bodies for further analyses using ArcMap. We carried out monitoring activities of birds on potential nesting sites, location and revisiting of nests using binoculars and spotting scopes. We always approached nests to a distance of 8–10 m using four-wheel drive cars (even within agricultural fields) to limit any possible disturbance to incubating birds. In addition to the locations of nests, we identified the agriculture habitat types used for nesting (Plate 1, Supplementary Table 1). We identified these using Calvo's (Reference Calvo1994) methodology based on the planting patterns of crops: we classified fields with crops planted in rows at least 60 cm apart as row crops, areas where crops were sown without leaving any row gaps as spring cover crops and areas that were left unsown after tilling as fallow. After locating the nests, we recorded the clutch size, nest cover and location coordinates for each nest and then placed a small stick c. 1 m from the nest to mark the nest location in the field. After locating a new nest, we consulted the owner or manager of the land. To prevent nest destruction by farming activities, we marked a buffer zone around the nests using 1.5 m-tall wooden poles. However, to avoid attracting the attention of potential nest predators, we only placed the nest markers during periods of active agricultural work (Zámečník et al., Reference Zámeĉník, Kubelka and Šálek2018). The sizes of these oval-shaped nest protection zones were 100 m2, which is sufficient to ensure adequate protection from agricultural machinery (Plate 2).

Plate 1 Main breeding habitats of collared pratincoles Glareola pratincola in Hungary: (a) row crop, (b) spring cover crop, (c) fallow land.

Plate 2 Protection zone around collared pratincole nest, marked temporarily by 1.5 m-tall wooden poles. This photograph was taken using a telephoto lens that distorts the actual distance between the two wooden poles.
During the incubation period, we checked all of the nests remotely using a spotting scope every other day. The incubation period was 18 days (based on Myhrvold et al., Reference Myhrvold, Baldridge, Chan, Sivam, Freeman and Ernest2015, and our field observations supplemented with use of the egg-floating methodology; see details in Székely et al., Reference Székely, Kosztolanyi and Küpper2006), and after the last nest visit, we classified each nest as hatched, predated, abandoned, unknown, flooded or destroyed by agricultural machinery. To identify the fate of each nest, we used the methods of Green et al. (Reference Green, Hawell and Johnson1987) to identify potential nest predators in addition to our field observations (Kiss et al., Reference Kiss, Molnár, Monoki, Kapocsi, Kovács and Aradi2018). We established successful hatching if there were small eggshell fragments or small chick droppings in the nest cup, predation if we found predator tracks or signs of predation by mammals or birds near the nest, desertion if the pair was not nearby and the egg was cold and abandonment because of rainfall if the egg was stuck in mud. We determined a nest successful if at least one chick hatched.
The survival and productivity of ground-nesting birds are influenced by predation (Martin, Reference Martin1993; Roodbergen et al., Reference Roodbergen, van der Werf and Hötker2012; Kubelka et al., Reference Kubelka, Šálek, Tomkovich, Végvári, Freckleton and Székely2018). To investigate relationships between pratincole breeding success and number of nest predators removed from the study area, we collected data from hunters. Potential huntable nest predators include mammals (red fox Vulpes vulpes, golden jackal Canis aureus, European badger Meles meles) and birds (Eurasian magpie Pica pica, hooded crow Corvus cornix). We requested hunting bag data from professional hunters for the period 2017–2021 and we aggregated the number of individuals killed for each year. Hunting bag data relate to the regional hunting district (c. 26,000 ha), which covers c. 65% of collard pratincole nest sites (National Game Management Database, 2022).
Estimating daily and total nest survival
To investigate the effects of year and agricultural habitat on nesting success, we estimated daily and total nest survival rates using calculations provided by Mayfield (Reference Mayfield1975). We applied this method to estimate the chances of a clutch surviving daily and for the full nesting period (egg laying + incubation period) by defining the daily nest survival rate as the number of failed nests divided by the sum of exposure days (Johnson, Reference Johnson1979). We calculated total nest survival using Mayfield's formula: (daily nest survival)nesting period in days, where the total nesting period is egg laying period + incubation (20 days in total). We defined exposure time as the number of days from finding a nest to the confirmed (or expected) last day of the breeding attempt. The incubation period started when the complete clutch had been laid. For those nests that hatched (i.e. at least one chick hatched), we calculated the exposure time from the day of nest finding to the confirmed (or predicted, using the egg floating methodology; Székely et al., Reference Székely, Kosztolanyi and Küpper2006) date of hatching (Mayfield, Reference Mayfield1975; Kubelka et al., Reference Kubelka, Šálek, Tomkovich, Végvári, Freckleton and Székely2018). For predated nests, we calculated exposure from the day of nest finding until the midpoint between the last positive and the first negative visit to the nest. For all other outcomes (i.e. unknown, abandoned, flooded, destroyed by agricultural machinery), we defined exposure time from the day of nest finding until the last positive nest visit, following standard protocols (Kubelka et al., Reference Kubelka, Šálek, Tomkovich, Végvári, Freckleton and Székely2018). We focused trail cameras on 116 nests to identify species of nest predators and the date of hatching.
Statistical analyses
We performed the statistical analyses in R 3.3.3 (R Core Team, 2021). To identify relationships amongst individual-level reproduction success metrics (i.e. nests hatched or failed and the number of hatched chicks), we used generalized linear models (GLMs) with factors of year, habitat type, Julian day of egg laying, distance from the closest field boundary, distance from the closest water body and colony density. The latter factor represented the mean distance from the focal nest to the three closest nests within the same breeding colony. As nesting success was a binary response variable (hatched or failed), we used a logistic regression GLM with the logit link error function. Field boundaries and water bodies were available in shape files. We computed the mean distance from the three neighbouring nests using the nndist spatial neighbourhood function available in the spatstat package in R. For the start of incubation, we used the Julian day of clutch completion at a given nest. We used ANOVA, implemented using the lm function, to analyse the associations between (1) clutch size and habitat type, (2) timing of hatching and habitat type, and (3) daily nest survival (aggregated for years and habitats).
Results
Timing of breeding and breeding success
During 2012–2021, we found 315 nests, for 212 of which we also determined the hatching date of the first chick. Egg laying started in late April and terminated in mid July (c. 2.5 months duration), with most nests (60%) laid during 25 May–15 June. The first eggs hatched on 16 May, which implies that the clutch was completed on 29 April. The latest hatch date was recorded on 3 August. The mean hatching date was 15 June ± SE 1 day (n = 212 nests; Table 1, Supplementary Fig. 1).
Table 1 Timing of breeding, clutch size and nesting success of collared pratincoles Glareola pratincola in agricultural habitats in Hungary (mean ± SE; Fig. 1). We found 315 nests over our study period, but as we were only able to establish hatching times for 212 of these nests, we show hatching times separately.
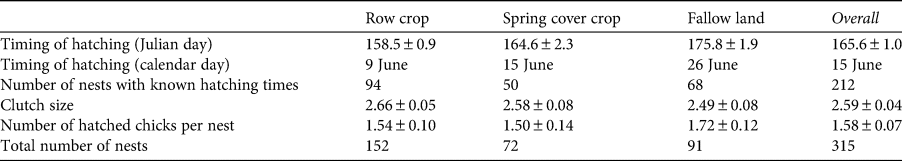
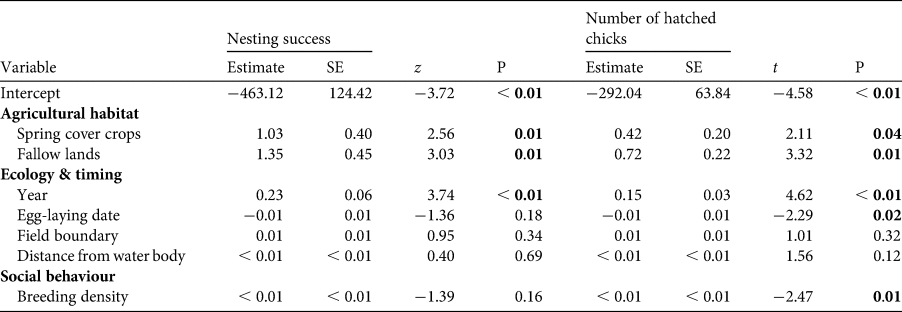
Collared pratincoles bred in three agricultural habitats: most nests were in row crops (48%), followed by fallow lands (29%) and spring cover crops (23%, n = 315 nests; Table 1). The timing of breeding was different between habitat types: nests in row crops or spring cover crops hatched earlier than in fallow lands (one-way ANOVA, b > 6.311, F 2,210 = 39.02, Pmin = 0.009; Supplementary Fig. 1). Similarly, clutch size was related to habitat type: the largest clutch sizes were in row crops (one-way ANOVA, b < −0.0491, F 2,268 = 2.601, Pmin = 0.0254; Table 1). A total of 75% (n = 68) of nests on fallow lands hatched at least one chick, whereas the corresponding figures were 69% (n = 50) on spring cover crops and 62% (n = 94) in row crops (Table 1). The overall nesting success was 67% (n = 315 nests). Nesting success was related to the type of habitat: nests in spring cover crops or fallow lands were more successful and produced more hatchlings than nests in row crops. In addition, nesting success was also associated with both time in the season and breeding density, as early nests and those in areas with higher breeding densities produced more chicks (Table 2).
Table 2 Nesting success and the number of hatched chicks of collared pratincoles in Hungary and their relationships to agro-technology, time, space and other ecological variables. Logistic and linear regression analyses were used to explore the relationship between these variables, as appropriate. Significant relationships are indicated in bold.
Daily nest survival increased significantly over the study period (linear regression, b = 0.0064, n = 8, P = 0.0189; Fig. 2). Total nest survival increased from 11.2 to 83.5% (Supplementary Table 2). However, using two-way ANOVAs we found no association between habitat type and daily nest survival (two-way ANOVA, b = −0.046, SE = 0.036, P = 0.21) or total nest survival (two-way ANOVA, b = −0.103, SE = 0.144, P = 0.48), using year as the unit of analysis. The highest level of total nest survival was recorded in fallow lands (Supplementary Table 2).

Fig. 2 Daily nest survival (mean ± SE) in relation to the study year (r 2 = 0.55, n = 10 years). The number of nests per year is indicated.
Causes of nest failure
Most nest failures (n = 102) were caused by predation (58%), followed by nest abandonment (23%) and flooding by heavy rainfall (17%), with 1% having an unknown fate. As a result of the nest-marking scheme, agricultural machinery destroyed only a few nests (1%; Supplementary Table 3). In total, 83% (n = 49) of all nest predation and 89% (n = 17) of all flooded nests were found in row crops and spring cover crops, respectively. The most common predators of eggs and chicks were red foxes, European badgers, hooded crows, western marsh harriers Circus aeruginosus and Caspian gulls Larus cachinnans.
Conservation action
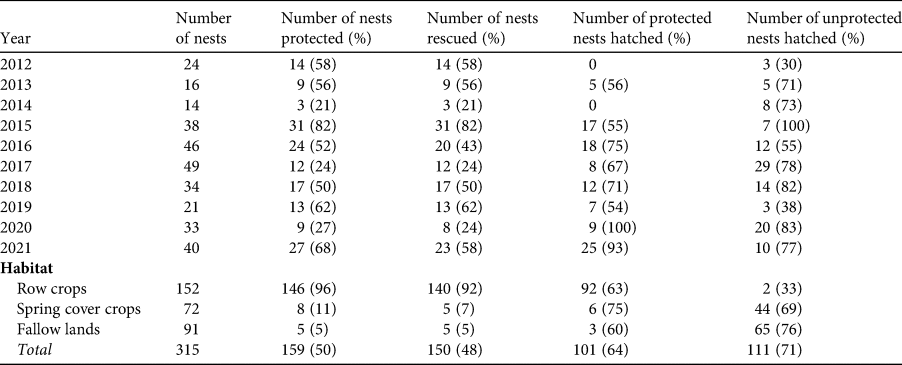
During the study period, we directly protected 159 nests (c. 50% of the total) with a protection zone. The number of protected nests fluctuated across years and habitats, although the largest proportion (92%) of protected nests was in row crops (Table 3). Nest protection was successful, as 64% of protected nests produced at least one chick (n = 101; Table 3). The number of nests we rescued via direct protection measures was 150 (Table 3).
Table 3 Nest protection activities for collared pratincoles in Hungary in relation to year and habitat type (n = 315 nests). Nests rescued refers to nests that would have been destroyed by agricultural machinery without direct protection.
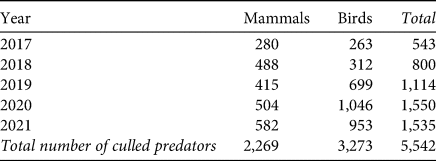
Hunters culled a mean of 454 mammals and 655 birds annually in the hunting district that overlapped with our study site (i.e. 1.7 mammals and 2.5 birds per 100 ha; Table 4). Daily nest survival was not predicted by the number of culled avian (linear regression, b = 0.0001, n = 5, P = 0.655) or mammalian predators (linear regression, b = 0.0005, n = 5, P = 0.503).
Table 4 Number of predators culled during 2017–2022 in the c. 26,000-ha hunting district that overlaps with the collared pratincole nest sites.
Discussion
Here we show that the nesting success of a ground-nesting bird is predicted by the agricultural habitat type of its nest as well as breeding density and timing of breeding. We increased daily nest survival of pratincoles over the study period through conservation measures, so that the majority of nests now hatch successfully.
Collared pratincoles that breed on fallow lands and in spring cover crops had significantly higher nesting success than those nesting in row crops. Nesting success for other shorebirds is also influenced by the timing and intensity of agricultural operations. For example, nest losses of northern lapwings Vanellus vanellus depend on the timing of spring tillage during the nesting period (independently of crop type; Sheldon et al., Reference Sheldon, Chaney and Tyler2007). We observed that predation pressure was lower in extensively used habitats compared with intensively treated areas. Similar patterns have been documented in black-tailed godwits Limosa limosa (Kentie et al., Reference Kentie, Both, Hooijmeijer and Piersma2015) and other ground-nesting species (Berg et al., Reference Berg, Lindberg and Källebrink1992). It is probable that the rise of modern intensive agriculture has favoured generalist predators (Pescador & Peris, Reference Pescador and Peris2001). In addition, rainfall was more likely to flood nests in fields of row crops, as the heavy agricultural machines used for these crops compact the soil and thus water drains more slowly in these habitats. The frequency of heavy rains appears to have declined during the study period, which could also have contributed to the higher nest survival we observed over these years.
Collared pratincoles breed in colonies of various sizes, and thus we expected inter-nest distance to be an important predictor of nesting success. A similar pattern was observed by Berg (Reference Berg1992), who found that predation risk was negatively correlated with the number of neighbours within a breeding colony of northern lapwings. Nest predation was also negatively correlated with nest density in other wader species (Macdonald & Bolton, Reference MacDonald and Bolton2008). In another colonial shorebird, the pied avocet Recurvirostra avosetta, nesting success was highest at intermediate densities (Hötker, Reference Hötker2000), suggesting that density might have a non-linear effect on breeding success in certain cases.
Several shorebird species are declining in their native breeding habitats across Europe, having been forced to choose riskier breeding sites as their preferred habitats are converted into agriculture (Berg, Reference Berg1992; Schifferli et al., Reference Schifferli, Spaar and Koller2006; Kentie et al., Reference Kentie, Both, Hooijmeijer and Piersma2015). For the collared pratincole and similar species such as the Eurasian curlew Numenius arquata, nesting success tends to be higher in their natural habitats than in agricultural ones (Berg, Reference Berg1992, Calvo, Reference Calvo1994; Vincent-Martin, Reference Vincent-Martin2007). In our study area, pratincoles only occupied agricultural habitats, which allowed us to compare the impacts of various types of human-made habitats on nesting success. However, we have no data on nest survival in the native grasslands of this species because these are now rare. In other studies that did include grasslands, higher nesting success was reported in these natural habitats (Calvo, Reference Calvo1994; El Malki et al., Reference El Malki, Hanane, Joulami and El Hamoumi2013).
Most fields in our study site were managed in different phases during the breeding season; therefore, the peak hatching dates occurred at different times. Nesting strategies were highly dependent on the local agricultural schedule because row crops and spring cover crops were sown first, followed by the ploughing of fallow lands. In row crops and spring cover crops, vegetation grows particularly uniformly and rapidly, reducing the time period during which the pratincoles can nest successfully. By contrast, vegetation on fallow lands grows heterogeneously in mosaic patches, creating more suitable nesting conditions. The highest number of nests was in row crops, which was the most common type of agricultural breeding habitat available for collared pratincoles and other shorebirds at our site.
The clutch size of pratincoles is similar in Hungary, Spain (Bertolero & Martinez Vilalta, Reference Bertolero and Marinez Vilalta1999) and France (Vincent-Martin, Reference Vincent-Martin2007) and higher than that in Ukraine and Morocco (Pozhidaeva & Molodan, Reference Pozhidaeva and Molodan1992; El Malki et al., Reference El Malki, Hanane, Joulami and El Hamoumi2013). However, the number of successfully hatched chicks was lower than in Ukraine (Pozhidaeva & Molodan, Reference Pozhidaeva and Molodan1992) and in Algeria (Bensaci et al., Reference Bensaci, Boutera, Cherief, Saheb, Moali and Houhamdi2014).
Conservation actions
Nesting success was different between the three major breeding habitats, with the results suggesting that the most productive habitats were spring crops and fallow fields. This difference persisted even though conservation measures focused on row crops, which increased the nesting success for this habitat. Separating the impact of conservation actions from that of the nesting habitats themselves would be difficult, as the majority of nest protection measures focused only on row crops. The finding that neither daily nest survival nor total nest survival was different between habitats might seem counterintuitive, although the analyses that we carried out at year level (rather than at the individual nest level as in the other analyses) would have lower statistical power. Nevertheless, we only had accurate data until the chicks hatched, so we can only draw conclusions regarding the effects of the habitats from the incubation period. Direct nest protection interventions increased nest survival similarly to other studies, such as the wood turtle Glyptemys insculpta conservation project that found nesting success can be increased by designing appropriate interventions (Bougie et al., Reference Bougie, Byer, Lapin, Peery, Woodford and Pauli2020). In the absence of direct nest protection, breeding success was probably low in critical habitats, similar to that described by Calvo (Reference Calvo1994), who found that as a result of changing agricultural practices, nesting success also improved. Nesting success and the daily survival rate of nests have increased significantly over the past decade probably because of conservation actions.
Although the number of culled predators did not predict nest survival, we believe this is because of the relatively crude nature of the data (i.e. hunting bag data) and small number of sample years. Predator control is likely to be important for the long-term survival of ground-nesting birds (Neuman et al., Reference Neuman, Page, Stenzel, Warriner and Warriner2004; Bolton et al., Reference Bolton, Tyler, Smith and Bamford2007). We are currently designing a project to investigate the effects of predator control on the nesting success of pratincoles. In addition, there are various other natural and human-induced factors that could influence the nest survival of ground-nesting birds, and we need more precise data on the effects of culling on the density of the most common nest predators.
Compared with unmarked nests, we did not find increased predation rates in nests marked with poles, similar to that observed by Zámeĉník et al. (Reference Zámeĉník, Kubelka and Šálek2018), perhaps because the poles were left near nests for only short time periods. Local farmers were supportive of this nest protection, and so none of the known nests were at serious risk during agricultural work. However, the collared pratincole conservation project should be further improved through the establishment of fields and fallow lands that are free from agricultural disturbance. As a result of the current agricultural scheme, agricultural land in Hungary and elsewhere in Europe tends to be used intensively, so arable fields are typically producing crops for most of the year (Tarjuelo et al., Reference Tarjuelo, Margalida and Mougeot2020).
Hortobágy National Park is involved in a species-focused recovery programme that includes improving alkaline grasslands to attract pratincoles, although the habitat restoration seems to have been unsuccessful so far (Kovács & Kapocsi, Reference Kovács, Kapocsi and Ecsedi2004). Based on the results presented here, we believe that joint actions by conservationists and farmers are key (Kiss et al., Reference Kiss, Molnár, Monoki, Kapocsi, Kovács and Aradi2018). Maintaining good relationships between farmers and conservationists is essential to achieving success in conservation projects (Logsdon et al., Reference Logsdon, Kalcic, Trybula, Chaubey and Frankenberger2015; Homberger et al., Reference Homberger, Duplain, Jenny and Jenni2017). Direct nest protection activities had to be implemented mostly in row crops because these types of agricultural land (especially sunflower and corn fields) are cultivated intensively during the breeding season. By contrast, in spring cover crops and fallow lands (with a few exceptions), we observed no disturbance by agricultural machinery after ploughing or sowing.
On a global scale, human activity can negatively influence the behaviour, productivity and nest survival of ground-nesting birds in various habitats, especially on farmland (Fahrig, Reference Fahrig1997; Donald et al., Reference Donald, Green and Heath2001; Colwell, Reference Colwell2010; Ward et al., Reference Ward, Semel and Herkert2010). We have set ourselves the goal of habitat development at the local level, as a result of which 50–100 ha of fallow lands are created every year to facilitate the settlement of shorebirds on the Nagykunság rice systems. These empty fields are created through disc ploughing from the middle to the end of April, and after the treatment there is no human disturbance during the breeding season. These areas are small in relation to the size of the total habitat, but this seems to be a promising project as increasing numbers of birds have nested and gathered in these fallow areas in recent years. Improvements could be supported through the development of targeted agricultural programmes, which would set management standards specifically for the arable lands used by the species and financially support the conservation efforts of farmers.
Our results suggest that direct conservation activities can achieve desired outcomes even in intensively farmed agricultural habitats. Without such interventions, a large proportion of farmland bird nests could be destroyed by agricultural machinery. We believe that the Eurasian populations of collared pratincoles are threatened considerably by anthropogenic pressures: for instance, even in their native breeding habitats the pratincoles are subject to adverse effects from climate change, pollution and an unnaturally high density of mesopredators. In addition to effective direct nest protection, it will be important to increase the proportion of safe fallow lands in the future as a specific agri-environmental protection measure, so that as many farmland birds as possible have the opportunity to choose this undisturbed agricultural habitat for breeding. As collared pratincoles nest in several places in artificial habitats across Europe, mainly close to wetlands such as rice fields, breeding habitats should be protected or restored more widely to maintain biodiversity in agricultural landscapes.
Acknowledgements
We thank Antal Széll, Miklós Lóránt and other national park rangers who participated in the conservation management of the species and data collection in Hungary; Fanni Takács, who supported our field data collection; William Jones and Tamás Székely Jr for their linguistic corrections; the farmers of Kisújszállás and Karcag, especially the workers of Nagykun 2000, Hubai és Társa and Indián Rizs Agro, Inc., who supported the protection of the species in Hungary through their patience, positive attitude and help with field site management; and the hunters association for providing data on predator control. VK and TSz were supported by ÉLVONAL-KKP 126949 of the Hungarian government. TSz was also funded by the Eötvös Lóránd Research Network (Grant no. 1102207) and VK was also supported by Junior Star GAČR project (31-2307692M_Kubelka). We thank Project TetraClim for the use of ELKH Cloud for our analyses.
Author contributions
Study design and fieldwork: ÁK, ÁM, IK, SzG, TSz; data analysis and writing: all authors.
Conflict of interest
None.
Ethical standards
This research abided by the Oryx guidelines on ethical standards.
Data availability
The dataset is available in the Dryad data repository at doi.org/10.5061/dryad.mgqnk9959.










