Introduction
Understanding the factors that influence the distribution and abundance of species is a fundamental theme in ecology (Andrewartha & Birch, Reference Andrewartha and Birch1954). These factors or determinants of niche space operate in a hierarchical manner, ranging from microsites to broad climatic regimes (Forman, Reference Forman1964). Knowledge of factors that influence habitat choice by animals, such as selection of vegetation patches, have assisted conservation planning and reserve design for metapopulation persistence (Lindenmayer, Reference Lindenmayer2000). Broad-scale distribution patterns of large mammalian herbivores are determined mainly by abiotic factors within which biotic mechanisms operate (Olff et al., Reference Olff, Ritchie and Prins2002). Herbivores are important components of many terrestrial ecosystems and human interventions are causing extinctions and major changes in composition of herbivore-assemblages across the world (Frank et al., Reference Frank, McNaughton and Tracy1998; Olff et al., Reference Olff, Ritchie and Prins2002).
The Trans-Himalaya, encompassing the Tibetan plateau and its marginal mountains, is one such ecosystem where pastoralism is altering native herbivore assemblages (Mishra et al., Reference Mishra, van Wieren, Heitkonig and Prins2002). Ladakh is the western extension of this unique high altitude Tibetan ecosystem, and harbours a diverse assemblage of eight wild ungulate species. Compared to the records of the 19th century explorers (Burrard, Reference Burrard1925) the current populations of these ungulates are low, probably resulting from past hunting and habitat degradation associated with increasing livestock populations (Fox et al., Reference Fox, Nurbu and Chundawat1991; Schaller, Reference Schaller1998).
One of the most threatened species is the small-sized, ruminant, Tibetan gazelle Procapra picticaudata (c. 15 kg), whose range once covered c. 20,000 km2 in Ladakh (Fox et al., Reference Fox, Nurbu and Chundawat1991). The species is categorized globally as Near Threatened on the IUCN Red List (IUCN, 2007) but it is on the brink of extinction in Ladakh, with <100 animals remaining in an area of c. 100 km2 in the Hanle Valley of Eastern Ladakh (Bhatnagar et al., Reference Bhatnagar, Mishra and Wangchuk2006a) and its population is also declining on the Tibetan plateau (Schaller, Reference Schaller1998; Xia et al., Reference Xia, Yang, Li, Wu and Feng2007). Despite the ban on its hunting in India, gazelle populations continue to decline, presumably because of intensified livestock grazing (Bhatnagar et al., Reference Bhatnagar, Mishra and Wangchuk2006a). The Hanle valley is an important production centre of pashmina or cashmere, a world-renowned fibre, and the population of the goats that produce it is increasing (Namgail et al., Reference Namgail, Bhatnagar, Mishra and Bagchi2007a). However, forage limitation (Bagchi et al., Reference Bagchi, Mishra and Bhatnagar2004; Mishra et al., Reference Mishra, van Wieren, Ketner, Heitkonig and Prins2004) and interference competition (Namgail et al., Reference Namgail, Fox and Bhatnagar2007b) from increasing livestock populations have probably caused local extinctions of native ungulates (Mishra et al., Reference Mishra, van Wieren, Heitkonig and Prins2002), and thus the issue of competition between gazelle and domestic livestock requires careful examination.
Here we examine the factors influencing the distribution of the largest known population of Tibetan gazelle in India. These data are relevant for developing a much needed in situ recovery programme for the species (Bhatnagar et al., Reference Bhatnagar, Mishra and Wangchuk2006a). We focused on understanding (1) the influence of terrain and vegetation characteristics on habitat choice by the gazelle, and (2) the nature of the interactions between gazelles and sympatric wild and domestic ungulates. We discuss the implications of our work for starting an in situ recovery programme.
Study area
The basin of the Hanle River, which is a major tributary of the Indus River (Fig. 1), is charcterized by low precipitation (200-400 mm), low temperatures (–30 to 25oC), and high elevations (4,700-5,100 m). Plant growth is confined to a short period (May-August) and vegetation is dry-alpine steppe with grasses, sedges, forbs and small shrubs (Bagchi et al., Reference Bagchi, Namgail and Ritchie2006). Besides gazelles, kiang Equus kiang (mean adult body mass 275 kg) also occur at an estimated density of 0.56 km2 (Bhatnagar et al., Reference Bhatnagar, Wangchuk, Prins, van Wieren and Mishra2006b). About 2,000 goats and sheep (25-30 kg) belonging to local nomadic herders also use the area of Kalak Tartar in the Hanle valley for a period of c. 30 days in early summer before they move upstream; lack of potable water curtails this grazing period. However, they return to the area in winter, with yaks, goats and sheep, following snowfall (Bhatnagar et al., Reference Bhatnagar, Mishra and Wangchuk2006a). Kiang use the area throughout the year alongside gazelles.
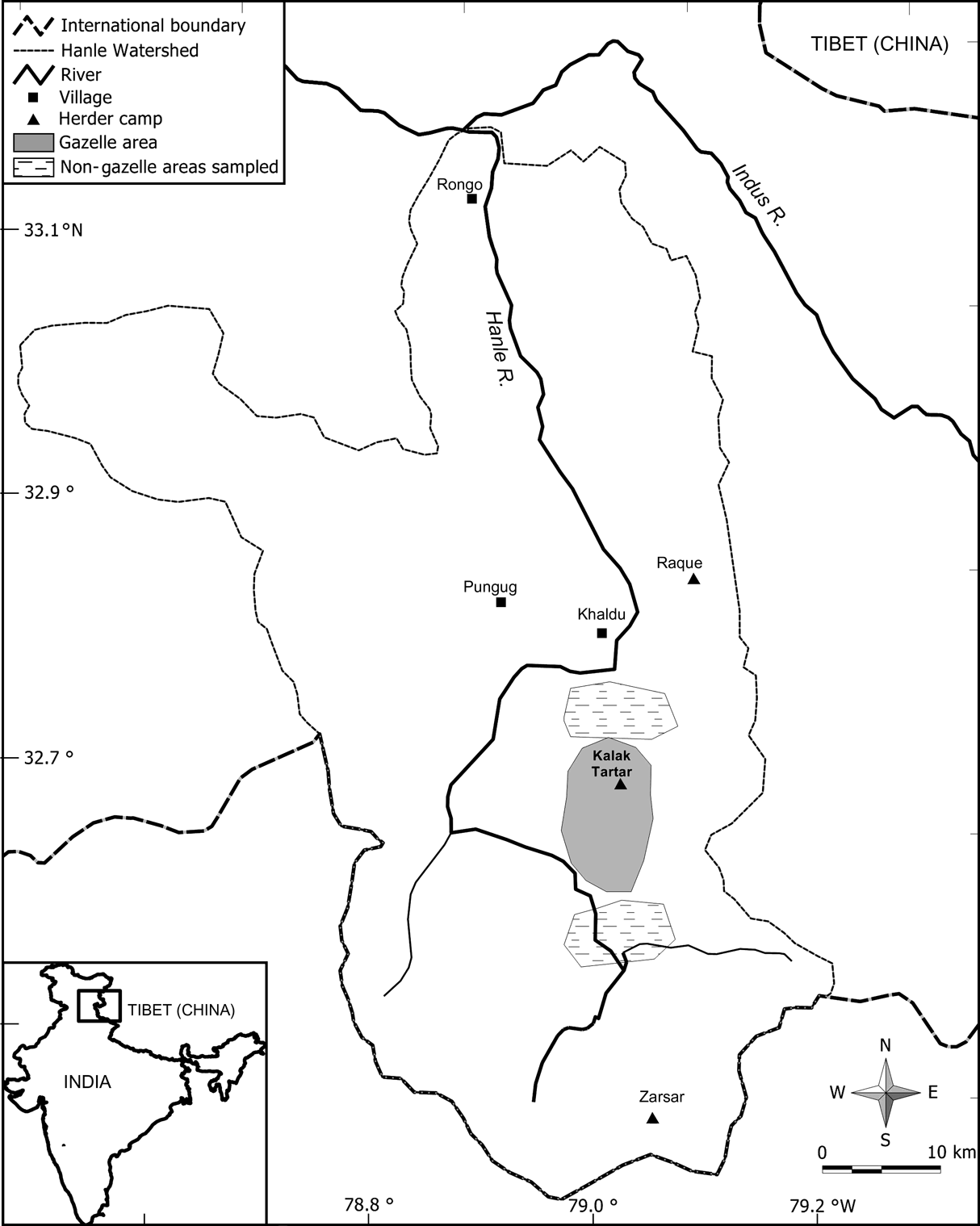
Fig. 1 Location of the study area in the Hanle valley, eastern Ladakh, showing the area with gazelles and also the two areas without gazelles that were sampled for vegetation cover and productivity. The inset indicates the location of the main figure in India.
Methods
Data for estimating gazelle habitat use were collected from direct sightings. Habitat variables such as slope angle, aspect and elevation were recorded at the animals’ locations during summer (May-August 2004) and winter (January-March 2005). Habitat affinities were assessed by the selection ratio (ratio of habitat use and availability) following Manly et al. (Reference Manly, McDonald, Thomas, McDonald and Erickson2002). The proportion of available habitat was determined from 51 random locations (Marcum & Loftsgaarden, Reference Marcum and Loftsgaarden1980). All variables were classified into categories, and the selection ratio and standard error were calculated for each category. The log-likelihood χ2 statistic was used to test for habitat selection (Manly et al., Reference Manly, McDonald, Thomas, McDonald and Erickson2002). In case of habitat selection, 95% confidence intervals were calculated for each habitat category as ŵi + Zα/(2I) SE(ŵi), where I is the number of habitat categories and SE(ŵi) is the standard error of the selection ratio. A habitat is used selectively if the confidence limits exclude unity. Under positive selection (preference) the interval is >1, and under negative selection (avoidance) it is <1.
From the presence/absence of gazelle pellets in randomly located plots as well as from direct sightings of animals during May-June 2004, we delineated areas that were selected by gazelles (of c. 45-50 km2), and two adjacent areas (Fig. 1) without gazelle (of c. 40-45 km2). To assess the difference between these two areas in vegetation cover during summer (June-August), we recorded the plant species or any other substrate at every 0.5 m interval along a 20 m transect (n = 38 in each area). We also investigated the difference in above-ground net primary productivity between these areas with 2*2 m exclosures (n = 7 in the area with gazelle and n = 5 in the areas without gazelle). We paired these exclosures with 2-m adjacent control plots (i.e. grazed and ungrazed) at the beginning of the growth season (May). At the end of the growth season (August), vegetation was clipped to ground level from two 1*1 m quadrats in each exclosure and control plot.
Tibetan gazelle are known to feed preferentially on forbs (Schaller, Reference Schaller1998). Such small ruminants rely on high-quality forage (van Soest, Reference van Soest1982) and fermentation of forbs is more viable compared to grasses (Foley & Cork, Reference Foley and Cork1992; Iason & van Wieren, Reference Iason, van Wieren, Olff, Brown and Drent1999). Therefore, we sorted plant biomass from the areas with and without gazelle into two functional groups: graminoids and forbs (herbs and small shrubs) and oven-dried them to obtain dry weights. We also assessed grazing intensity in the two areas from the difference in biomass between fenced and control plots, and compared the data from the two areas with General Linear Models, using SAS v. 9.0 (SAS Institute, Cary, USA). Data are presented as ± SE and statistical significance adjudged at α = 0.05 in all cases.
We recorded spatial distribution of gazelles in different pastures of the Kalak Tartar area using pellet counts (Neff, Reference Neff1968) in 123 randomly located 5*10 m quadrats in the area with gazelle. The influence of sympatric ungulates (goats, sheep, yak and kiang) on the spatial distribution of gazelle was assessed using data on the presence or absence of their faecal pellets in plots, which were compared to a null model following Bagchi et al. (Reference Bagchi, Mishra and Bhatnagar2004). Faecal pellets of gazelle (average length 9.4 ± SE 1.7 mm) are visibly smaller and easily distinguishable from those of adult goats and sheep (13.7 ± SE 1.8 mm; Bhatnagar et al., Reference Bhatnagar, Mishra and Wangchuk2006a). Confusion with the faeces of juvenile goats and sheep was precluded because young and juveniles are herded separately near the pastoral camps. However, we did not distinguish between pellets of goats and sheep, and considered them as one group. Plot-based co-occurrence between gazelles and other species was assessed by calculating the C-score index as Cij = (ri-S)(rj-S), where ri is the number of plots with species i and rj is the number of plots with species j, with S being the number of shared sites (Stone & Roberts, Reference Stone and Roberts1990). This measures the tendency of any two species not to co-occur in the same plot, with larger values indicating greater separation between them. Deviation of observed C-scores due to chance was assessed by Monte Carlo simulations using Ecosim 6.10 (Gotelli & Entsminger, Reference Gotelli and Entsminger2001). In this null-model, species were assigned randomly to plots (1,000 iterations) so that occurrence of one species was independent of others.
Results
Sixty-three observations were made of gazelles during summer, and 30 during winter. Gazelles used relatively flat areas (6-15°) disproportionately during both summer ![]() and winter
and winter ![]() . They preferred south-facing slopes and avoided north-facing slopes during winter
. They preferred south-facing slopes and avoided north-facing slopes during winter ![]() . There was a weak preference for lower areas (4,751-4,900 m) in winter
. There was a weak preference for lower areas (4,751-4,900 m) in winter ![]() , whereas relatively higher areas were avoided during both summer and winter within the available altitudinal range (Table 1).
, whereas relatively higher areas were avoided during both summer and winter within the available altitudinal range (Table 1).
Table 1 Estimated seasonal habitat selection indices (see text for details) for the Tibetan gazelle in the Hanle Valley, Ladakh, India.

1 ŵi, estimated habitat selection ratio; SE(ŵi) SE of selection ratio; ŵi(l) and ŵi(u) 95% lower and upper confidence limits, respectively
2 Inference less reliable because of few observations
3 Preference;
4 Avoidance (see methods for further details)
Five species of graminoids and 17 forbs were recorded. Plots in the area with gazelle had higher overall vegetation cover (45.2 ± SE 1%) than those in the area without gazelle (27.2 ± SE 1%; F 1,75 = 133.3, P <0.001). Net above-ground primary productivity was marginally higher in the gazelle area (38.7 ± 2 g m−2) compared to (29.5 ± 2 g m−2) the area without gazelles (F 1,10 = 4.67, P = 0.056). After accounting for correlation between forb biomass and total biomass (R = 0.69, P = 0.01) we found a significantly higher proportion of forbs in the gazelle area compared to the non-gazelle area (ANCOVA, F 1,9 = 6.65, P = 0.02). Comparisons of fenced and control plots showed that 47 ± SE 6 % of plant biomass was consumed by herbivores (wild and domestic) in areas outside gazelle range, whereas only 29 ± SE 5% was consumed in plots inside the area with gazelle (F 1,10 = 5.05, P = 0.04).
Of the 123 plots, gazelle dung pellets were recorded in 38%, that of kiang in 45%, of yak in 34%, and of goats and sheep in 71%. Pair-wise comparisons showed that co-occurrence of gazelles and goats and sheep was significantly lower than expected by chance, whereas gazelle tended to co-occur with kiang and domestic yak (Table 2).
Table 2 Spatial co-occurrence, measured by the C-score index (Stone & Roberts, Reference Stone and Roberts1990), of the Tibetan gazelle with sympatric livestock and kiang in the Kalak Tartar area of Ladakh, India. All values were significantly different (P <0.01) from random as adjudged by a null-model.
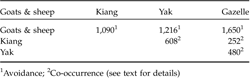
1 Avoidance;
2 Co-occurrence (see text for details)
Discussion
Many native ungulates of the Trans-Himalaya have a long co-evolutionary history (Schaller, Reference Schaller1977, Reference Schaller1998) and exhibit resource partitioning under sympatry (Namgail et al., Reference Namgail, Fox and Bhatnagar2004). In comparison, domestic livestock have been introduced relatively recently (1,000-3,000 years ago, Goldstein & Beal, Reference Goldstein and Beal1990) and their interactions with native species are more likely to be competitive. Our data suggest that gazelles have a predominantly competitive relationship with small-sized goats and sheep, and a possible indirect facilitative interaction with large-sized wild kiang and domestic, but native, yak (facilitation through habitat modification; Vesey-Fitzgerald, Reference Vesey-Fitzgerald1960; Bell, Reference Bell1971). However, because our data on co-occurrence patterns are based on distribution of pellet-groups there could be potential bias if gazelles maintain latrine sites, although we encountered gazelle pellets throughout the study area.
The negative relationship between the distribution of gazelle and domestic goats and sheep at localized scales in the study area is in contrast to patterns observed at regional scales on the Tibetan plateau where the species’ encounter rate is higher in areas with relatively high livestock presence (Fox & Bårdsen, Reference Fox and Bårdsen2005). This discrepancy could be related to the difference in the spatial scales between the two studies, and the indices used for livestock presence. More specifically, although gazelle and livestock may co-occur at a regional scale, they may segregate at the habitat level as found in our study. Comparative data from the Tibetan plateau collected at finer scales could clarify this further.
Forage competition between livestock and wild herbivores in the Trans-Himalaya is increasingly being documented (Bagchi et al., Reference Bagchi, Mishra and Bhatnagar2004; Mishra et al., Reference Mishra, van Wieren, Ketner, Heitkonig and Prins2004). Estimates show that large herds of goats and sheep, as in our study area, can consume >200 kg of forage per day (dry weight) of which 50-55% can be forbs (Bagchi et al., Reference Bagchi, Mishra and Bhatnagar2004). Gazelles depend on high-quality forage (forbs) compared to fibrous forage (grasses, Foley & Cork, Reference Foley and Cork1992; Iason & van Wieren, Reference Iason, van Wieren, Olff, Brown and Drent1999), with 70-90% of their diet consisting of forbs (Harris & Miller, Reference Harris and Miller1995; Schaller, Reference Schaller1998; Miller & Schaller, Reference Miller, Schaller and Stellrecht1998). This constraint on smaller bodied ungulates such as the Tibetan gazelle arises because metabolic requirements (M) scale as M ∝ B3/4 of the body-mass (B), whereas the gut-capacity (G) varies as G ∝ B1 (Demment & van Soest, Reference Demment and van Soest1985). These allometric relationships make smaller ruminants such as the gazelle dependant on forbs. Therefore, high rates of removal of forbs by livestock can disproportionately affect gazelles. Additionally, goats and sheep are husbanded in large herds, and are accompanied by herders and guard dogs that may cause direct disturbance and interference competition (Namgail et al., Reference Namgail, Fox and Bhatnagar2007b). On the other hand, bulk-feeding on graminoids by kiang and yak (58-95% of their diet; Schaller, Reference Schaller1998; Mishra et al., Reference Mishra, van Wieren, Ketner, Heitkonig and Prins2004) could potentially favour the growth of forbs and attract gazelles into such areas.
Our data and analyses provide an improved understanding of the ecological requirements of the Tibetan gazelle at a critical time when the species is threatened with local extinction in Ladakh. The habitat characteristics required by the Tibetan gazelle, as outlined here, can be used to identify potentially suitable gazelle habitat in areas adjoining Kalak Tartar. Once identified, these areas need to be relieved of livestock pressures to facilitate colonization by gazelles. Acquisition and protection of even small-sized patches can be of high conservation importance (Berger, Reference Berger2003). We have initiated this process by organizing workshops with the Wildlife Protection Department and local nomads to communicate our research results and initiate the formulation of strategies for a recovery programme for the Tibetan gazelle (Bhatnagar et al., Reference Bhatnagar, Seth, Takpa, Ul-Haq, Namgail, Bagchi and Mishra2007).
Acknowledgements
We thank the Rufford Foundation, the Whitley Fund for Nature, and the Ford Foundation for supporting our work. Additional support came from the Wildlife Conservation Society (to TN) and Syracuse University (to SB). We thank C.M. Seth, Jigmet Takpa and Salim Ul-Haq of the Department of Wildlife Protection, M.P. Singh, Dorjey, Tsewang and Sanjeev of the Indian Astrophysics Observatory, and Tsetan Paljor and Angdus of Hanle for their support. R. Raghunath helped in preparing the map.
Biographical sketches
Tsewang Namgail and Sumanta Bagchi are studying the ecology of mountain ungulates and rangeland dynamics in the Trans-Himalayan mountains, and both are involved in community-based conservation initiatives in the region. Charudutt Mishra and Yash Veer Bhatnagar study various aspects of high altitude ecology, including grazing systems and human-wildlife conflicts, run community-based conservation programmes, and work on conservation policy development.





