Introduction
Populations of three species of Gyps vultures endemic to South Asia fell by more than 97% between the early 1990s and 2007 (Prakash et al., Reference Prakash, Pain, Cunningham, Donald, Prakash and Verma2003, Reference Prakash, Green, Pain, Ranade, Saravanan and Prakash2007, Reference Prakash, Bishwakarma, Chaudhary, Cuthbert, Dave and Kulkarni2012), leading to their being categorized as Critically Endangered in the IUCN Red List (BirdLife International, 2014). Research has established that veterinary use of diclofenac, a non-steroidal anti-inflammatory drug (NSAID), was the principal cause of this collapse in vulture numbers (Oaks et al., Reference Oaks, Gilbert, Virani, Watson, Meteyer and Rideout2004; Green et al., Reference Green, Newton, Shultz, Cunningham, Gilbert, Pain and Prakash2004, Reference Green, Taggart, Senacha, Raghavan, Pain, Jhala and Cuthbert2007; Shultz et al., Reference Shultz, Baral, Charman, Cunningham, Das and Ghalsasi2004). Diclofenac is nephrotoxic at low doses to all species of Gyps vultures tested (Oaks et al., Reference Oaks, Gilbert, Virani, Watson, Meteyer and Rideout2004; Swan et al., Reference Swan, Cuthbert, Quevedo, Green, Pain and Bartels2006a). Residues of the drug were found in carcasses of domesticated ungulates available to vultures in India (Green et al., Reference Green, Taggart, Senacha, Raghavan, Pain, Jhala and Cuthbert2007; Taggart et al., Reference Taggart, Senacha, Green, Jhala, Raghavan and Rahmani2007, Reference Taggart, Senacha, Green, Cuthbert, Jhala and Meharg2009) and vultures were exposed when they consumed carcasses of ungulates treated shortly before death (Oaks et al., Reference Oaks, Gilbert, Virani, Watson, Meteyer and Rideout2004; Green et al., Reference Green, Taggart, Das, Pain, Sashi Kumar, Cunningham and Cuthbert2006). Post-mortem examination of Gyps vultures killed by diclofenac poisoning in experiments showed extensive visceral gout and necrosis of kidney tissues similar to that seen in a high proportion of vultures found dead in the wild (Oaks et al., Reference Oaks, Gilbert, Virani, Watson, Meteyer and Rideout2004; Shultz et al., Reference Shultz, Baral, Charman, Cunningham, Das and Ghalsasi2004; Meteyer et al., Reference Meteyer, Rideout, Gilbert, Shivaprasad and Oaks2005; Swan et al., Reference Swan, Cuthbert, Quevedo, Green, Pain and Bartels2006a). Bans on the veterinary use of diclofenac have been in force since 2006 in India, Pakistan and Nepal, and since 2010 in Bangladesh. The safety to Gyps vultures of meloxicam, an alternative veterinary NSAID, has been established experimentally (Swan et al., Reference Swan, Naidoo, Cuthbert, Green, Pain and Swarup2006b; Swarup et al., Reference Swarup, Patra, Prakash, Cuthbert, Das and Avari2007). In India the proportion of ungulate carcasses contaminated with diclofenac fell by about half within 4 years of the introduction of the ban (Cuthbert et al., Reference Cuthbert, Taggart, Prakash, Chakraborty, Deori and Galligan2014). In association with this decrease in diclofenac prevalence, the rate of decline of Gyps vulture populations in India, Nepal and Pakistan has slowed (Jamshed et al., Reference Jamshed, Chaudhry, Ogada, Malik, Virani and Giovanni2012; Prakash et al., Reference Prakash, Bishwakarma, Chaudhary, Cuthbert, Dave and Kulkarni2012), as has the decline in two other vulture species, red-headed vulture Sarcogyps calvus and Egyptian vulture Neophron percnopterus, which may also be affected by diclofenac (Galligan et al., Reference Galligan, Amano, Prakash, Kulkarni, Shringarpure and Prakash2014).
Here we report the findings of necropsies of dead vultures collected in India during 2000–2012, which includes periods before and after the diclofenac ban in 2006. We re-evaluate the previously observed perfect association of visceral gout with diclofenac and other NSAIDs by comparing necropsy results with measurements of the concentrations of nine NSAIDs in liver and/or kidney. We evaluate changes over time in the prevalence of visceral gout and NSAID contamination in carcasses of vultures found dead in the wild.
Methods
Collection of vulture carcasses
Carcasses of vultures were collected from July 2000 to April 2012 in nine states of India (Assam, Gujarat, Haryana, Himachal Pradesh, Jharkhand, Madhya Pradesh, Maharashtra, Rajasthan and Uttarakhand), extending from the western to the eastern extremes of the country. We report here only results for specimens for which the year of collection was known and results of necropsy, NSAID assay or both were available. A total of 62 vultures were examined at necropsy for visceral gout (one cinereous vulture Aegypius monachus, one Eurasian griffon vulture Gyps fulvus, three Himalayan griffon vultures Gyps himalayensis, 17 long-billed vultures Gyps indicus and 40 oriental white-backed vultures Gyps bengalensis). Liver and/or kidney samples from 48 vultures were assayed for NSAIDs (one A. monachus, one G. fulvus, three G. himalayensis, 10 G. indicus and 33 G. bengalensis). Carcasses of 47 vultures (1 A. monachus, 1 G. fulvus, 3 G. himalayensis, 10 G. indicus and 32 G. bengalensis) were assessed for both gout and NSAIDs. No NSAID assays were available for seven G. indicus and eight G. bengalensis with necropsy results, and one G. bengalensis with an NSAID assay did not have a necropsy result. Vulture carcasses were collected by staff of the Bombay Natural History Society and volunteers, local conservation NGOs and individuals. Two carcasses of G. bengalensis were obtained as a result of special efforts to collect and treat the large numbers of birds killed and injured by collisions with kite strings during the annual kite-flying festival in the city of Ahmedabad (Gujarat), but the others were found opportunistically, dead or dying in the field. After their population decline became apparent, vultures in India were strictly protected under Schedule I of the Wildlife Protection Act (1972), which requires permits before live or dead specimens can be collected. This restriction influenced both the number of carcasses obtained and the distribution of collection localities. Our study included all 23 vulture carcasses collected in India for which data were reported by Shultz et al. (Reference Shultz, Baral, Charman, Cunningham, Das and Ghalsasi2004).
Necropsies of vulture carcasses and assessment of visceral gout
Detailed necropsy examinations were undertaken by trained veterinarians and field biologists who followed a standard protocol (Cunningham et al., Reference Cunningham, Prakash, Pain, Ghalsasi, Wells and Kolte2003). This included external and internal visual examination for gross abnormalities, and the collection of liver and/or kidney tissues for subsequent NSAID residue analysis. Where possible, morbid tissues fixed in 10% NBF were processed by a conventional technique to obtain 4 μm thick paraffin embedded sections (Luna, Reference Luna1972). The sections stained with routine hematoxylin and eosin stain were examined microscopically for pathological changes associated with nephrotoxicity. De Galantha stain was employed for demonstration of urate crystals. Images of representative changes were documented. Samples of kidney and/or liver were removed and frozen for NSAID assays.
In some cases, when carcasses were found in remote locations or where trained personnel were not available, full necropsies could not be performed and less detailed examinations were conducted in the field. We consider these examinations, together with the detailed post mortems, sufficient for the detection of the presence or absence of visceral gout based upon observation of white crystals of uric acid deposited on the surfaces of organs such as the liver (see Oaks et al., Reference Oaks, Gilbert, Virani, Watson, Meteyer and Rideout2004 and Swan et al., Reference Swan, Cuthbert, Quevedo, Green, Pain and Bartels2006a for illustrations). In reviewing the post-mortem reports, we found two cases of white deposits on internal organs, like those seen in gout, in which this was considered at the time to be possibly due to an unidentified fungal infection in one case and candidiasis (a specific fungal infection) in another. In both cases, fungal infection was not confirmed by microscopy. In the case of the bird with suggested candidiasis, subsequent histopathological examination of the kidney revealed severe gout nephrosis. This suggested that white deposits observed on macroscopic examination had been misidentified as fungal infection. We therefore judged that gout had been mistakenly identified as fungal disease in both cases and reassigned both as having visceral gout.
Measurement of NSAID residues
Samples of frozen liver and kidney (c. 0.5 g) were thawed and weighed (to ±1 mg) into new glass test tubes and homogenized with 2 ml of HPLC grade acetonitrile. This mixture was then centrifuged at 1000 g for 5 minutes and the supernatant filtered using disposable PTFE syringe filter units of 0.45 micron. The filtered extract was stored in LC vials at −20°C until analysis. Samples collected up until June 2004 were analysed for diclofenac only by LC–ESI/MS (liquid chromatography–electrospray ionization mass spectrometry) using an Agilent 1100 LC and 1946D MS, following methods in Taggart et al. (Reference Taggart, Senacha, Green, Jhala, Raghavan and Rahmani2007). The limit of quantification (LOQ) for this technique (back-calculated to wet tissue concentration) was 0.01 mg kg−1. Samples collected after June 2004 were analysed for nine different veterinary NSAIDs (carprofen, diclofenac, flunixin, ibuprofen, indometacin, ketoprofen, meloxicam, naproxen and nimesulide) that were selected based on their potential risk to avian species, presence within pharmacies in India and likelihood of entering the veterinary sector in the region (Taggart et al., Reference Taggart, Senacha, Green, Cuthbert, Jhala and Meharg2009; Cuthbert et al., Reference Cuthbert, Dave, Chakraborty, Kumar, Prakash, Ranade and Prakash2011). They were analysed by LC–ESI/MS in negative ion mode (utilizing a C18 column) following methods described in Taggart et al. (Reference Taggart, Senacha, Green, Cuthbert, Jhala and Meharg2009). LOQ values for these nine NSAIDs were 0.005–0.02 mg kg−1 (see Supplementary Table S1 in Taggart et al., Reference Taggart, Senacha, Green, Cuthbert, Jhala and Meharg2009). The LOQ for diclofenac was the same (0.01 mg kg−1) in the analyses conducted before and after June 2004. Of the 48 carcasses for which diclofenac measurements were made, values were available for both liver and kidney for 37 carcasses, liver only for eight carcasses and kidney only for three carcasses.
Statistical analysis
The statistical significance of associations between the presence of gout and the detection of diclofenac residues in liver and/or kidney was assessed using the Fisher exact test for a 2 × 2 contingency table (Siegel & Castellan, Reference Siegel and Castellan1988). The two-tailed exact probability was calculated for the observed outcome under a null hypothesis of no association.
We estimated trends over time in the prevalence of diclofenac residues in the liver and/or kidney and the prevalence of visceral gout in vultures by logistic regression analysis, (Crawley, Reference Crawley2007) with the presence/absence of diclofenac contamination or the presence/absence of gout as binary dependent variables and calendar year of collection as an independent variable. It was necessary to allow for species and age class in these analyses because previous studies have indicated that the prevalence of both gout and diclofenac contamination of Gyps vultures in the Indian subcontinent vary with those variables. Both prevalences tend to be higher in G. bengalensis than in G. indicus (Green et al., Reference Green, Newton, Shultz, Cunningham, Gilbert, Pain and Prakash2004; Shultz et al., Reference Shultz, Baral, Charman, Cunningham, Das and Ghalsasi2004) and higher in adults than immatures (Gilbert et al., Reference Gilbert, Virani, Watson, Oaks, Benson and Khan2002; Green et al., Reference Green, Newton, Shultz, Cunningham, Gilbert, Pain and Prakash2004). For example, in a large sample of G. bengalensis found dead in Pakistan, the prevalence of visceral gout increased progressively with age, being 13% in nestlings, 19% in fledglings, 63% in subadults and 80% in adults (Gilbert et al., Reference Gilbert, Virani, Watson, Oaks, Benson and Khan2002).
The proportions of carcasses of different Gyps species changed over time. For example, after 2004 a larger proportion of the carcasses examined were of G. bengalensis rather than G. indicus (Table 1). Given that the prevalence of gout and diclofenac tended to be higher in G. bengalensis than in G. indicus when the species are compared during the same period (see above), ignoring the change over time in species composition in the regression analysis would bias the estimated trend to be more positive (less negative) than it should be.
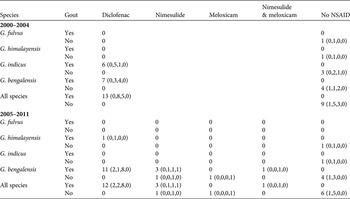
Table 1 Co-occurrence of visceral gout and residues of NSAIDs in carcasses of wild Gyps vultures collected in India during 2000–2011. Results are shown separately for a period when assays were only performed for diclofenac (2000–2004) and a later period (2005–2011) when carprofen, flunixin, ibuprofen, indometacin, ketoprofen, meloxicam, naproxen and nimesulide were also assayed. For these additional drugs, only residues of nimesulide and meloxicam were detected. Numbers of nestlings, immatures, adults and birds of unknown age, respectively, are shown in brackets.
The proportion of adult vultures in the sample examined was slightly higher after 2004 than before (Table 1), and this would tend to bias the trend in the opposite direction. To allow for these differences we fitted multiple logistic regression models with the main effects of species (G. himalayensis, G. indicus and G. bengalensis) and age class (nestling, immature [i.e. juvenile and sub-adult] and adult) each included as three-level factors in addition to time (collection year) as a continuous variable.
We excluded from the logistic regression models the results for the single specimens of A. monachus and G. fulvus because the small sample size for these species did not allow us to fit reliable statistical models of the effects of species differences. We also excluded results for two Gyps bengalensis carcasses whose recovery was associated with a kite festival (see above). Carcasses whose age class was not recorded were also excluded (six carcasses from the gout dataset and two carcasses from the diclofenac dataset).
We expected that the prevalence of diclofenac contamination and visceral gout would decline with increasing time during this period, based upon independent information on the downward trend in diclofenac contamination of ungulates (Cuthbert et al., Reference Cuthbert, Taggart, Prakash, Chakraborty, Deori and Galligan2014). Hence, we used one-tailed t-tests in these analyses to assess the statistical significance of trends over time. We plotted the expected proportions from the models against time for adult G. bengalensis and adult G. indicus.
Comparison of the decline in diclofenac prevalence in vulture carcasses with the decline expected from surveys of diclofenac in ungulate carcasses
We calculated the expected probability of death caused by diclofenac per vulture meal (C) in mid 2005 and the annual survival rate of adults in the absence of diclofenac using the simulation model of a vulture population described by Green et al. (Reference Green, Newton, Shultz, Cunningham, Gilbert, Pain and Prakash2004). We obtained values of C and survival that gave a value for the annual rate of population decline for G. bengalensis in India equal to the value observed at that time (population multiplication rate λ = 0.520; Green et al., Reference Green, Newton, Shultz, Cunningham, Gilbert, Pain and Prakash2004) and a value for the proportion of dead adult G. bengalensis with diclofenac in mid 2005 equal to that from the multiple logistic regression fitted to post-mortem data (see preceding section), which was 85.2%. We then estimated C for mid 2009 by reducing the mid 2005 estimate by 66%, which is the change in this parameter estimated for the 4 years between mid 2005 and mid 2009 from the surveys of diclofenac concentrations in liver samples of ungulate carcasses available to vultures in India by Cuthbert et al. (Reference Cuthbert, Taggart, Prakash, Chakraborty, Deori and Galligan2014). We used the method given by Green et al. (Reference Green, Newton, Shultz, Cunningham, Gilbert, Pain and Prakash2004) to calculate the expected proportion of deaths of adult G. bengalensis caused by diclofenac in mid 2009 from this reduced mid 2009 value of C. We obtained confidence limits for this expected proportion from the bootstrap confidence limits for the change in expected death rate per meal given by Cuthbert et al. (Reference Cuthbert, Taggart, Prakash, Chakraborty, Deori and Galligan2014; see their Table 2). We performed a significance test of the difference between the expected proportion of deaths of adult G. bengalensis caused by diclofenac in mid 2009 based upon vulture necropsies and the estimate of the same quantity derived from the surveys of diclofenac in ungulate livers. To do this we generated lists of 10,000 bootstrap estimates of the proportions of vultures with diclofenac for each of the two methods. We aligned the two randomly-ordered lists, calculated the absolute difference between values for each pair of bootstrap replicates and took the proportion of replicates in which the difference had the opposite sign to that observed in the real data to be the probability of observing by chance a difference as large as or larger than that obtained from the original calculations.
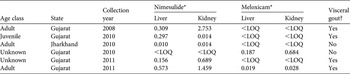
Table 2 Concentrations (mg kg−1) of nimesulide and meloxicam and the presence of visceral gout in all six carcasses (of G. bengalensis) in which either or both of these drugs was detected. Other NSAIDs (diclofenac, carprofen, flunixin, ibuprofen, indometacin, ketoprofen and naproxen) were assayed in all these birds but none was detected.
* <LOQ, below the limit of quantification of the assay
Results
Prevalence of visceral gout
Visceral gout was not present in either of the single A. monachus or G. fulvus carcasses examined. In the other three species the overall proportions with gout were 33% for G. himalayensis (1/3), 53% for G. indicus (9/17) and 73% for G. bengalensis (29/40).
Co-occurrence of visceral gout and NSAID residues in the liver and/or kidney
During the period 2000–2004, when diclofenac was the only NSAID being measured, diclofenac residues were present in liver and/or kidney tissue in all Gyps vulture carcasses in which visceral gout was identified, and in none of the carcasses with no gout (Table 1). This perfect association between diclofenac and visceral gout is highly significant (Fisher exact test P < 0.0001). In the later period, 2005–2011, in which other NSAIDs were also assayed in addition to diclofenac, all Gyps vulture carcasses with diclofenac residues also had visceral gout and no carcasses without gout had diclofenac. However, four of the 16 carcasses with gout did not have measurable levels of diclofenac, but did have residues of other NSAIDs (Table 1). All four of these carcasses had nimesulide in both the liver and kidney and one of them had meloxicam, as well as nimesulide, in both tissues. Of eight carcasses without gout, one had very low residues of nimesulide and one had residues of meloxicam (Table 1). Hence, during 2005–2011 the perfect association between diclofenac and gout found in the earlier period became weaker, though it remained statistically significant (Fisher exact test P = 0.0013). A logistic regression analysis of the 29 vulture carcasses with gout, in which the presence/absence of diclofenac was the binary dependent variable and year of collection was the independent variable, suggested a tendency for the proportion of vulture carcasses with gout that had no diclofenac residues to increase over time, but this trend was not statistically significant (t 27 = 1.48, P = 0.15).
Concentrations of NSAIDs in liver and kidney of vultures
In the 37 vulture carcasses for which diclofenac assays were performed for both liver and kidney, diclofenac was detected above the limit of quantification in both tissues in all 17 cases in which it was detected in either tissue, and was below the limit of quantification in both tissues in the remaining 20 cases. In the 17 cases with diclofenac above the LOQ in both tissues, there was a significant positive correlation between the concentrations in liver and kidney (r = 0.663, t 15 = 3.43, P = 0.003, Supplementary Fig. S1). The arithmetic means of the concentrations of diclofenac in liver and kidney in this subset of individuals were similar and not significantly different (liver, mean 0.181 mg kg−1, range 0.010–0.797 mg kg−1; kidney, mean 0.253 mg kg−1, range 0.010–0.872 mg kg−1; Wilcoxon signed ranks test, P = 0.124).
In the five vulture carcasses in which nimesulide was detected, levels were above the limit of quantification in both liver and kidney (Table 2). There was a weak and non-significant positive correlation between the nimesulide concentrations in the liver and kidney (r = 0.491, t 3 = 0.97, P = 0.402, Supplementary Fig. S2). Four of the five carcasses with measurable residues of nimesulide had visceral gout. The carcass with nimesulide and no gout had low concentrations of nimesulide near to the limit of quantification in both tissues, whilst the four birds with gout had considerably higher concentrations in one or both tissues (Table 2). In the two vulture carcasses in which meloxicam was detected, it was above the limit of quantification in both liver and kidney. In one of these carcasses there was visceral gout, but the concentration of meloxicam was very low in both liver and kidney whereas the concentration of nimesulide in both tissues was very high. The other carcass with meloxicam had high concentrations of the drug in both liver and kidney but no sign of visceral gout (Table 2). Meloxicam was the only NSAID detected in this bird.
Changes over time in the prevalence of diclofenac and visceral gout
Logistic regression analysis of the trend in the prevalence of diclofenac in liver and/or kidneys of vultures indicated a decline that was close to statistical significance (Fig. 1a). In a multiple regression model with effects of species and age accounted for, the logarithm of the odds of a vulture carcass having a measurable level of diclofenac declined by 0.2019 per year (1 SE = 0.1246, t 36 = 1.620, one-tailed P = 0.057). The proportion of adult G. bengalensis expected from the regression model to have a measurable level of diclofenac fell from 85% in the middle of 2005, just before the diclofenac ban, to 72% four years later in mid 2009 (Fig. 1a). For G. indicus, the equivalent change was from 77 to 59%.
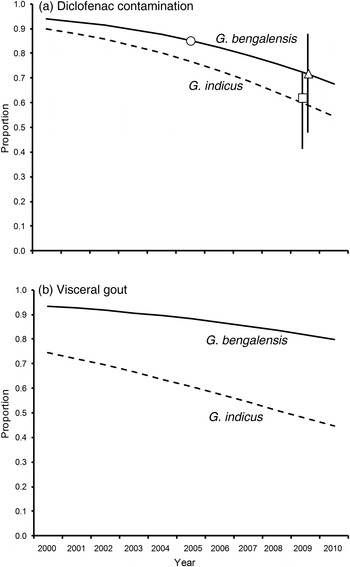
Fig. 1 (a) Proportions of carcasses of wild adult Gyps bengalensis and G. indicus contaminated with diclofenac, in relation to the year of collection. Curves represent expected values from a logistic regression model that included the main effects of species, age class and year. Symbols show the expected proportions of adult G. bengalensis contaminated with diclofenac in mid 2005 (circle) and mid 2009 (triangle) from the data from vulture carcasses, and the expected proportion of adult G. bengalensis deaths caused by diclofenac in mid 2009 (square) based upon the results of surveys of diclofenac contamination of carcasses of domesticated ungulates. Vertical lines are 95% confidence limits. (b) Proportions of carcasses of wild adult G. bengalensis and G. indicus with visceral gout, in relation to the year of collection. Curves represent expected values from a logistic regression model that included the main effects of species, age class and year.
Logistic regression analysis of the trend in the prevalence of visceral gout indicated a non-significant decline at a slower rate than that for diclofenac (Fig. 1b). In a multiple regression model with effects of species and age accounted for, the logarithm of the odds of a vulture carcass having gout declined by 0.1289 per year (1 SE = 0.1038, t 36 = 1.242, one-tailed P = 0.110). The proportion of adult G. bengalensis expected from the regression model to have gout fell from 88% in the middle of 2005 to 82% four years later in mid 2009 (Fig. 1b). For G. indicus the equivalent change was from 61 to 48%.
Comparison of the decline in diclofenac prevalence in vulture carcasses with the decline expected from surveys of diclofenac in ungulate carcasses
The expected vulture death rate per meal estimated from surveys of diclofenac residues in liver samples from ungulate carcasses declined by 66% in the 4 years between mid 2005 and mid 2009 (see Table 2 of Cuthbert et al., Reference Cuthbert, Taggart, Prakash, Chakraborty, Deori and Galligan2014). This change in expected death rate per meal, when used in the vulture population model of Green et al. (Reference Green, Newton, Shultz, Cunningham, Gilbert, Pain and Prakash2004; see Methods), gave an expected proportion of deaths of adult G. bengalensis caused by diclofenac in mid 2009 of 62.0% (95% confidence limits 41.5–72.2%). The estimated proportion of adult G. bengalensis carcasses with diclofenac in mid 2009 derived from the logistic regression model of vulture necropsy results was 71.9% (95% confidence limits 48.2–87.5%) whereas the same proportion derived from the surveys of diclofenac in ungulate carcasses was 62.0%, if it is assumed in both cases that the proportion of vulture carcasses with diclofenac in mid 2005 was 85.2% (Fig. 1a). The decline in the proportion of vulture carcasses with diclofenac derived from vulture necropsies was smaller than that derived from diclofenac surveys of ungulate carcasses (85.2–71.9% cf. 85.2–62.0%, respectively). However, the difference between the estimates derived from the two methods does not approach statistical significance (bootstrap P = 0.201).
Discussion
Our results indicate that diclofenac remained a significant cause of mortality for India's vultures and that the drug has continued to kill birds long after the 2006 regulations to prevent its veterinary use. The proportion of Gyps vultures found dead in the wild in India that had measurable levels of diclofenac in their tissues showed only a small and non-significant decline in the 5 years following the ban on the veterinary use of diclofenac covered by our study. The estimated size of the decline was broadly consistent with an independent estimate based upon measurements of the change in the prevalence and concentration of diclofenac in carcasses of domesticated ungulates available to vultures (Cuthbert et al., Reference Cuthbert, Taggart, Prakash, Chakraborty, Deori and Galligan2014). Continued mortality of vultures in India caused by diclofenac after the ban is consistent with the continued availability of the drug in pharmacies. Based upon surveys conducted between November 2007 and June 2010 in eleven Indian states, Cuthbert et al. (Reference Cuthbert, Dave, Chakraborty, Kumar, Prakash, Ranade and Prakash2011) reported that diclofenac formulated for non-veterinary use was offered for sale for veterinary use in 36% of pharmacies that sold any type of NSAID.
All vultures with measurable diclofenac in liver and/or kidney had visceral gout and this association was highly statistically significant. Wild Asian Gyps vultures that died with visceral gout and with measurable diclofenac in their tissues in our study had similar concentrations of diclofenac in the kidney to those that died in similar circumstances in the study of Oaks et al. (Reference Oaks, Gilbert, Virani, Watson, Meteyer and Rideout2004). Means and ranges were similar in our study of wild G. bengalensis and G. indicus carcasses from India (mean = 0.253 mg kg−1, range = 0.010–0.872 mg kg−1), the study of Oaks et al. (Reference Oaks, Gilbert, Virani, Watson, Meteyer and Rideout2004) of wild G. bengalensis carcasses from Pakistan (mean = 0.271 mg kg−1, range =0.064–0.642 mg kg−1) and the study of Oaks et al. (Reference Oaks, Gilbert, Virani, Watson, Meteyer and Rideout2004) of captive G. bengalensis that died after experimental administration of meat from water buffalo or goat given a veterinary dose of diclofenac shortly before death (mean = 0.388 mg kg−1, range = 0.070–0.906 mg kg−1). We therefore consider that diclofenac was likely to have been the cause of death of all of the vultures reported in the present study in which the drug was detected at above the limit of quantification.
The estimated decline in the prevalence of visceral gout in Gyps vultures found dead in India was smaller than that for diclofenac prevalence, and did not approach statistical significance. This may be because cases of visceral gout that were not associated with the presence of diclofenac in liver and/or kidney tissues began to occur from 2008 onwards. Four G. bengalensis with gout collected during 2008–2011 had no measurable diclofenac, but all had high concentrations of nimesulide in the liver and/or kidney. One of these carcasses also contained meloxicam residues but at concentrations so low that involvement of meloxicam in the death of the bird is unlikely. Another G. bengalensis carcass had a very low concentration (near LOQ) of nimesulide in both liver and kidney and no visceral gout. Hence, wild vultures in India are being exposed to nimesulide. Exposure at a high level is associated with visceral gout and death. Reddy et al. (Reference Reddy, Anjaneyulu, Sivasankari and Rao2006) suggested that nimesulide is likely to be safe for vultures, based upon a comparison of the toxicity of nimesulide and diclofenac to domestic fowl Gallus domesticus. However, there are large differences in the toxicity of NSAIDs among bird species (Cuthbert et al., Reference Cuthbert, Pain, Green, Swan and Swarup2006), so the safety of nimesulide in domestic fowl cannot be taken to indicate its safety to distantly-related vultures.
Nimesulide is legally approved for veterinary use in India and was offered for sale for this purpose in 48% of pharmacies that sold any type of NSAID in surveys conducted during 2007–2010 in eleven Indian states (Cuthbert et al., Reference Cuthbert, Dave, Chakraborty, Kumar, Prakash, Ranade and Prakash2011). The prevalence of nimesulide in pharmacies was particularly high in Gujarat, where it was offered for sale for veterinary use in 80% of shops visited (28 out of 35 shops; R.J. Cuthbert, unpubl. data). It is therefore notable that all four vultures with both nimesulide residues and visceral gout were collected from Gujarat. However, there is little evidence of nimesulide in carcasses of domesticated ungulates in India. Taggart et al. (Reference Taggart, Senacha, Green, Cuthbert, Jhala and Meharg2009) did not find any nimesulide residues in liver samples from 1,488 ungulate carcasses collected between April and December 2006. After including further samples collected up until July 2010 (total n = 3,150), only three ungulate carcasses with low (<0.04 mg kg−1) levels of nimesulide were found (0.1 mg kg−1; M.A. Taggart, unpubl. data).
Comparison of the time to maximum plasma concentration (t max) and elimination half-life (T 1/2) of nimesulide in cattle following intramuscular injection shows that its pharmacokinetics are broadly similar to those of diclofenac (EMEA, 2003; Mahapatra et al., Reference Mahapatra, Sahoo, Panda and Parija2009). Hence, the elimination of nimesulide in cattle is unlikely to be much more rapid than for diclofenac. To date, the liver has been the only organ routinely sampled in surveys of NSAIDs in ungulate carcasses in India (Taggart et al., Reference Taggart, Senacha, Green, Jhala, Raghavan and Rahmani2007, Reference Taggart, Senacha, Green, Cuthbert, Jhala and Meharg2009; Cuthbert et al., Reference Cuthbert, Taggart, Prakash, Chakraborty, Deori and Galligan2014). Nimesulide concentrations in ungulate livers may be unusually low relative to other tissues. Alternatively, the exposure pathway for vultures may involve sources of carrion that have not been surveyed, such as poultry waste. These questions should be addressed through further field sampling and experiments to study tissue distribution of nimesulide in ungulates.
Another NSAID found in vulture carcasses was meloxicam, which is the only drug established by experiment to be of low toxicity to Gyps vultures and other scavenging birds (Swan et al., Reference Swan, Naidoo, Cuthbert, Green, Pain and Swarup2006b; Cuthbert et al., Reference Cuthbert, Parry-Jones, Green and Pain2007; Swarup et al., Reference Swarup, Patra, Prakash, Cuthbert, Das and Avari2007). One vulture carcass with visceral gout had a low concentration of meloxicam and a high concentration of nimesulide in liver and kidney tissues, as described above. Another vulture carcass had a high concentration of meloxicam in liver and kidney and no trace of any other NSAID, and it had no evidence of gout. The cause of death of this bird was recorded to be diphtheroid enteritis. Therefore our findings are consistent with the safety to vultures of veterinary use of meloxicam indicated by experimental studies on captive birds. However, carcasses of wild vultures should continue to be monitored to check that this is the case under field conditions.
The NSAIDs carprofen, flunixin, ibuprofen, indometacin, ketoprofen and naproxen were not detected in any of the 25 vulture carcasses assayed for them. In experiments on the African vultures Gyps coprotheres and Gyps africanus, ketoprofen has been shown to be nephrotoxic at doses that are likely to be encountered by wild vultures (Naidoo et al., Reference Naidoo, Wolter, Cromarty, Diekmann, Duncan and Meharg2010). A wild G. fulvus was recently found dead in Spain with visceral gout associated with high levels of flunixin residues in liver and kidney tissues (Zorrilla et al., Reference Zorrilla, Martinez, Taggart and Richards2015). This augments previous evidence from surveys of the therapeutic use of NSAIDs on captive vultures in zoos, rehabilitation centres and other collections (Cuthbert et al., Reference Cuthbert, Parry-Jones, Green and Pain2007) that flunixin may be nephrotoxic to Gyps vultures. Cuthbert et al. (Reference Cuthbert, Parry-Jones, Green and Pain2007) also found evidence of nephrotoxicity of carprofen in one Gyps vulture. Experiments on captive vultures to measure the toxicity of flunixin and carprofen have not yet been conducted.
Taggart et al. (Reference Taggart, Senacha, Green, Cuthbert, Jhala and Meharg2009) found that some liver samples from domesticated ungulates available to vultures in India during April–December 2006 contained residues of ibuprofen and ketoprofen, but flunixin was not detected (Table 2 in Taggart et al., Reference Taggart, Senacha, Green, Cuthbert, Jhala and Meharg2009). In surveys conducted during 2007–2010 in 11 Indian states by Cuthbert et al. (Reference Cuthbert, Dave, Chakraborty, Kumar, Prakash, Ranade and Prakash2011), flunixin was being offered for sale for veterinary use in 7% of pharmacies that sold any type of NSAID, ibuprofen in 32% and ketoprofen in 29%. The fact that we did not find residues of these drugs in the sample of 25 vulture carcasses assayed for them may reflect the small size of our sample rather than their true absence from vulture carcasses. There is a 5% probability of our survey finding no contamination with any of these drugs, even if the true prevalence of the drugs in vulture carcasses had been as high as 11% ((1–0.113)25 = 0.05).
Our study highlights the continuing threat to Asia's vultures from veterinary use of diclofenac and identifies a new potential threat from nimesulide. Toxicity testing of nimesulide on Gyps vultures is needed to establish whether or not the compound is nephrotoxic. Illegal misuse in the veterinary sector of diclofenac products labelled ‘for human use only’ is the cause of much of the ongoing threat from that drug and further action to eliminate this has been recommended (Cuthbert et al., Reference Cuthbert, Dave, Chakraborty, Kumar, Prakash, Ranade and Prakash2011). Identification of NSAIDs that are effective on cattle and also safe for vultures at the maximum likelihood exposure level would be valuable for vulture conservation, but so far the only known example of such a drug is meloxicam.
Acknowledgements
We thank the Ministry of Environment and Forests and the Chief Wildlife Wardens for the states of Assam, Gujarat, Haryana, Himachal Pradesh, Jharkhand, Madhya Pradesh, Maharashtra, Rajasthan and Uttarakhand for permissions, and Kartik Shastri, Ruchi Dave, S. Saravaran, Satya Prakash, Sumantha Ghosh, Puja Basu, Bhrigu Neog and Vibhu Prakash for collecting vulture carcasses. Andrew Cunningham, Romain Pizzi, Yedra Feltrer, Devojit Das, Percy Avari, Jeherul Islam and Devendra Podadhe performed the necropsies. Ian Newton provided useful comments. We gratefully acknowledge financial support and assistance for the project from the Director, Indian Veterinary Research Institute, the UK Government's Darwin Initiative and the Royal Society for the Protection of Birds.
Biographical sketches
Richard J. Cuthbert did research on the conservation of Asian vultures and seabirds of oceanic islands before moving to the Wildlife Conservation Society. Mark Taggart studies levels of contamination and the impacts of pollutants on wildlife species. Mohini Saini, Anil Sharma and Asit Das conduct research on nutrition and the effects of animal diseases and pollutants on the health of wildlife in India. Mandar Dilip Kulkarni studies conservation genetics of Gyps vultures. Parag Deori works as a veterinarian on the Bombay Natural History Society's vulture programme. Sachin Ranade works on ex situ conservation and ecology of Gyps vultures and carries out population surveys. Rohan N. Shringarpure is studying microflora in Gyps vultures. Toby H. Galligan and Rhys E. Green research the conservation of Asian vultures and other bird species.





