Introduction
The Global Strategy for Plant Conservation (GSPC, 2008) and the European Plant Conservation Strategy (Planta Europa, 2008) aim to halt the continuing loss of plant diversity and, as part of this, the development of conservation strategies is an issue that needs to be urgently addressed at the national level (GSPC, 2008; Sharrock & Jones, Reference Sharrock and Jones2009). To develop a conservation strategy for a species, assessment of conservation status is the first step (Planta Europa, 2008) and the now accepted standard for doing this is the categories and criteria of the IUCN Red List of Threatened Species (IUCN, 2001; Grammont de & Cuarón, Reference Grammont de and Cuarón2006; Rodrigues et al., Reference Rodrigues, Pilgrim, Lamoreux, Hoffmann and Brooks2006; Hoffman et al., Reference Hoffman, Brooks, da Fonseca, Gascon, Hawkins and James2008).
In situ conservation measures such as the protection and restoration of natural habitats are the best methods of preserving biological diversity (Lande, Reference Lande1988; Francisco-Ortega et al., Reference Francisco-Ortega, Santos-Guerra, Kim and Crawford2000). However, in urgent situations ex situ conservation becomes an alternative way to prevent immediate extinction. One of the most effective ways to conserve ex situ plant diversity is storage in a seed bank, which is the most practical method for preserving large amounts of genetic material in a small space and with minimum risk of genetic damage (Iriondo & Pérez, Reference Iriondo, Pérez and Bowes1999).
Within the Mediterranean biodiversity hotspot (Myers et al., Reference Myers, Mittermeier, Mittermeier, da Fonseca and Kents2000), central northern Sardinia, including the Gennargentu massif, has been identified as one of 52 putative glacial refugia (sensu Médail & Diadema, Reference Médail and Diadema2009). The flora of this massif, however, has been little investigated. One of the elements of this flora is Lamyropsis microcephala (Moris) Dittrich & Greuter (Family Asteraceae), a perennial rhizomatous herb endemic to an extremely restricted area on the north (Pisargiu, Fonni) and south-western (Rio Aratu, Desulo) slopes of Mount Bruncu Spina (Diana Corrias, Reference Diana Corrias1977; Camarda, Reference Camarda2006; Bacchetta et al., Reference Bacchetta, Fenu, Mattana and Ulian2007, Reference Bacchetta, Fenu, Mattana and Ulian2008).
L. microcephala has been reported to have low seed set and low seed germination and to mainly propagate vegetatively (Diana Corrias, Reference Diana Corrias1977). Based on seed germination requirements (i.e. pre-chilling and high germination temperatures) Mattana et al. (Reference Mattana, Daws and Bacchetta2009) demonstrated that L. microcephala is adapted to a temperate climate and suggested that its distribution is still contracting under the present Mediterranean climate. The two localities in which the species was previously known to occur, being isolated both ecologically and genetically, are considered separate populations (G. Bacchetta et al., unpubl. data).
Global warming is affecting the distribution of species (Iverson & Prasad, Reference Iverson and Prasad1998; Bakkenes et al., Reference Bakkenes, Alkemade, Ihle, Leemans and Latour2002), and particularly of endemic plants (Pickering et al., Reference Pickering, Wendy and Ken2008), causing latitudinal and altitudinal shifts (Gottfried et al., Reference Gottfried, Pauli, Reiter and Grabeherr1999). Climatic variations can alter distribution areas and reduce population sizes and thus increase the risk of extinction of threatened species (Davis & Shaw, Reference Davis and Shaw2001). In addition, warmer temperatures affect seed germination of mountain species such as L. microcephala (Mattana et al., Reference Mattana, Daws and Bacchetta2009) that require a pre-chilling period to germinate (Kazakisv et al., Reference Kazakisv, Ghosn, Vogiatzakis and Papanastasis2007).
L. microcephala is considered one of the most threatened endemic plant species in Sardinia because of the negative effects of a ski run, built in the plant’s habitat, and associated touristic activities (Arrigoni, Reference Arrigoni1974; Pignatti et al., Reference Pignatti, Menegoni and Giacanelli2001). The species is categorized as Critically Endangered on the National (Conti et al., Reference Conti, Manzi and Pedrotti1992), Regional (Conti et al., Reference Conti, Manzi and Pedrotti1997) and global Red Lists (Camarda, Reference Camarda2006), is one of the top 50 threatened plants species of the Mediterranean Islands (Montmollin de & Strahm, Reference Montmollin de and Strahm2005), and listed in Appendix I of the Bern Convention and as a priority species in Annex II of the European Habitats Directive (DIR 92/43/EEC). L. microcephala has recently been included on the European threatened plant list (Sharrock & Jones, Reference Sharrock and Jones2009) but no conservation actions, either in situ or ex situ, have been previously implemented (Camarda, Reference Camarda2006).
Considering the urgency of conservation action for L. microcephala and the lack of ecological and conservation studies on the endemic flora of the Gennargentu massif, the main aim of this study was to develop an in situ and ex situ conservation strategy for this Critically Endangered endemic species. We estimated the species’ distribution and population size, characterized its habitat, identified threats, reassessed the species’ conservation status and established ex situ conservation measures.
Study area
The c. 30,000 ha Gennargentu massif has a maximum altitude of 1,834 m and is characterized by several ridges higher than 1,500 m. The bedrock consists of Paleozoic metamorphytes and granodiorites. Available climate data (from Fonni at 900 m) indicates a typical Mediterranean annual pattern of temperature and precipitation, with a dry summer. Bioclimatically this area is classified as temperate sub-Mediterranean (Bacchetta et al., Reference Bacchetta, Fenu, Mattana and Ulian2008).
Methods
The distribution of L. microcephala was verified by field surveys during 2006–2009 in the localities for which herbarium specimens (in the herbaria of CAG, Università degli Studi di Cagliari; CAT, Università di Catania; FI, Museo di Storia Naturale, Florence; SASSA, Università di Sassari (Faculty of Sciences); SS, Università di Sassari (Faculty of Pharmacy); TO, University of Turin; and in GBIF, 2011) and/or published data (Diana Corrias, Reference Diana Corrias1977; Bacchetta et al., Reference Bacchetta, Fenu, Mattana and Ulian2007, Reference Bacchetta, Fenu, Mattana and Ulian2008) were available. When a locality was confirmed or discovered, the following studies were undertaken.
The geographical limits of localities were mapped each year, with a global positioning system, and areas estimated, using ArcView v. 3.2 (ESRI, Redlands, USA), to detect any annual changes in area occupied. For each locality we noted the altitudinal range, slope, aspect and habitat type according to the European Habitat Directive (DIR 92/43/EEC).
Eighty-one permanent plots of 2 × 1 m were randomly established in each locality in 2009 to estimate ramet densities. We estimated the number of ramets per locality by counting the number in each plot and then extrapolating the mean density per plot to the whole area. All the ramets found in the plots were monitored monthly from July to September and the number of capitula per reproductive ramet determined in August. The non-parametric Kruskal-Wallis test was used to analyse differences in density, and a one-way ANOVA used to examine mean number of capitula per ramet, followed by a post hoc Duncan’s test to examine differences between group means. All statistical tests were performed using Statistica v. 6.0 (Statsoft, Tulsa, USA).
The threats to L. microcephala in each locality were determined from field observations and categorized following the IUCN threats classification scheme (IUCN, 2010b). A grid of 2 × 2 km was used for assessing area of occupancy (AOO, defined as the area within the extent of occurrence, EOO, that is occupied by a taxon, where EOO is defined as the area contained within the shortest continuous imaginary boundary that can be drawn to encompass all the known sites of occurrence of a taxon, excluding cases of vagrancy; IUCN, 2001), following IUCN (2008) and Rossi & Gentili (Reference Rossi and Gentili2008). EOO and conservation status were assessed following IUCN (2008).
The conservation measures adopted or proposed for the species were based on our field observations and on published data (Diana Corrias, Reference Diana Corrias1977; Camarda, Reference Camarda2006; Bacchetta et al., Reference Bacchetta, Fenu, Mattana and Ulian2007, Reference Bacchetta, Fenu, Mattana and Ulian2008) and reported following the IUCN Conservation Actions Classification Scheme (IUCN, 2010a). After obtaining the permits required by European and National laws we harvested seeds from all of the confirmed and newly discovered localities. Seeds were stored in the Sardinian Germplasm Bank (BG-SAR) where, once cleaned by removing the pappus and separating any empty aborted seeds, they were placed in a dry room at 15% relative humidity and 15°C and then at 5°C (the active collection) and at -25°C (the base collection), the latter comprising a black-box collection (material stored for future use) and a check-up collection (seed lots for testing viability during storage). We also commenced an education and awareness programme with the local people and relevant authorities at the regional and local level, offering lectures and seminars to increase awareness of the importance of conserving threatened plant species.
Results
We confirmed the presence of L. microcephala in the two already known sites, at Rio Aratu and Pisargiu, both of them on the Bruncu Spina ridge, and found two new sites, at Bruncu Spina and Bau ‘e Laccos (Fig. 1, Table 1). The area of the four localities varied from 200 m2 to 240,000 m2, at altitudes of 1,450–1,820 m, on slopes of 15–45° with north to west aspects, with the plants growing preferentially along catchment areas or in moderate hygrophilous conditions (Table 1). Our field surveys during 2006–2009 in the two previously known localities found little variation in the extent of the populations between years (data not presented here). The vegetation community where L. microcephala grows is perennial grassland, with hemicryptophytes and cushion chamaephytes being dominant, characterized as the endemic Carici–Genistetea lobelioidis vegetation class (Pignatti et al., Reference Pignatti, Pignatti, Nimis and Avanzini1980). In the European Habitat Directive (DIR 92/43/EEC) this vegetation type is Endemic Oro-Mediterranean heaths with gorse (code 4090) and the subtype Cyrno-Sardinian hedgehog-heaths (code 31.75). In the two larger localities (Rio Aratu and Bau ‘e Laccos) L. microcephala was found also in hygrophilous phytocoenosis of the endemic Caricion microcarpae alliance and, in Bau ‘e Laccos, partly under Alnus glutinosa (L.) Gaertn. riparian woodlands.

Fig. 1 The study area, showing the main peaks of the Gennargentu massif. The white lines and dot indicate the four areas where Lamyropsis microcephala has been found (Rio Aratu, Pisargiu, Bruncu Spina and Bau ‘e Laccos). The rectangle on the inset indicates the location of the main figure in Sardinia.
Table 1 The four sites where Lamyropsis microcephala has been located, with altitude range, mean slope (and range in parentheses), aspect and identified threats (IUCN, 2010b). Other threats (with * in the table) are coded in an earlier threats classification scheme (IUCN, 2007).
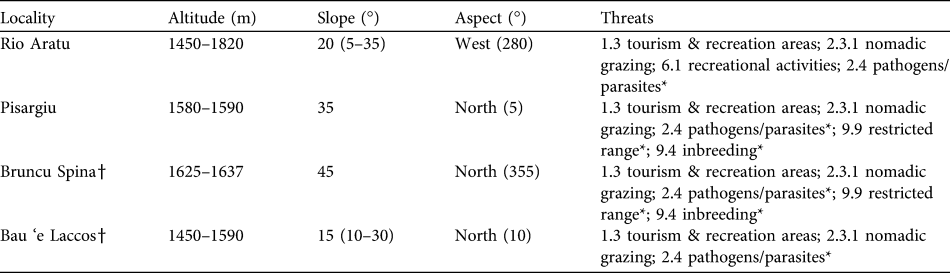
† Localities newly discovered in this study
The same threats were detected in all localities, with tourism and other outdoor activities the mean threats, followed by expansion of pastoral activities and nomadic grazing of sheep, cattle, horses and pigs (Table 1). Other detected pressures included natural threats and in particular the abundance of the parasitic plant Cuscuta spp..
The results of the plot surveys in 2009 are given in Table 2. The estimated number of ramets per locality varied considerably between the four localities but the mean densities (8.29–10.33 m-2) were not significantly different. The mean percentage of ramets that were reproductive did not differ greatly between localities (52.3–59.2%). However, the mean number of capitula per reproductive ramet was 1.98–2.69 and was significantly lower at Pisargiu than at the other localities.
Table 2 For each of the four localities of L. microcephala in 2009 the area, number of 2 x 1 m monitoring plots, mean density of ramets, estimated population size, percentage of ramets reproductive, and mean number of capitula per reproductive ramet.
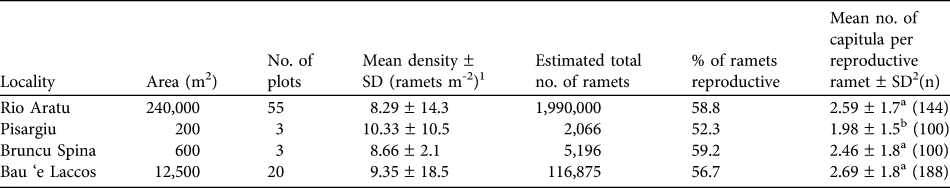
1 Test for differences in mean density: Kruskal-Wallis test (df = 3, H = 3.202; P = 0.362)
2 Test for differences in mean number of capitula per reproductive ramet: one-way ANOVA (df = 3, F = 3.6263, P = 0.013), followed by post hoc Duncans' test for pairwise analysis (values with the same letters are not different at P > 0.05)
Table 3 presents the conservation measures adopted and proposed for L. microcephala. Those that we have already adopted are ex situ conservation (the seed collections) and formal education, and awareness and communications, with several lectures and seminars involving the local populace. We collected seed annually from 2006 to 2009 in Rio Aratu, from Pisargiu in 2006, 2007 and 2009, and from Bruncu Spina and Bau ‘e Laccos in 2009 (Table 4). Depending on the total amount of seeds, sub-lots of each accession were made to create a base collection. Seeds were also sent to the Seed Conservation Department, Royal Botanic Gardens, Kew, Wakehurst Place, West Sussex, UK (Table 4).
Table 3 Conservation measures carried out in this study, or proposed, for L. microcephala. Conservation actions are coded following IUCN (2010a).

* The conservation measures reported in Camarda (Reference Camarda2006).
Table 4 Seed lots of L. microcephala stored in the Sardinian Germplasm Bank and sent to the Seed Conservation Department, Kew.
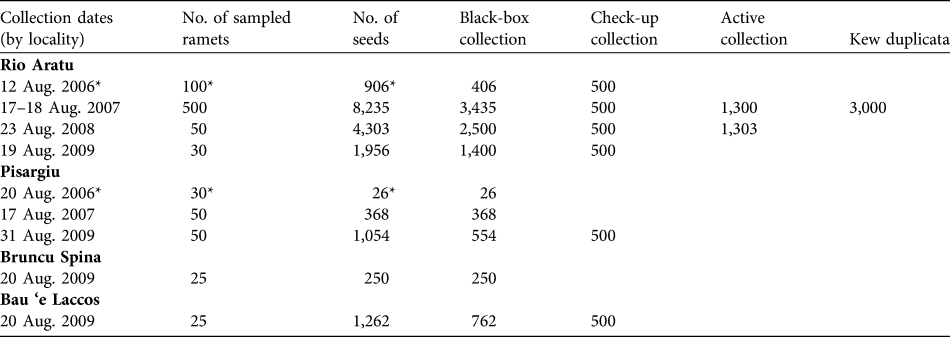
* Reported in Bacchetta et al. (Reference Bacchetta, Fenu, Mattana and Ulian2007)
Based on the extent of occurrence (1.62 km2), area of occupancy (4 km2), number of locations (one, sensu IUCN, 2008) and an inferred decline due to habitat loss and fragmentation of the original population, we confirmed the Red List categorization of Critically Endangered for L. microcephala, based on criteria B1ab(i,ii,iii,v) + 2ab(i,ii,iii,v).
Discussion
Our discovery of two previously unknown localities for L. microcephala supports the hypothesis that these sites may constitute the remnants of a formerly larger population on the Bruncu Spina slopes in the area where the ski run was built in 1974, as suggested by herbarium specimen collected in 1972 on the north slope of this mountain at 1,750 m (Diana Corrias, Reference Diana Corrias1977). The major threats to L. microcephala are habitat loss from the expansion of infrastructure for tourism, and grazing. Tourism and recreational activities are the main threat to ecosystems in the Mediterranean area (Allen, Reference Allen2001) and typically lead to habitat fragmentation (Gibbs, Reference Gibbs2001).
Although the effects of trampling on Mediterranean endemic plants has been rarely investigated, there have been several studies of the effects of grazing. Overgrazing affects plant density and survival rate, flowering season and recruitment rate (Lavergne et al., Reference Lavergne, Debussche and Thompson2005; Ramula, Reference Ramula2008; Sletvold & Grindeland, Reference Sletvold and Grindeland2008), whereas if grazing is regulated or limited to certain seasons it improves population growth (Bullock et al., Reference Bullock, Hill and Silvertown1994; Ehrlén et al., Reference Ehrlén, Syrjänen, Leimu, García and Lethilä2005; Picó et al., Reference Picó, Quintana-Ascencio, Mildénd, Ehrlén and Pfingsten2008). A positive effect of regulated grazing has been reported for rhizomatous plants (Bullock et al., Reference Bullock, Hill and Silvertown1994; Jongejans et al., Reference Jongejans, de Vere and Kroon de2008). Nevertheless, as a precautionary measure, grazing and the tourism activities should be regulated in the four known localities of L. microcephala, and no new pathways opened (Table 3).
Habitat fragmentation increases extinction risk for rare species (Holsinger, Reference Holsinger, Young and Clark2000; Matthies et al., Reference Matthies, Bräuer, Maibom and Tscharntke2004; Schleuning & Matthies, Reference Schleuning and Matthies2009), interferes with distribution, fitness and seedling recruitment (Lienert, Reference Lienert2004; Kolb & Diekmann, Reference Kolb and Diekmann2005; Benito et al., Reference Benito, Martínez-Ortega, Muñoz, Lorite and Peñas2009; Vere de et al., Reference Vere de, Jongejans, Plowman and Williams2009), reduces the number of breeding individuals and gene flow (Dudash & Fenster, Reference Dudash, Fenster, Young and Clarke2000) and pollination efficiency (Duncan et al., Reference Duncan, Nicotra, Wood and Cunningham2004). Although G. Bacchetta et al. (unpubl. data) detected a moderate level of genetic diversity in the populations of L. microcephala at Rio Aratu and Pisargiu, the low number of capitula per ramet that we found is probably related to the small population size, as demonstrated in several studies (Fischer & Matthies, Reference Fischer and Matthies1998; Oostermeijer et al., Reference Oostermeijer, Luijten, Krenova and Den Nijis1998).
The discovery of L. microcephala in two new localities has enlarged the previously reported EOO (0.32 km2; Bacchetta et al., Reference Bacchetta, Fenu, Mattana and Ulian2008). Nevertheless this has not altered the current categorization of the species as Critically Endangered, although the criteria are different. Our implementation of ex situ conservation measures for L. microcephala continues the work started by Bacchetta et al. (Reference Bacchetta, Fenu, Mattana and Ulian2007). Considering the significant degree of genetic differentiation between the two historical L. microcephala localities (G. Bacchetta et al., unpubl. data) the creation of a black-box seed collection for all four localities now known will ensure the conservation of the genetic variability of this threatened species. The seeds collected could be used for future reinforcement, restoration or reintroduction of the species in suitable areas of the Gennargentu massif.
This study of a Critically Endangered extremely narrow endemic plant of the Mediterranean area, together with population genetic studies in progress, is the first part of a long-term integrated conservation programme for the species. The approach described here could serve as a model for determining conservation status and developing conservation strategies for other narrowly distributed, threatened species for which little is known of their distribution and reproductive biology.
Acknowledgements
This research was funded by RAS–Assessorato Difesa Ambiente. The support of the Millennium Seed Bank Project (Seed Conservation Department, Royal Botanic Gardens, Kew) is gratefully acknowledged. We thank T. Ulian and A. Congiu for help with fieldwork.
Biographical sketches
Giuseppe Fenu has a particular interest in conservation of the endemic and threatened plants of Sardinia. Efisio Mattana is interested in ex situ conservation and germination ecophysiology of the endemic flora of Sardinia. Gianluigi Bacchetta carries out geobotanical analyses in the western Mediterranean area.






