Data on species occupancy, distribution and abundance across different habitats are fundamental for effective management and conservation. The scale of human perturbations, particularly habitat loss and degradation, is such that there is an urgent need to develop non-invasive survey techniques that can be deployed over large areas (Gompper et al., Reference Gompper, Kays, Ray, Lapoint, Bogan and Cryan2006).
Scat surveys paired with DNA-based analyses have been used to investigate occupancy and food habits of a variety of rare species, particularly carnivores, and to derive abundances and obtain data on species distribution and habitat use (Piggott & Taylor, Reference Piggott and Taylor2003; Wilson et al., Reference Wilson, Frantz, Pope, Roper, Burke, Cheeseman and Delahay2003; Walker et al., Reference Walker, Novaro and Branch2007; Napolitano et al., Reference Napolitano, Bennett, Johnson, O’Brien, Marquet and Barría2008).
The success of scat surveys in temperate climates, often conducted by specially trained dogs (Smith et al., Reference Smith, Ralls, Hurt, Adams, Parker and Davenport2003; Long et al., Reference Long, Donovan, Mackay, Zielinski and Buzas2007), has generated interest in using scat surveys to determine patterns of habitat use in tropical areas (Gonzalez & Duarte, 2007; Furtado et al., Reference Furtado, Carrillo-Percastegui, Jácomo, Powell, Silveira, Vynne and Sollmann2008). However, to compare species occupancy between habitats the detection probability of scats should be the same across strata but coprophagy by vertebrates can violate such assumptions in temperate regions (Livingston et al., Reference Livingston, Gipson, Ballard, Sanchez and Krausman2005).
In addition to vertebrate consumers scats are removed, to varying degrees, by insects in the tropics (Freymann et al., Reference Freymann, Buitenwerf, Desouza and Olff2008; Nichols et al., Reference Nichols, Spector, Louzada, Larsen, Amezquita and Favila2008) with removal of fresh faeces by dung beetles occurring within minutes (D. Norris, pers. obs.). Such removal is likely to restrict the effectiveness of scat surveys as there is no way to compare the occurrence of species or individuals between habitats with different coprophagous vertebrate/invertebrate communities. We conducted scat removal experiments to answer the following questions: (1) does scat removal vary between different habitat types, and (2) does scat type influence these removal rates?
Experiments were conducted in three sites surrounding the town of Alta Floresta, Mato Grosso State, Brazil, during June–September 2008 (see Michalski et al., Reference Michalski, Nishi and Peres2007, for further study area details). To investigate the influence of scat type we obtained samples from two species with contrasting diets: carnivore (jaguar) and omnivore (human). Human and jaguar scat samples were placed along 50-m transects in three habitats at each site: forest > 1,000 ha, riparian corridor connecting forest areas > 1,000 ha, and pasture. Each transect was placed 50 m perpendicular to the border with the pasture matrix. Pasture transects were placed 50 m from the border with any forest type. As our narrowest corridor was 100 m wide it was not possible to position transects further from the border. To ensure spatial independence transects were separated by a minimum of 500 m. In each transect we deployed three sample sites separated by 25 m. Sample sites were a circular area of 50 cm in diameter cleared of dead sticks and loose leaves. Jaguar scats were obtained from five captive-bred individuals (with complete veterinary history and up to date vaccinations) in the Parque Zoológico de Sapucaia do Sul, Rio Grande do Sul, Brazil. The diet of the captive individuals was raw meat, including bones and fur, allowing direct comparison with wild counterparts. Fresh jaguar scats were collected by zoo staff, avoiding direct handling, frozen and transported to our study area where they were kept frozen. To ensure freezing did not bias removal rates between scat types, human scats (from two individuals) were kept frozen for the same length of time.
Samples were prepared the day before each experiment by homogenizing the scats required for each type so that samples of the same type had the same consistency and composition. Scats were refrigerated overnight in plastic moulds to provide a standardized form and quantity (50 g per sample). Two samples were placed at each site. One was simply placed and the other, as a control, was protected from insects. Each control was covered with a plastic net (2-mm squares) supported by a plastic frame forming a protective tower (15–20 cm high). The net and frame were embedded in the ground, preventing insects accessing the sample but retaining exposure to sun and wind. Our experiments were carried out during the dry season and therefore rainfall did not influence removal.
We had a total of 108 samples (open n = 54 and control n = 54) in 18 transects (nine with human and nine with jaguar scats) across three habitat types (two transects per habitat) in three sites (Fig. 1). Samples were placed between 05.55 and 06.15 hours and checked at intervals of +1, +2, +4, +8 and +24 hours. At each sampling interval we recorded the presence of insects and whether the sample had been removed or not.

Fig. 1 Location of the study region in northern Mato Grosso (see black rectangle on inset for location in Brazil), showing transects with jaguar (white fill) and human (black fill) scat samples in three habitat types.
We used a generalized linear model (GLM, with binomial error distribution) to investigate the relationship between sampling interval and removal probability (Fig. 2). We compared the timing of two events: (1) ≥ 50% and (2) 100% removal. As no samples in pasture habitats or controls experienced removal > 50%, they were not included in this analysis. The GLM revealed that the variance of the probability increased with the mean; therefore we explored the response of complete sample removal (binary, n = 54) to influences of habitat and scat type modelled as fixed effects within a generalized linear mixed effect model (GLMM). We modelled sampling interval and transect as uncrossed random effects to account for non-normal (autocorrelated) errors resulting from the grouping of samples and repeated sampling (Pinheiro & Bates, Reference Pinheiro and Bates2000). All analyses were carried out with R v. 2.8.1 (R Development Core Team, 2008).
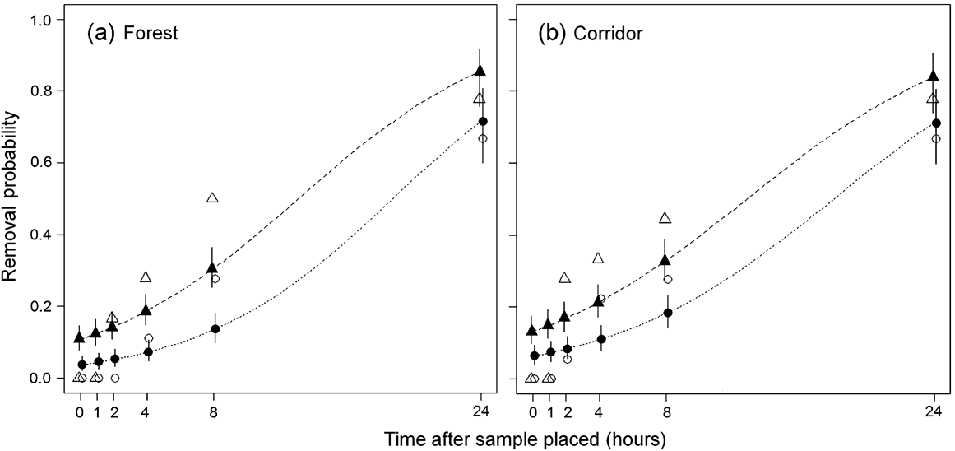
Fig. 2 Comparison of scat removal probability during 24 hours in forest and corridor habitats. The probability of > 50% removal (GLM predictions: dashed line and solid triangles; observed means: open triangles) and 100% removal (GLM predictions: dotted line and solid circles; observed means: open circles) of human and jaguar scat samples in (a) forest (n = 18) and (b) corridor (n = 18) habitats.
Of the 54 open samples 24 (44%) were removed within 24 hours across all habitats (Fig. 2). The removal of scats showed a strong association with both habitat and scat type. No samples were removed in pasture habitats whereas 12 of 18 samples (67%) in both forest and corridor transects were removed (Fig. 2). Human scats accounted for 15 (62.5%) of the 24 samples removed. Our GLMM showed that habitat type had a stronger influence than scat type, with both forest (slope = 6.10 ± SE 2.25), Z = 2.71, P < 0.01) and corridor (slope = 6.80 ± SE 2.25, Z = 3.02, P < 0.01) having significantly higher removal compared with pasture. Human scat samples had a higher removal compared with jaguar (slope = 2.92 ± SE 1.49, Z = 1.95, P < 0.05) with the lower Z values suggesting a weaker influence compared with habitat type.
The removal of samples was most strongly correlated with the presence of dung beetles (polychoric correlation rho = 0.60) compared with the presence of individuals from other insect orders (polychoric correlation rho: lepidoptera, 0.31; coleoptera excluding dung beetles, 0.19; hymenoptera, 0.11; orthoptera, -0.02 and diptera, -0.57). From the 24 samples removed, dung beetles (tunnellers and rollers) were directly observed removing 17 (71%) and ants were present on the other seven but were only directly observed removing one jaguar scat.
Insect communities, particularly dung beetles, directly influenced detection probability of scat samples. We found a clear contrast between pasture and forested habitats but the differences in removal probability both between habitats and species are likely to be conservative. Previous studies have shown that the composition of tropical dung beetle communities is strongly influenced by distance from edge (Didham et al., Reference Didham, Hammond, Lawton, Eggleton and Stork1998). Placing samples at 50 m from the border between forest and pasture therefore means our samples in forest habitats were exposed to a subset of species, limiting our ability to detect differences between forest and corridor habitat types.
Generally, our results agree with previous findings that scat removal rates vary with habitat and scat type in temperate climates (Livingston et al., Reference Livingston, Gipson, Ballard, Sanchez and Krausman2005); however, in our study, the primary removal agents were insects rather than vertebrates. Livingston et al. (Reference Livingston, Gipson, Ballard, Sanchez and Krausman2005) also found strong seasonal effects on scat removal caused by rainfall and variation in inter/intraspecific coprophagy. Although seasonal effects were beyond the scope of our study, we predict that removal rates will also vary in tropical regions that have seasonal climates and/or seasonal variation in species' diet. The consistency in results across temperate and tropical regions suggests that similar patterns of variation in removal rates will occur in a variety of biomes.
In addition to varying removal rates there are a number of other known biases that scat surveys should address. Not only are there differences in observers (both human and canine) but the visibility and detection of scat samples are also likely to vary between habitats. For example, a human observer will probably be able to detect more samples in open forest compared with pasture areas (where pasture tufts conceal scat samples), whereas dogs will have a higher detection probability in pasture areas where the wind can carry scents over greater distances (Wasser et al., Reference Wasser, Davenport, Ramage, Hunt, Parker, Clarke and Stenhouse2004). It is therefore necessary to standardize survey efforts (which could be done post-survey via the analysis of a subset of samples that were collected within a fixed effective detection distance from a survey transect) to compare results between habitats.
Results from non-invasive scat surveys have the potential to address fundamental conservation and management questions. However, based on removal rates in tropical forested habitats, such techniques may not be cost effective for species that occur at low densities. Additionally, surveys that rely on non-invasive sampling of scat samples in the tropics must account for differences in scat removal rates between habitats and target species before conclusions can be drawn regarding patterns of habitat use. To do this, we recommend that any monitoring programme that uses scat surveys must apply a standardized methodology similar to that presented here to quantify and incorporate such differences into species occupancy models.
Acknowledgements
This research was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo to FM (FAPESP: 2007/01252-2), the Wildlife Conservation Society, Conservation, Food and Health Foundation, Cleveland Metroparks Zoo and The Cleveland Zoological Society, and The Rufford Small Grants Foundation. We thank Raquel Von Hohendorff and staff at the Parque Zoológico de Sapucaia do Sul for providing jaguar scat samples. We are deeply indebted to the landowners who contributed to this study and to Geraldo Araújo and Linda Maria Weber for their field assistance. Rob Ewers and Toby Gardner provided valuable suggestions on experimental design, and comments from two anonymous referees improved this article.
Biographical sketches
Darren Norris studies spatial and temporal risk analysis and investigates the effects of forest fragmentation on Neotropical mammals. His current research includes monitoring the distribution of giant and Neotropical otters. Fernanda Michalski has a particular interest in the ecological consequences of habitat fragmentation and conservation biology. For the past 8 years she has been developing and coordinating research projects in the Brazilian Amazon that explore faunal and floral responses to anthropogenic perturbations.




