Introduction
In the tropics South-east Asia has the largest proportion of endemic mammals and is also the region where mammals face the greatest threat of extinction (Sodhi et al., Reference Sodhi, Posa, Lee, Bickford, Koh and Brook2009). The scientific community has reached a consensus that the region is facing a biodiversity crisis and therefore requires strategic conservation planning and implementation of conservation actions (Sodhi et al., Reference Sodhi, Koh, Brook and Ng2004; Koh & Sodhi, Reference Koh and Sodhi2010; Duckworth et al., Reference Duckworth, Batters, Belant, Bennett, Brunner and Burton2012). Estimating animal population abundance using surveys can have several implications for addressing biodiversity crises (Ogutu et al., Reference Ogutu, Bhola, Piepho and Reid2006; Hassel-Finnegan et al., Reference Hassel-Finnegan, Borries, Larney, Umponjan and Koenig2008). Identifying important populations is a key step in setting conservation priorities and is necessary for monitoring population status over time (Plumptre & Cox, Reference Plumptre and Cox2006). The lack of resources devoted to the conservation of lesser known yet highly threatened species means that the conservation status assigned to many taxa is based on single short-term surveys or has not been verified.
The red-shanked douc Pygathrix nemaeus, categorized as Endangered on the IUCN Red List (IUCN, 2012), is an Asian colobine monkey that belongs to a monophyletic group, along with two other douc species (Pygathrix nigipes and Pygathrix cinerea). All three are endemic to Lao People's Democratic Republic (PDR), Cambodia and Vietnam (occurring in one, two or all three of these countries, depending on the species). P. nemaeus occurs in Vietnam and Lao PDR and perhaps Cambodia (Rawson & Roos, Reference Rawson and Roos2008; Coudrat et al., Reference Coudrat, Duckworth and Timmins2012). With the remaining populations in Vietnam estimated at no more than 30 individuals (Bach Ma National Park; Nadler, Reference Nadler, Nadler, Rawson and Thinh2010) to c. 2,000 individuals (Phong Nha-Ke Bang National Park; Haus et al., Reference Haus, Vogt, Forster, Vu and Ziegler2009), and with constant hunting pressure on douc species for traditional medicine, local consumption and international trade, the security of the species in Vietnam is uncertain. The largest population of P. nemaeus is known to occur in Lao PDR, in particular in the central-eastern part of the country, in the foothills of the Annamite mountain range and some adjacent lowland areas (Timmins & Duckworth, Reference Timmins and Duckworth1999; Coudrat et al., Reference Coudrat, Duckworth and Timmins2012). However, there is no population estimate for the species, which is increasingly threatened by hunting pressure in these remote, relatively large and dense forests (Coudrat et al., Reference Coudrat, Duckworth and Timmins2012). Baseline density estimates for P. nemaeus in the region will facilitate long-term monitoring and assessment of conservation success.
To provide baseline data for the conservation of this species we conducted line-transect surveys over one year during 2011–2012 in Nakai–Nam Theun National Protected Area. We combined species distribution modelling and distance sampling (Buckland et al., Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001) to estimate the density and population abundance of P. nemaeus, and based on our results we suggest a conservation action plan for the world's largest remaining population of red-shanked douc.
Study area
Nakai–Nam Theun National Protected Area (c. 3,500 km2) is located in central-eastern Lao PDR, in the Annamite mountain range (Fig. 1). The Protected Area remains largely forested, with c. 80% forest cover (Robichaud et al., Reference Robichaud, Sinclair, Odarkor-Lanquaye and Klinkenberg2009) and a variety of habitat types, including mixed semi-evergreen/coniferous, upper montane, dry evergreen and wet evergreen forests (Timmins & Evans, Reference Timmins and Evans1996). Elevation in the Protected Area is 500–2,300 m. Annual precipitation is 1,865–2,620 mm and annual mean temperature is 14–24 °C, with extremes of 4–32 °C. Five main rivers cross Nakai–Nam Theun. There are land delimitation zones around the 31 enclave villages for local use of forest products, under the Wildlife and Aquatic Law and Forestry Law (National Assembly Lao PDR, 2007a,b).

Fig. 1 Nakai–Nam Theun National Protected Area, where we carried out transect surveys at 10 sites during 2011–2012. Habitat suitability for the red-shanked douc Pygathrix nemaeus was modelled using MAXENT. Continuous logistic suitability was reclassified to obtain the binary map, under the minimum training presence threshold, resulting in a suitable habitat of 1,578 km2. The rectangle on the inset shows the location of the main map in LAO PDR.
The heterogeneity of habitats makes the wildlife community in Nakai–Nam Theun one of the most diverse in the region, with numerous globally threatened species, including recently discovered large mammals, > 430 species of birds, nine species of primates and key carnivore species (Timmins & Evans, Reference Timmins and Evans1996). This makes the area a priority for wildlife conservation in Lao PDR and the Indo–Burma region (Robichaud et al., Reference Robichaud, Marxh, Southammakoth and Khounthikoummane2001; Tordoff et al., Reference Tordoff, Baltzer, Fellowes, Pilgrim and Langhammer2012).
Methods
Data collection
In 2011–2012 we visited 10 sites across the Protected Area (Fig. 1). We chose these study sites on the basis of their relative accessibility (2–4-days to reach camp). We pre-set transects arbitrarily, without seeking more accessible terrain, on a 1:50,000 topographic map, with a total of 81 transects across the Protected Area. For logistical reasons we set transects approximately perpendicular to a watercourse, and transects were 1–2 km long, 400–500 m apart and parallel to each other. We kept the pre-set bearing for each transect, using a compass, except when we had to go around obstacles (e.g. rivers with no crossing point, large fallen tree trunks). At each study site we walked 4–20 transects, some of which were replicated two or three times, resulting in a total of 176 transects and 310 km walked (Table 1). The variation in the number of transects and replications between sites was a result of adjustment of the methodology following the first field trip, time constraints and poor weather conditions. We followed distance sampling line-transect methodology (Buckland et al., Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001, Reference Buckland, Plumptre, Thomas and Rexstad2010): for each group sighting of P. nemaeus we recorded the perpendicular distance from animal to transect, using a laser range-finder (sometimes calculated from sin[animal-transect angle]*animal-to-observer distance). We also recorded the coordinates with a geographical positioning system (GPS), time and, whenever possible, an estimate of group size. Dense canopy, movement of the animals (often involving group splitting) and group spread prevented an exact assessment of the centre of a group (Marshall et al., Reference Marshall, Lovett and White2008) and therefore we always attempted to record distance to an animal at the centre of all other visible individuals. We moved slowly along transects, given the rugged terrain (walks were of 1.5–5.5 hours duration). We occasionally stopped to rest but attempted to limit time spent at each resting location. A group of 2–4 people walked each transect.
Table 1 Study sites visited in Nakai–Nam Theun National Protected Area (Fig. 1) during 2011–2012, with the area covered, the total number of transects, the total survey effort, and the number of observations at each site.
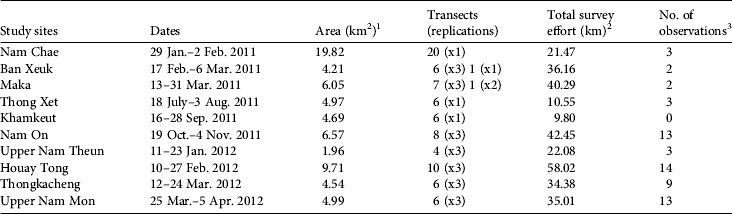
1 The size of the study site was estimated using the maximum convex polygon around the transects
2 Total survey effort includes replications
3 Seven observations that may have been of a group previously counted on the same transect walk were discarded from the analysis (n = 55; N = 62)
Species distribution modelling
We used the maximum entropy general-purpose machine learning method, which has been adapted for species distribution modelling (MAXENT v. 3.3.3k; Phillips et al., Reference Phillips, Dudík and Schapire2004, Reference Phillips, Anderson and Schapire2006). The method combines biological data of species occurrence (presence-only data, e.g. GPS coordinates) with environmental characteristics to estimate a probability distribution of maximum entropy (i.e. closest to uniform), subject to the set of constraints provided (i.e. environmental characteristics where the species occurs; Phillips et al., Reference Phillips, Anderson and Schapire2006). We used a ten-fold cross-validation replication (Kohavi, Reference Kohavi1995), using MAXENT's default parameters (Phillips & Dudik, Reference Phillips and Dudik2008).
We included 25 variables in the model: 19 bioclimatic layers (Hijmans et al., Reference Hijmans, Cameron, Parra, Jones and Jarvis2005), elevation, land cover (from 2002, with 14 categories), percentage of forest cover, slope, and distance from water and from villages. To avoid model over-fitting (Veloz, Reference Veloz2009; Merckx et al., Reference Merckx, Steyaert, Vanreusel, Vincx and Vanaverbeke2011; Dormann et al., Reference Dormann, Elith, Bacher, Buchmann, Carl and Carré2012), occurrence data were corrected for spatial autocorrelation by selecting one locality per 2 km2, resulting in 36 occurrence points for the model (n = 82, including opportunistic sightings beyond transects). Outputs were analysed using ArcGIS v. 9.3 (ESRI, Redlands, USA). We assessed the model's predictive power using the area under the curve of the receiver-operating characteristic and the Boyce Index (Boyce et al., Reference Boyce, Vernier, Nielsen and Schmiegelow2002; Hirzel et al., Reference Hirzel, Le Lay, Helfer, Randin and Guisan2006). To estimate the distribution range of P. nemaeus in Nakai–Nam Theun Protected Area we created a binary map, using the minimum training presence threshold.
Distance sampling
We used DISTANCE 6.0 (Thomas et al., Reference Thomas, Buckland, Rexstad, Laake, Strindberg and Hedley2010) to analyse survey data, using the conventional distance-sampling engine. We pooled all data collected during the study (Table 1). We removed from the analysis the observations that may have been double counts of the same group on the same transect (of a total of 62 observations). As we could not count group sizes because of poor visibility and fleeing behaviour, we modelled group density rather than individual density and estimated population density post hoc based on the mean group size calculated. Transects that were replicated were analysed together as single lines and their survey effort corresponded to the transect length multiplied by the number of replications (Buckland et al., Reference Buckland, Plumptre, Thomas and Rexstad2010; Table 1).
We followed the line transect analysis steps of Buckland et al. (2001: 135). We plotted our data (perpendicular distances) in a frequency histogram of 10 m intervals to select five interval cut-off points (0, 20, 40, 60, 80 and 104 m, corresponding to the largest value recorded) and created a grouped data set. We then ran six models with different combinations of key function and adjustment terms: (1) half-normal + cosine, (2) half-normal + simple polynomial, (3) half-normal + Hermite polynomial, (4) uniform + cosine, (5) uniform + simple polynomial, and (6) uniform + Hermite. We selected the best model according to the Akaike information criterion (AIC), the χ2 goodness-of-fit statistical test, and the coefficient of variation of the density estimates (Buckland et al., Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001).
Results
Our predicted model yielded an area under the curve of 0.789. The Boyce Index validation method, with 100 classes, indicated a significant (P < 0.01) predictive power (r = 0.511). The resulting predicted distribution range (under the minimum presence training threshold) was 1,578 km2, 44.4% of the total area (Fig. 1).
The model with the half-normal key function and cosine adjustment fitted our data the best and resulted in a mean density of 2.8 groups per km2 (Table 2). Using this group density and the predicted area of suitable habitat we estimated there are c. 4,420 groups in Nakai–Nam Theun National Protected Area (Table 3).
Table 2 Distance analysis results for different models, calculated with 55 observations and data grouping with five intervals (cut-off points: 0, 20, 40, 60, 80, 104 m), with density estimate, coefficient of variation, probability of detection, ΔAIC, AIC, and χ2 goodness-of-fit P value.
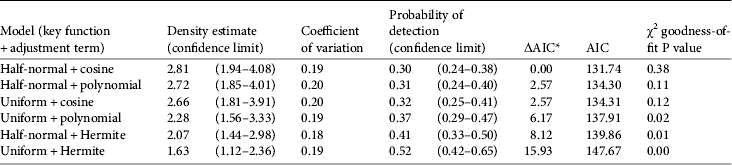
* Delta AIC = model AIC−lowest AIC of all models
Table 3 Abundance estimates calculated by the MAXENT model for various parameters, based on estimated density and range size in Nakai–Nam Theun National Protected Area.

1 Mean of the encounter rates for each transect walk; ∑i n=176 [number of sightings/transect walki length]/total number of walks n, calculated for the total number of observations (62)
2 Under the minimum presence training threshold
Discussion
Survey limitations
Our analysis yielded a density of 2.8 groups of P. nemaeus per km2 and 4,418 groups in Nakai–Nam Theun National Protected Area, with our distribution model of an estimated c. 1,600 km2 of suitable habitat. Our habitat suitability model may be underestimated as a result of survey bias (Phillips et al., Reference Phillips, Dudík, Elith, Graham, Lehmann, Leathwick and Ferrier2009), mainly in the central regions; it should therefore be considered as a conservative estimate. Given their suspected multi-level social organization, involving regular travel of more than one group together, group size can vary widely (four to c. 30 in our study, excluding solitary animals; Hoang, Reference Hoang2007; Rawson, Reference Rawson2009). Distance sampling has been used for several species of primates but it is difficult to assess reliability because it is rarely possible to assess true density. Using this method for primates can also be challenging because of their behaviour and the difficulty of obtaining sufficiently large and random sample sizes in rainforest habitats, often leading to violation of the technique's assumptions (Buckland et al., Reference Buckland, Plumptre, Thomas and Rexstad2010). Our results may therefore not represent the true density of the species in the area. However, our study included a large number of transects and replications across the area, resulting in sufficient observations for the models.
Other assumptions could also have been violated: (1) Poor visibility as a result of forest density meant that we were sometimes unable to ascertain the distance to the group centre. By instead estimating the distance to the first animal seen we may have overestimated the density (Marshall et al., Reference Marshall, Lovett and White2008; Buckland et al., Reference Buckland, Plumptre, Thomas and Rexstad2010). (2) Some groups may have remained undetected because of disturbance from transect-cutting on the first walk, occasional noisy movement through denser habitat (e.g. bamboo) or poor weather conditions in some areas (e.g. the Khamkeut site). Our detection probability was low (30%). However, a commonly observed behavioural response of doucs to threat involves at least some individuals remaining hidden in trees, which may have influenced the probability of detection. (3) Most groups were detected while fleeing, which may have affected the density estimate (Marshall et al., Reference Marshall, Lovett and White2008) but did not prevent us from identifying species. We were able to identify all 126 primate groups detected (including groups and solitary animals; 12 gibbons, 52 macaques, 62 doucs) at least to genus, doucs being the easiest to identify by their striking colour, locomotion and calls. (4) Some groups may have been counted twice along the same transect. We compensated for this by discarding all sightings that we suspected may have been counted twice (as assessed from the direction of group flight from previous sighting). Overall, the limitations of our study may be balanced between overestimation and underestimation of the density. Although alternative survey designs have been suggested to overcome the difficulties involved in surveying primates (Buckland et al., Reference Buckland, Plumptre, Thomas and Rexstad2010), they remain a challenge to implement in practice and it is likely that most estimates of primate density involve violations of some of the assumptions. Estimated densities can still be indicative of relative abundance and can be compared between studies when survey biases are similar. Hence it is essential to assess potential violations of assumptions in all distance sampling studies. Distance sampling remains the best method available to estimate density of colobines.
Conservation of P. nemaeus in Nakai–Nam Theun National Protected Area
Our density estimate for P. nemaeus in Nakai–Nam Theun National Protected Area falls within the range of that of other colobines elsewhere in South-east Asia (Table 4). Detection frequencies from surveyed areas can also be indicative of the relative abundance of a species in an area, which is in general proportionally equivalent to group density estimates (Table 4).
Table 4 Comparison of estimates of colobine density in South-east Asia, with species, location, overall detection frequency, group density, and source. Note that methodologies differ between studies, which can lead to violation of some of the assumptions of the distance sampling method (refer to the specific studies for details of methodologies).
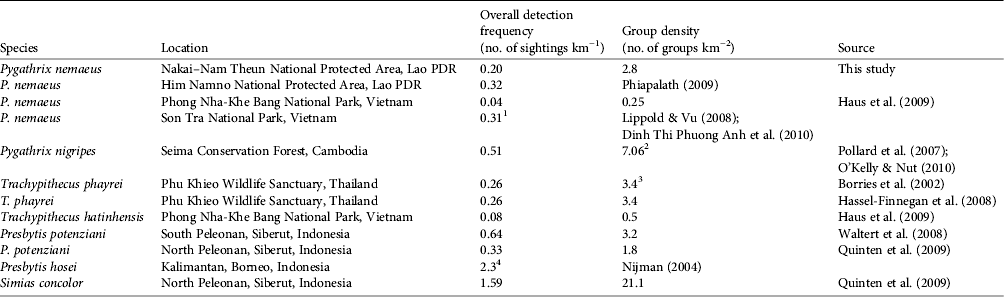
1 Inferred from 13 groups encountered over a total forested area of 41.9 km2
2 Mean group density for annual estimates from 2005 to 2010
3 Calculated with strip transect method (NRC, 1981)
4 Result for primary hill forest survey, estimated using effective distance method (Whitesides et al., Reference Whitesides, Oates, Green and Kluberdanz1988)
The conservative estimate of suitable habitat for the species in Nakai–Nam Theun is c. 1,600 km2, which is probably larger than areas of suitable habitat where the species occurs in Vietnam and Cambodia. In Vietnam the largest remaining populations of P. nemaeus are relatively small (Table 4) as a result of continuous deforestation and high hunting pressure (Lippold & Vu, Reference Lippold and Vu2008; Blair et al., Reference Blair, Sterling and Hurley2011; Coudrat et al., Reference Coudrat, Duckworth and Timmins2012). The species is already locally extinct in several areas in Vietnam. The population in the central region of the limestone-dominated Phong Nha-Ke Bang National Park may remain naturally protected because poor accessibility prevents overhunting. However, the population of Son Tra National Reserve is located in an increasingly human-dominated landscape and the species will only be maintained there by ongoing species-focused conservation projects (Lippold & Vu, Reference Lippold and Vu2008; Dinh Thi Phuong Anh et al., Reference Dinh Thi, Nguyen Dinh, Huynh Thi, Nadler, Rawson and Thinh2010; Ulibarri & Streicher, Reference Ulibarri and Streicher2012). If no action is taken the same situation is likely to occur in Lao PDR, at least in the most accessible areas, as the value of the species in international trade increases as it becomes increasingly rare and the human population grows, putting more pressure on the species and its natural habitat.
In Seima Biodiversity Conservation Area, in eastern Cambodia, a population of the closely related black-shanked douc Pygathrix nigripes has an estimated density of c. 7 groups per km2 (Pollard et al., Reference Pollard, Clements, Nut, Ko and Rawson2007; O'Kelly & Nut, Reference O'Kelly and Nut2010). This population is the largest in the world for this species and is considered to be secure as a result of long-term conservation efforts in the area and low hunting pressure (WCS, 2009; O'Kelly & Nut, Reference O'Kelly and Nut2010). The discovery of this large population was covered in the media, which helped promote the importance of the area for wildlife conservation. In 2009 a core area within the Conservation Area was designated as the Seima Conservation Forest by the Cambodian government, and a relatively successful conservation project was implemented (Evans et al., Reference Evans, O'Kelly, Men, Nut, Pet, Pheakdey, Pollard, Sunderland, Sayer and Hoang2012). This example demonstrates the importance of communicating, both nationally and internationally, the findings from such studies. A similar case could be made for Nakai–Nam Theun National Protected Area.
The population of P. nemaeus in Lao PDR is the world's largest and offers the best hope for the species’ conservation (Coudrat et al., Reference Coudrat, Duckworth and Timmins2012). However, it is far from secure because of the lack of or failure of management strategies. Wildlife in Nakai–Nam Theun National Protected Area has been under increasing hunting pressure from local and Vietnamese hunters, both for local consumption and the lucrative international trade (Nooren & Claridge, Reference Nooren and Claridge2001; Robichaud et al., Reference Robichaud, Sinclair, Odarkor-Lanquaye and Klinkenberg2009). As a result of the demand from Vietnam and China for colobine bones for use in traditional medicine (Nooren & Claridge, Reference Nooren and Claridge2001), douc populations are decreasing in Vietnam and the threat to Lao PDR populations (especially near the border with Vietnam) is likely to increase. Doucs are often traded in Lao PDR (Davidson et al., Reference Davidson, Robichaud, Tizard, Vongkhamheng and Wolstencroft1997; Nooren & Claridge (Reference Nooren and Claridge2001); Phiapalath, Reference Phiapalath2009; Coudrat et al., Reference Coudrat, Duckworth and Timmins2012).
The Asian Species Action Partnership established to tackle the ongoing South-east Asian species extinction crisis highlights the importance of effective site-based species-focused projects (Duckworth et al., Reference Duckworth, Batters, Belant, Bennett, Brunner and Burton2012). There is currently no species-specific conservation of P. nemaeus in Lao PDR but a long-term conservation project is planned for Nakai–Nam Theun National Protected Area (Coudrat, Reference Coudrat2012). This will be the country's first such research project for this little-known species and will apply best practices to secure its conservation (Sunderland et al., Reference Sunderland, Sayer and Hoang2012). The project has the potential to ensure the survival of the red-shanked douc in Lao PDR as well as other threatened and unique species of the Annamite mountain range.
Acknowledgements
Permission to undertake the field survey in Nakai–Nam Theun National Protected Area was granted by the Department of Forestry of the Ministry of Agriculture and Forestry of Lao PDR. The NT2–WMPA provided support throughout CNZC's PhD research in Nakai–Nam Theun; we thank Phouthone Sophathilath, Thong-Eth Phayvanh and Soukhatha Vannalath. We thank the local people who assisted during data collection; Will Duckworth, Bill Robichaud, Latsamay Sylavong and Phaivanh Phiapalath, who provided advice and support; and Prof. Bounthob and Onvilay Souliya of The National University of Laos, who provided logistical support. CNZC's PhD research was funded by the Mohamed Bin Zayed Species Conservation Fund, International Primatological Society, American Society of Primatologists, Primate Society of Great Britain, Primate Action Fund (through Conservation International), Primate Conservation Inc., and Idea Wild. We thank the reviewers for their comments.
Biographical sketches
Camille N. Z. Coudrat has conducted field work in Cambodia and Lao PDR since 2009. Her main research focus is the conservation and ecology of primates and other wildlife, and conservation education. She is continuing her research in Lao PDR, focusing on the conservation and behavioural ecology of the red-shanked douc and the white-cheeked gibbon in Nakai–Nam Theun National Protected Area. Chanthalaphone Nanthavong was responsible for wildlife monitoring and patrolling at the Nam Theun 2 Watershed Management and Protection Authority during 2006–2012. His research is currently focused on the ecology of the southern white-cheeked gibbon Nomascus siki in Nakai–Nam Theun National Protected Area. K. Anna I. Nekaris has studied Asian mammals in the wild and in captivity for more than 15 years. She has conducted field studies of all currently recognized taxa of slow and slender lorises and has initiated conservation awareness and capacity-building projects in numerous loris range countries. She is the director of the Little Fireface Project and the Nocturnal Primate Research Group.






