Introduction
Halting biodiversity loss depends largely on developing effective conservation policies and planning (Johnson et al., Reference Johnson, Balmford, Brook, Buettel, Galetti and Guanchum2017). Evidence-based, inclusive, participatory conservation strategies are recommended when specific actions are needed to save species from extinction (IUCN, 2017). Key species can act as umbrellas or flagships, transforming species-level conservation plans into ecosystem-wide benefits (Superina et al., Reference Superina, Cortés Duarte and Trujillo2018).
The northern and the southern Darwin's frog (Rhinoderma rufum and Rhinoderma darwinii) are named after Charles Darwin, who was the first to collect R. darwinii, in 1834. These species are the only known amphibians in which the males brood their offspring within their vocal sacs (Plate 1). In R. rufum the later larval stages develop in water, whereas in R. darwinii the entire larval development, lasting up to 8 weeks and including metamorphosis, takes place within the male's vocal sac (Formas et al., Reference Formas, Pugín and Jorquera1975; Formas, Reference Formas2013; Supplementary Fig. 1). Endemic to the Austral temperate forests of South America, both species are highly threatened as a result of dramatic population declines, particularly during the last 4 decades (Crump & Veloso, Reference Crump, Veloso, Smith-Ramírez, Armesto and Valdovinos2005; Bourke et al., Reference Bourke, Busse and Böhme2012; Soto-Azat et al., Reference Soto-Azat, Valenzuela-Sánchez, Collen, Rowcliffe, Veloso and Cunningham2013a). The habitat of Darwin's frogs is an ecoregion characterized by a high degree of endemism and is thus of high conservation priority (Myers et al., Reference Myers, Mittermeier, Mittermeier, da Fonseca and Kent2000). Rhinoderma rufum has not been recorded since 1981 and remaining populations of R. darwinii are small and isolated (Soto-Azat et al., Reference Soto-Azat, Valenzuela-Sánchez, Collen, Rowcliffe, Veloso and Cunningham2013a; IUCN, 2019). Darwin's frogs are found only in native forest (generally old-growth), and R. darwinii abundance has been positively correlated with the structural complexity of its forest habitat (Valenzuela-Sánchez et al., Reference Valenzuela-Sánchez, Schmidt, Pérez, Altamirano, Toledo and Pérez2019a). Although habitat loss is a threat, population declines and extirpations have also been documented within protected areas and undisturbed ecosystems (Crump & Veloso, Reference Crump, Veloso, Smith-Ramírez, Armesto and Valdovinos2005; Soto-Azat et al., Reference Soto-Azat, Valenzuela-Sánchez, Collen, Rowcliffe, Veloso and Cunningham2013a).

Plate 1 A brooding male southern Darwin's frog Rhinoderma darwinii in a typical humid substrate of the Valdivian temperate forest.
Recently, there has been growing concern about Darwin's frogs, evidenced by several independent and uncoordinated research and conservation efforts. The majority (75%) of all publications on Darwin's frogs indexed in the Web of Science were published during 20010–2019, when a number of in situ and ex situ conservation projects were established for R. darwinii. Thus, we identified an opportunity for collaboration to provide efficient and cost-effective conservation outcomes for these unique and highly threatened frogs. In 2017 the Chilean section of the IUCN SSC Amphibian Specialist Group convened stakeholders to develop a conservation plan for Darwin's frogs, and as a result the Binational Conservation Strategy for Darwin's Frogs was launched in 2018. Here we summarize the process of the strategy's development, present its main findings and recommendations and discuss the major challenges and opportunities of implementation. This work adds to the scarce peer-reviewed literature on species conservation planning and seeks to stimulate its use as a biodiversity conservation tool.
Study area
The Austral temperate forests, which include the habitat of Darwin's frogs (32–47 °S), cover > 20 million ha, mainly in Chile but also in Argentina (4.6 and 16.0 million ha for R. rufum and R. darwinii, respectively; IUCN, 2019). Rhinoderma rufum is endemic to the coastal range of Chile at 0–500 m altitude (Bourke et al., Reference Bourke, Busse and Böhme2012; Soto-Azat et al., Reference Soto-Azat, Valenzuela-Sánchez, Collen, Rowcliffe, Veloso and Cunningham2013a; Cuevas, Reference Cuevas2014). Rhinoderma darwinii is found in both the coastal range of Chile (including Mocha Island and the Chiloé Archipelago) and the Andes of Chile and Argentina (Soto-Azat et al., Reference Soto-Azat, Valenzuela-Sánchez, Collen, Rowcliffe, Veloso and Cunningham2013a) at 0–1,340 m altitude (Úbeda & Pastore, Reference Úbeda and Pastore2015). Historical distributions of Rhinoderma spp. are shown in Fig. 1.
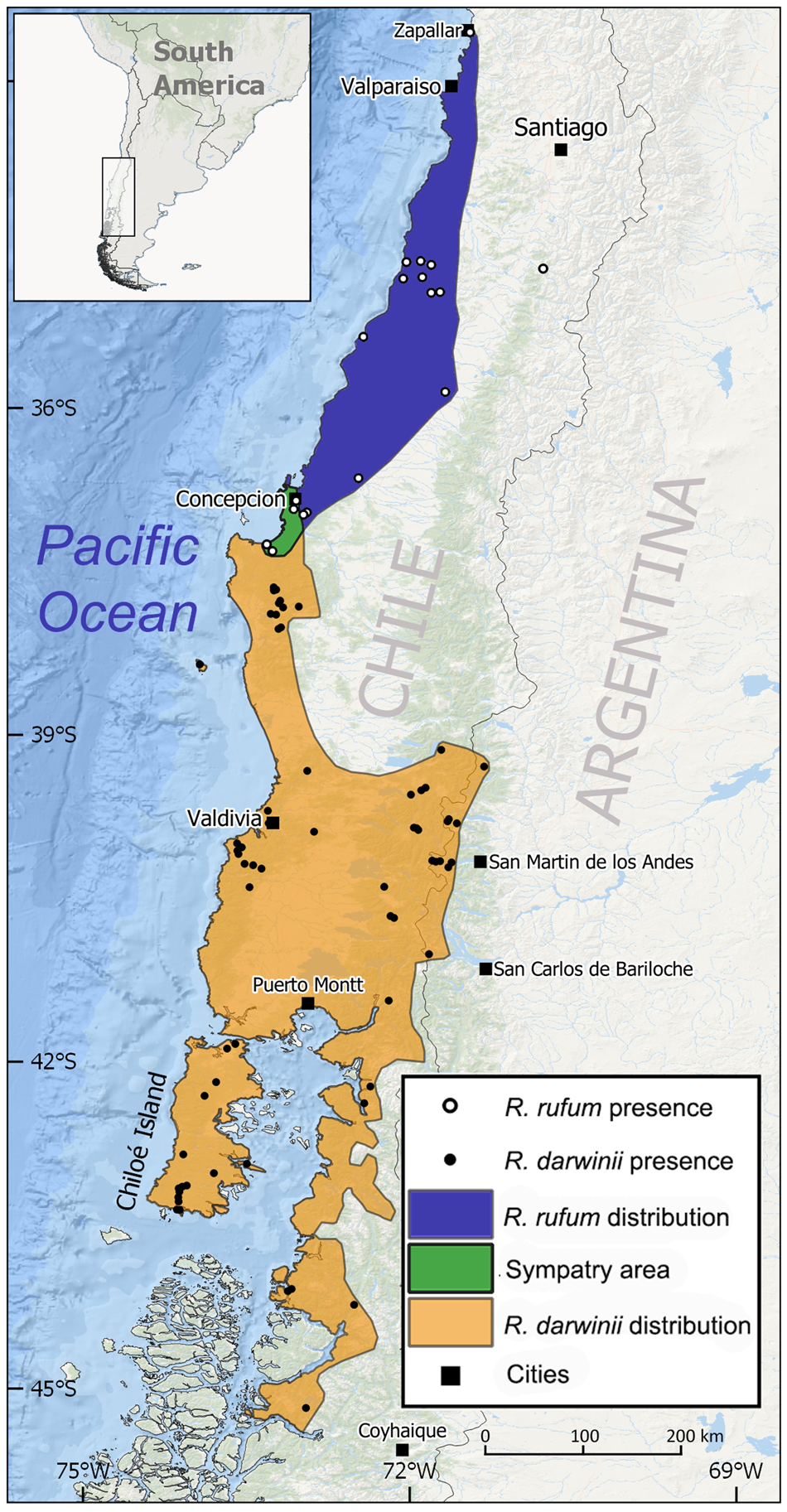
Fig. 1 Distribution of Darwin's frogs in Chile and Argentina. Historical distribution of Rhinoderma rufum, Rhinoderma darwinii and the area of sympatry around Concepción. Localities with known current presence of R. darwinii are shown in solid dots. No population of R. rufum is currently known, but historical localities are shown in open dots. There is one unusual historical record for R. rufum in the Chilean foothills of the Andes (Barros, Reference Barros1918).
Methods
Conservation assessment
In July 2015, 19 Chilean herpetologists met at Universidad Andres Bello in Santiago to update the IUCN Red List assessment of Chilean amphibians (Soto-Azat et al., Reference Soto-Azat, Valenzuela-Sánchez, Ortiz, Díaz-Páez, Castro and Charrier2015). This work highlighted the urgent need for conservation planning for Darwin's frogs. Re-assessments of R. darwinii and R. rufum followed the IUCN Red List methodology: literature and data searches, assessments by experts and external review.
Development of the strategy
Development of the Binational Conservation Strategy for Darwin's Frogs was facilitated by an Amphibian Specialist Group co-chair, a programme officer and a thematic group chair and followed IUCN guidelines for species conservation planning (IUCN, 2017). Initially, a 1-day symposium in September 2017 convened 292 participants interested in the conservation of Darwin's frogs. Here, 27 national and international speakers presented advances on Rhinoderma spp. research and conservation, and discussed IUCN guidelines for effective conservation planning (IUCN, 2017). Subsequently, 30 stakeholders that had been selected for their expertise, influence, and representation of relevant organizations, attended a 3-day conservation strategy workshop where we: (1) formulated the strategy's vision, (2) defined the strategy's time frame, (3) discussed the current status of Darwin's frogs, (4) assessed the challenges, barriers and threats to their conservation, (5) established working groups (see below), (6) developed conservation goals, objectives and actions, (7) prioritized objectives and actions and (8) elected the coordination structure. We formed four working groups based on identified conservation needs: (1) habitat loss, (2) captive breeding, research and climate change, (3) policy and education, and (4) diseases and invasive species. Following a SMART (Specific, Measurable, Achievable, Realistic and Time-bound) approach, each working group proposed goals, objectives and actions, which were later discussed, validated and prioritized by all workshop participants. After the workshop, a draft of the strategy was distributed among participants and others involved in the development of the strategy, for comment and approval.
Results
Conservation assessment
Rhinoderma rufum is categorized as Critically Endangered (Possibly Extinct) based on criterion D because its population size is estimated to be < 50 mature individuals (IUCN, 2019). The species has not been recorded since 1981 despite intense searches across its historical range (Busse, Reference Busse2002; Bourke et al., Reference Bourke, Busse and Böhme2012; Soto-Azat et al., Reference Soto-Azat, Valenzuela-Sánchez, Collen, Rowcliffe, Veloso and Cunningham2013a; Cuevas, Reference Cuevas2014). Rhinoderma darwinii is categorized as Endangered following criteria B2ab(iii) (IUCN, 2019) because (1) its current area of occupancy is estimated to be 224 km2 (< 500 km2 threshold; B2), (2) extant populations are small and isolated (a), and (3) the extent and quality of its remaining habitat continues to decline (b(iii); Crump & Veloso, Reference Crump, Veloso, Smith-Ramírez, Armesto and Valdovinos2005; Soto-Azat et al., Reference Soto-Azat, Valenzuela-Sánchez, Collen, Rowcliffe, Veloso and Cunningham2013a; Uribe-Rivera et al., Reference Uribe-Rivera, Soto-Azat, Valenzuela-Sánchez, Bizama, Simonetti and Pliscoff2017; Bourke et al., Reference Bourke, Busse and Böhme2018). Only R. darwinii has been kept and bred in captivity.
A conservation strategy
Under the vision ‘Darwin's frogs, unique in the world for their reproductive peculiarity, are conserved and valued as an emblem for the protection of the native forests of southern Chile and Argentina’, the Binational Conservation Strategy for Darwin's Frogs was launched in October 2018. The strategy is divided into two main components: a status review of Rhinoderma spp., and the conservation strategy itself, comprising a threat assessment (Fig. 2) and a list of prioritized conservation actions. The strategy aims to achieve the following goals by 2028: (1) obtain key information on the biology, management and status of Rhinoderma populations, (2) reduce the main threats to Darwin's frogs, and (3) provide the financial, legal and societal support needed for the proposed conservation actions. To this end, the strategy contains 39 actions, grouped under 12 objectives (Supplementary Table 1). Each conservation action lists responsible stakeholders, deadlines, indicators, potential collaborators and funding sources. The strategy (IUCN ASG–Chile, 2018) has been distributed among relevant authorities, conservation organizations, local communities and the general public.

Fig. 2 Conceptual model showing a threat assessment for Darwin's frogs (R. rufum and R. darwinii). We identified direct and indirect threats, barriers presented by lack of knowledge, contributing factors and pressures, and plotted their interactions with each other and within the binational conservation strategy. *OIE = World Animal Health Organization.
Website
The full Binational Conservation Strategy for Darwin's Frogs can be downloaded from the strategy's website (see full reference in IUCN ASG–Chile, 2018). This website provides information on Rhinoderma biology and conservation along with relevant literature and other resources. The strategy is intended to be a dynamic and adaptive document, and the website will help with the coordination of identified actions.
Darwin's Frog Alliance
A key outcome of the conservation planning process was the creation of the Darwin's Frog Alliance, a network of 47 individuals, representing 30 institutions and a diverse array of stakeholders (from academia, government, zoological institutions, local communities, NGOs and the private sector; Supplementary Table 2), to enhance collaboration for the conservation of Darwin's frogs. The Alliance is led by members of the Amphibian Specialist Group, and endorsed by the Chilean and Argentinian Ministries of Environment.
Threat assessment
The status review supported previous claims that the decline of Darwin's frogs has been largely driven by habitat loss, chytridiomycosis and climate change (Bourke et al., Reference Bourke, Ulmer, Mutschmann, Busse, Werning and Böhme2010, Reference Bourke, Busse and Böhme2012, Reference Bourke, Busse and Böhme2018; Soto-Azat et al., Reference Soto-Azat, Valenzuela-Sánchez, Collen, Rowcliffe, Veloso and Cunningham2013a, Reference Soto-Azat, Valenzuela-Sánchez, Clarke, Busse, Ortiz and Barrientos2013b; Uribe-Rivera et al., Reference Uribe-Rivera, Soto-Azat, Valenzuela-Sánchez, Bizama, Simonetti and Pliscoff2017; Valenzuela-Sánchez et al., Reference Valenzuela-Sánchez, Schmidt, Uribe-Rivera, Costas, Cunningham and Soto-Azat2017). Here we provide a brief synthesis of this review.
Status of populations
Using species distribution modelling, Bourke et al. (Reference Bourke, Busse and Böhme2012) identified areas with potential remnant R. rufum populations, providing guidance for future efforts to rediscover this species. Soto-Azat et al. (Reference Soto-Azat, Valenzuela-Sánchez, Collen, Rowcliffe, Veloso and Cunningham2013a) dated its extinction to 1982 (95% CI: 1980–2000) using historical sightings. In contrast, R. darwinii is found in small and isolated populations (Soto-Azat et al., Reference Soto-Azat, Valenzuela-Sánchez, Collen, Rowcliffe, Veloso and Cunningham2013a; Valenzuela-Sánchez et al., Reference Valenzuela-Sánchez, Cunningham and Soto-Azat2015). During the development of the strategy, we identified 56 extant populations in Chile and 10 in Argentina (Fig. 1). In Chile, R. darwinii has recently disappeared from, or drastically declined in, many localities where it was abundant only decades ago (Crump & Veloso, Reference Crump, Veloso, Smith-Ramírez, Armesto and Valdovinos2005; Soto-Azat et al., Reference Soto-Azat, Valenzuela-Sánchez, Collen, Rowcliffe, Veloso and Cunningham2013a). The size of extant populations is c. 10–145 reproductive individuals (Crump, Reference Crump2002; Soto-Azat et al., Reference Soto-Azat, Valenzuela-Sánchez, Collen, Rowcliffe, Veloso and Cunningham2013a; Valenzuela-Sánchez et al., Reference Valenzuela-Sánchez, Harding, Cunningham, Chirgwin and Soto-Azat2014, Reference Valenzuela-Sánchez, Schmidt, Uribe-Rivera, Costas, Cunningham and Soto-Azat2017, Reference Valenzuela-Sánchez, Schmidt, Pérez, Altamirano, Toledo and Pérez2019a). In Argentina, the species has been less well studied but, based on museum collections, it was probably much more abundant in the past (Úbeda & Pastore, Reference Úbeda and Pastore2015).
Habitat loss
The original habitats of R. rufum, the Coastal Mediterranean and Maulino deciduous forests (32–36 °S), have been almost completely replaced by exotic pine and eucalypt plantations and agriculture, with < 7% of Maulino forest remaining (Smith-Ramírez, Reference Smith-Ramírez2004; Echeverría et al., Reference Echeverría, Coomes, Salas, Rey-Benayas, Lara and Newton2006). The Valdivian temperate rainforest (36–47 °S) is the typical habitat of R. darwinii. To the north, the situation for R. darwinii is similar to that for R. rufum, but further south the native forest becomes more continuous as the coverage of protected areas increases, thus providing more suitable habitat for the species.
Amphibian chytridiomycosis
Caused by the fungus Batrachochytrium dendrobatidis, this emerging disease is known for its catastrophic and ongoing impacts on amphibian populations worldwide (Scheele et al., Reference Scheele, Pasmans, Berger, Skerrat, Martel and Beukema2019). This pathogen has been identified from museum specimens of wild Chilean amphibians collected since the 1970s (Soto-Azat et al., Reference Soto-Azat, Valenzuela-Sánchez, Clarke, Busse, Ortiz and Barrientos2013b). This coincides with the documented onset of South American amphibian declines (Scheele et al., Reference Scheele, Pasmans, Berger, Skerrat, Martel and Beukema2019). Surveys in Chile have demonstrated that B. dendrobatidis is infecting R. darwinii in the wild (Bourke et al., Reference Bourke, Ulmer, Mutschmann, Busse, Werning and Böhme2010), with evidence of lethal chytridiomycosis (Soto-Azat et al., Reference Soto-Azat, Valenzuela-Sánchez, Clarke, Busse, Ortiz and Barrientos2013b), which leads to extirpation of infected populations (Valenzuela-Sánchez et al., Reference Valenzuela-Sánchez, Schmidt, Uribe-Rivera, Costas, Cunningham and Soto-Azat2017).
Climate change
Because of its specific habitat requirements (Valenzuela-Sánchez et al., Reference Valenzuela-Sánchez, Schmidt, Pérez, Altamirano, Toledo and Pérez2019a), slow life-history strategy (Valenzuela-Sánchez et al., Reference Valenzuela-Sánchez, Schmidt, Uribe-Rivera, Costas, Cunningham and Soto-Azat2017) and dispersal limitations (Valenzuela-Sánchez et al., Reference Valenzuela-Sánchez, Harding, Cunningham, Chirgwin and Soto-Azat2014, Reference Valenzuela-Sánchez, Cayuela, Schmidt, Cunningham and Soto-Azat2019b), Rhinoderma spp. are expected to be particularly susceptible to climate change (Soto-Azat et al., Reference Soto-Azat, Valenzuela-Sánchez, Collen, Rowcliffe, Veloso and Cunningham2013a). Using a dispersal-constrained species distribution model, Uribe-Rivera et al. (Reference Uribe-Rivera, Soto-Azat, Valenzuela-Sánchez, Bizama, Simonetti and Pliscoff2017) estimated that during 1970–2010, climate change led to a reduction of suitable habitat for this species by 23–40%. Bourke et al. (Reference Bourke, Busse and Böhme2018) predicted an expansion of climatically suitable areas for R. darwinii by 2080, especially in the south of its range. However, unless assisted by translocations, R. darwinii would not naturally colonize most of the emerging suitable areas (Uribe-Rivera et al., Reference Uribe-Rivera, Soto-Azat, Valenzuela-Sánchez, Bizama, Simonetti and Pliscoff2017). Incorporating dispersal limitations analyses of climate change projections for 2050 and 2080 show reductions of 33–56% in the potential distribution of R. darwinii (Fig. 3; Uribe-Rivera et al., Reference Uribe-Rivera, Soto-Azat, Valenzuela-Sánchez, Bizama, Simonetti and Pliscoff2017).

Fig. 3 Boxplot (median, 25th, and 75th percentiles) of relative changes in the extent of potential habitat (suitable and accessible) of R. darwinii, projected to two temporal windows (2050 and 2080) and two climate change scenarios (Relative Concentration Pathway 4.5 and 8.5; IPCC, Reference Pachauri and Meyer2014). The dashed line represents a scenario of no change compared to the present situation.
Other threats
Collection of wild Rhinoderma spp., mainly for hobbyists and museums, was common in the past (J.C. Ortiz, pers. obs., 1988; Soto-Azat et al., Reference Soto-Azat, Valenzuela-Sánchez, Collen, Rowcliffe, Veloso and Cunningham2013a; Supplementary Fig. 1). Other threats and barriers include livestock farming in forest habitats, non-compliance with current legislation, and lack of public awareness and engagement (Fig. 2).
Ongoing conservation
Although there are 30 protected areas (28 in Chile and two in Argentina) within the range of R. darwinii, none protect any of the historical sites of R. rufum. Since 2009, three in situ conservation projects have been implemented for R. darwinii: in Huilo Huilo, Tantauco and Melimoyu private parks. Currently, there are two independent ex situ conservation projects for R. darwinii: one led by Universidad de Concepción in conjunction with Zoo Leipzig, Germany (since 2009), and another by the National Zoo of Chile (since 2010). There are also several education initiatives focused on Darwin's frogs: one at the National Zoo of Chile (visited by > 860,000 people in 2018), a Darwin's frog education centre in Huilo Huilo (> 100,000 visitors in 2018), and an education programme run by NGO Ranita de Darwin, which has reached > 15,000 people in local communities within the range of Rhinoderma spp.
Discussion
Multi-pronged strategies that combine research, management, education and policy are required to achieve successful species conservation (Superina et al., Reference Superina, Cortés Duarte and Trujillo2018). The Binational Conservation Strategy for Darwin's Frogs is a multi-sectoral, participatory effort and follows an evidence-based process to achieve the long-term conservation of Darwin's frogs. Additionally, this strategy promotes these species as non-traditional flagships for the conservation of the Austral temperate forest, which has been identified as one of the world's 25 biodiversity hotpots (Myers et al., Reference Myers, Mittermeier, Mittermeier, da Fonseca and Kent2000).
Conservation challenges
Habitat protection
The coastal range of central Chile has the greatest terrestrial species richness in the country, but almost entirely lacks protection and has experienced a rapid loss of biodiversity (Smith-Ramírez, Reference Smith-Ramírez2004), especially since the 1970s (Echeverría et al., Reference Echeverría, Coomes, Salas, Rey-Benayas, Lara and Newton2006). If R. rufum is rediscovered, it will be challenging to guarantee its immediate in situ protection, considering that it may occur on private land. In contrast, protected areas have allowed the persistence of R. darwinii. In Chile, 93% of known populations (52 out of 56; Fig. 1) are within areas with some level of protection, mostly private parks (43%). Although < 5% of the range of R. darwinii lies in Argentina, all known populations (10) in this country are in two large and contiguous national parks: Lanín and Nahuel Huapi (Úbeda & Pastore, Reference Úbeda and Pastore2015). Private reserves (which cover 1.5 million ha in central and southern Chile) are key for the conservation of Darwin's frogs. Similarly, a partnership with the forestry sector can boost the protection of Darwin's frog habitat. Forestal Arauco is the largest forestry company in South America (owning 1.4 million ha in Chile and Argentina) and a participating member of the Binational Conservation Strategy for Darwin's Frogs. Most of their land is planted with exotic pines and eucalypts, but > 110,000 ha of native forests are protected as conservation areas, five of which currently protect populations of R. darwinii (Arauco, 2017). Improving the conservation status of Darwin's frogs depends on increasing the area and connectivity of its habitat (Soto-Azat et al., Reference Soto-Azat, Valenzuela-Sánchez, Collen, Rowcliffe, Veloso and Cunningham2013a). The traditional approach to achieving this would be to create or expand protected areas (Smith-Ramírez, Reference Smith-Ramírez2004), but a novel initiative is being implemented in southern Chile. In collaboration with local landowners and regional government, NGO Ranita de Darwin promotes amphibian conservation by voluntary agreements (Ranita de Darwin, Reference Ranita de Darwin2020a) to encourage planting of native forest, habitat management and monitoring of the Darwin's frog population by landowners (Santangeli et al., Reference Santangeli, Arroyo, Dicks, Herzon, Kukkala and Sutherland2016).
Managing wildfires
Wildfires are considered an emerging threat to Darwin's frogs. During 2017 and 2018, central and southern Chile experienced the largest wildfires in recent history; > 500,000 ha were burnt in 2017, 20% of which involved native forest (CONAF, 2017). Climate change projections predict a trend of increasing damage by wildfires (Urrutia-Jalabert et al., Reference Urrutia-Jalabert, González, González-Reyes, Lara and Garreaud2018). Fire prevention or rapid containment is a key aspect of future conservation management plans for Rhinoderma spp.
Reintroductions
There are plans to reintroduce R. darwinii individuals from captive breeding projects to areas from which they have been extirpated, or to use them for population reinforcement. Translocation success will depend on evidence-based management of the threats that led to the extirpations or declines at the release sites (IUCN, 2013; Molina-Burgos et al., Reference Molina-Burgos, Valenzuela-Sánchez, Alvarado-Rybak, Klarian and Soto-Azat2018).
Policy and public engagement
The Chilean Ministry of Environment administers the legal instrument of Recovery, Conservation and Management (RECOGE) Plans to execute research, protection and conservation programmes for threatened species. The Ministry has been part of the development of the Binational Conservation Strategy for Darwin's Frogs since its inception; inclusion of RECOGE criteria in the strategy will facilitate its adoption. In Argentina, where R. darwinii is present only in two national parks, the National Park Administration is responsible for implementing the strategy. Once signed by the environment authorities of both countries, the strategy will facilitate interdisciplinary and international working amongst public agencies. Another area in which both countries can take action involves animal health departments, with the enforcement of amphibian import regulations and the implementation of biosecurity protocols aimed at limiting the spread of B. dendrobatidis (and other invasive species) both at national and local levels (Valenzuela-Sánchez et al., Reference Valenzuela-Sánchez, O'Hanlon, Alvarado-Rybak, Uribe-Rivera, Cunningham and Fisher2018; Bacigalupe et al., Reference Bacigalupe, Vásquez, Estay, Valenzuela-Sánchez, Alvarado-Rybak and Peñafiel-Ricaurte2019). Official recognition of the strategy is also relevant for establishing nation-wide conservation education programmes. Improving the public's knowledge of and attitudes towards Darwin's frogs will be critical to achieve the strategy's objectives (Márquez-García et al., Reference Márquez-García, Jacobson and Barbosa2018).
Future research
Studies on population trends and threats to R. darwinii in Argentina are lacking and little is known about the genetic diversity of Rhinoderma. There have been no genetic studies of R. rufum and limited information is available for R. darwinii (Crump & Veloso, Reference Crump, Veloso, Smith-Ramírez, Armesto and Valdovinos2005). A characterization of the genetic structure of Rhinoderma spp. across their range would inform in situ management and assessment of potential reintroductions using captive-bred animals (IUCN, 2013).
Other critical investigations include improving our understanding of two known major threats: amphibian chytridiomycosis and climate change (Soto-Azat et al., Reference Soto-Azat, Valenzuela-Sánchez, Clarke, Busse, Ortiz and Barrientos2013b; Uribe-Rivera et al., Reference Uribe-Rivera, Soto-Azat, Valenzuela-Sánchez, Bizama, Simonetti and Pliscoff2017). For R. darwinii, research is underway to assess mitigation actions addressing infections with B. dendrobatidis (Ranita de Darwin, Reference Ranita de Darwin2020b). Besides phenotypic plasticity, amphibians have two options to deal with climate change: evolutionary adaptation and dispersal (Uribe-Rivera et al., Reference Uribe-Rivera, Soto-Azat, Valenzuela-Sánchez, Bizama, Simonetti and Pliscoff2017). No information exists concerning evolutionary or phenotypic responses to rapid and ongoing climate change (IPCC, Reference Pachauri and Meyer2014); studies addressing this issue will allow us to predict, and potentially mitigate, the impacts of climate change on Rhinoderma.
Conclusions
The Binational Conservation Strategy for Darwin's Frogs is the first conservation strategy exclusively dedicated to amphibians at the species level in both Chile and Argentina. It provides an informative case study of a comprehensive programme for iconic, yet under-appreciated, fauna and an example of how small ectothermic animals can become flagship species for conservation, a role usually assigned to larger charismatic mammals. The development of the strategy is a constructive example of stakeholders, including local communities and industry, working together to generate a robust instrument to combat the amphibian extinction crisis. This multi-disciplinary conservation planning initiative is an effort to coordinate existing work in Chile and Argentina and to catalyse further conservation actions based on scientific evidence. Successful implementation of the strategy will help to ensure the long-term viability of these unique frogs and, by extension, of their habitat, the Austral temperate forest.
Acknowledgements
We thank Zoo Leipzig, Huilo Huilo Foundation, Universidad Andres Bello, Fundación MERI, Forestal Arauco, Darwin Vineyards and Cerveza Tropera for financial support for the development of the Binational Conservation Strategy for Darwin's Frogs, and Anne Baker (Amphibian Ark) for her support with the preparation of facilitation materials. CA and AV-S are supported by Fondecyt grants no. 1181758 and 3180107, respectively.
Author contributions
Writing: CA, AV-S, AAC; revision: all authors.
Conflict of interests
None.
Ethical standards
This work abided by the Oryx guidelines on ethical standards.






