There has been no confirmed sighting of the Javan tiger Panthera tigris sondaica since the 1970s and the subspecies has been declared extinct (Seidensticker, Reference Seidensticker, Tilson and Seal1987; Jackson & Nowell, Reference Jackson and Nowell1996, Reference Jackson and Nowell2008). In 2019 a putative encounter with a tiger was reported near a community plantation in West Java, and DNA from a single hair sample collected from a fence nearby was subsequently analysed (Wirdateti et al., Reference Wirdateti, Yulianto, Raksasewu and Adriyanto2024). Our re-examination of the genetic data from the paper raises concerns regarding the credibility of the data and hence the reliability of the conclusion.
Wirdateti et al. amplified and sequenced a 1,043 bp cytochrome b mitochondrial DNA (mtDNA) segment from the hair collected and compared it to those of leopards and tiger subspecies of known origin, including a Javan tiger museum specimen collected in 1930. Phylogenetic trees showed that the hair sample aligned most closely with the Javan tiger museum specimen, forming a clade distinct from other tiger subspecies and the Javan leopard. Based on the results the authors concluded that the hair belongs to the Javan tiger, implying that this tiger subspecies is not extinct. However, after reanalysing the data presented by Wirdateti et al., we conclude there is no support for the authors' conclusions, for the following three reasons: (1) the sequences that the authors obtained are not genuine tiger mtDNA, (2) the sequences are probably nuclear pseudogene copies of mtDNA (Numt), and (3) the sequences generated from the putative and the control Javan tiger specimens are more divergent from one another than the mean difference between other tiger sequences, yet readers cannot evaluate the reliability of the original data because few details concerning quality control were provided in the paper.
Firstly, the sequences that the authors obtained are not tiger mtDNA segments. In the paper, the genetic clade including the hair sample in question (NCBI Accession OQ601561.1) and the Javan tiger museum specimen from 1930 (OQ601562.1) is an outgroup to the tiger mtDNA clade and is phylogenetically equidistant from both tigers and leopards, which is a pattern that was not observed in previous studies involving the Javan tiger (Xue et al., Reference Xue, Yamaguchi, Driscoll, Han, Bar-Gal and Zhuang2015; Sun et al., Reference Sun, Liu, Tiunov, Gimranov, Zhuang and Han2023). To investigate this issue, we conducted a phylogenetic analysis of the two putative Javan tiger sequences produced by Wirdateti et al., along with published mtDNA sequences from Panthera species (28 Panthera tigris including one Panthera tigris sondaica museum specimen, three Panthera pardus, three Panthera leo, three Panthera onca and three Panthera uncia; Table 1). We used MUSCLE 5.1 (Edgar et al., Reference Edgar2022) for multi-sequence alignment of the 42 sequences. We manually trimmed 72 bp from both sides of the alignment, resulting in a 971 bp nucleotide DNA sequence matrix without missing data.
Table 1 Panthera species mitogenome DNA sequences assessed in this study.
We constructed a maximum likelihood phylogenetic tree using IQ-TREE 2.3.0 (Nguyen et al., Reference Nguyen, Schmidt, von Haeseler and Minh2015), with the HKY+G model selected by jModelTest 2.1.10 (Darriba et al., Reference Darriba, Taboada, Doallo and Posada2012), and we evaluated statistical support based on 10,000 bootstraps. Prima facie, our results (Fig. 1) appear to recapitulate the pattern documented by Wirdateti et al. (Reference Wirdateti, Yulianto, Raksasewu and Adriyanto2024), in which the clade including OQ601561.1 and OQ601562.1 is an outgroup of the tiger mtDNA clade. The clade exhibits an unusually elongated branch length in comparison to those of all other tiger subspecies. This pattern was not observed in previous studies based on partial (Xue et al., Reference Xue, Yamaguchi, Driscoll, Han, Bar-Gal and Zhuang2015) or full (Sun et al., Reference Sun, Liu, Tiunov, Gimranov, Zhuang and Han2023) mtDNA sequences from Javan tiger specimens of known origin, and therefore suggests that the two sequences generated by the authors do not originate from Javan tiger mtDNA.
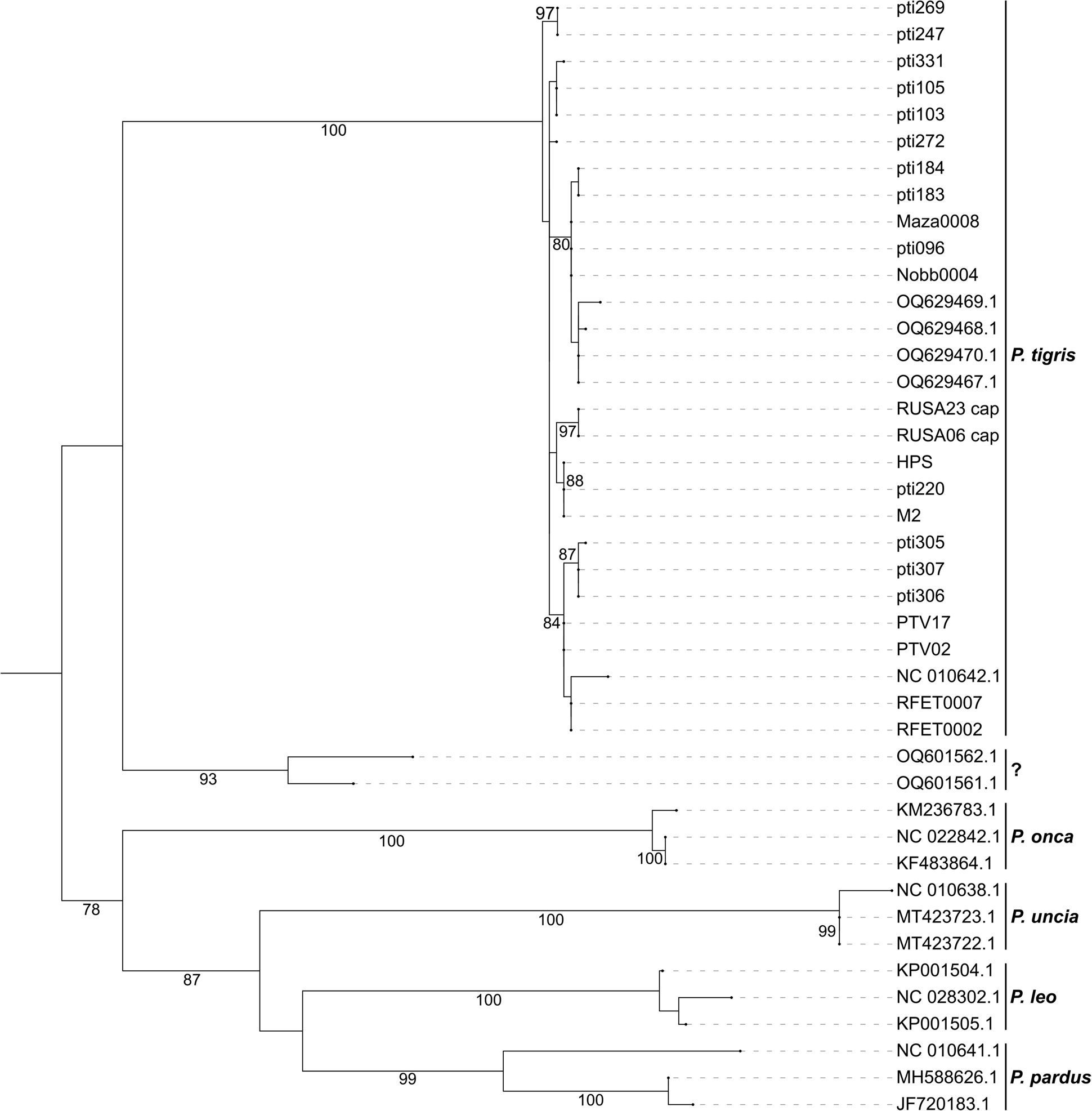
Fig. 1 A maximum likelihood phylogenetic tree inferred from the 971-bp mtDNA cytochrome b sequence assessed in this study. Haplotypes are labeled by their sample IDs. The sequences in question (OQ601561.1 and OQ601562.1) are marked with a question mark. Nodes with bootstrap values larger than 70% based on 10,000 bootstrap replicates are labelled. The reference Panthera tigris sequences included all nine subspecies (Table 1).
We further evaluated the pairwise genetic distances (p-distances) amongst the sequences using Biopython 1.83 (Cock et al., Reference Cock, Antao, Chang, Chapman, Cox and Dalke2009). The mean distance amongst the 28 published tiger mtDNA sequences is 5.645 × 10−3 ± SD 2.733 × 10−3 (378 pairwise distance calculations), whereas the mean distance between the putative Javan tiger sequences generated by the authors and the published tiger mtDNA sequences is 0.07353 ± SD 2.872 × 10−3 (56 pairwise distance calculations), which is 13 times greater than the mean between-tiger genetic distance. For comparison, the mean mtDNA genetic distance between a non-tiger Panthera species and a tiger is 0.1049 ± SD 4.854 × 10−3 (336 pairwise distance calculations), which is only slightly greater than the level of genetic distance between the putative Javan tiger and published tiger subspecies.
From the perspectives of both phylogenetic pattern and genetic distance, the two putative Javan tiger sequences generated by the authors exhibit significant disparities from the mtDNA sequences of all tiger subspecies, including the published Javan tiger mtDNA haplotype (Maza0008; Sun et al., Reference Sun, Liu, Tiunov, Gimranov, Zhuang and Han2023). Such differences cast doubt on the genuine mtDNA origins of the two Javan tiger sequences. It is improbable for these two sequences to have originated from tiger mtDNA (let alone Javan tiger mtDNA). This would explain why the two sequences do not cluster with other tigers.
Secondly, the sequences that the authors obtained are probably nuclear mtDNA segments. Nuclear mtDNA pseudogene segments (Numts) result from the transfer of cytoplasmic mtDNA (Cymt) copies into the nuclear DNA, a scenario that is often found in the tiger and Panthera genomes (Luo et al., Reference Luo, Kim, Johnson, van der Walt, Martenson and Yuhki2004; Kim et al., Reference Kim, Antunes, Luo, Menninger, Nash, O'Brien and Johnson2006). Given their common origin, there is a possibility that both Cymt and Numt segments can be amplified. This issue is particularly common in the Panthera genus as Numt co-amplifications frequently appear in Cymt-targeting PCR experiments in these species (Zhang et al., Reference Zhang, Zhang, Shen, Hou, Lv and Yue2006; Creecy et al., Reference Creecy, Coil and Hickey2024). In GenBank, some pseudogene tiger Numt sequences are mistakenly labelled as tiger Cymt sequences (Morgan et al., Reference Morgan, Ewart, Nguyen, Sitam, Ouitavon and Lightson2021).
A BLASTn 2.14.1 (Camacho et al., Reference Camacho, Coulouris, Avagyan, Ma, Papadopoulos, Bealer and Madden2009) search against the latest tiger genome assembly pti1_mat1.1 (NCBI Accession GCF_018350195.1) indicates Numts are the most probable sources of OQ601561.1 and OQ601562.1. The best (NC_056676.1:5,568,675-5,569,645) and second best (NC_056676.1:5,585,283-5,586,253) matched regions of OQ601561.1 and OQ601562.1 are both located on an autosomal scaffold corresponding to tiger chromosome F2. The nucleotide sequence identities of these matches are all > 97.4% across the 971 bp trimmed sequences, whereas the similarity to the tiger mtDNA (NC_010642.1) is ≤ 92.5%. In contrast, mtDNA segments from a previously published Javan tiger sequence (Maza0008; Sun et al., Reference Sun, Liu, Tiunov, Gimranov, Zhuang and Han2023) and the Sumatran tigers Panthera tigris sumatrae acquired by the authors (OQ629467.1 and OQ629468.1) matched the tiger mtDNA, with > 98.75% sequence similarity (Fig. 1, Table 2). These results suggest that the two supposed Javan tiger sequences generated by the authors are not completely derived from Cymt but more likely from Numt or a mixture of the two.
Table 2 BLASTn results of the mtDNA sequences assessed in this study.
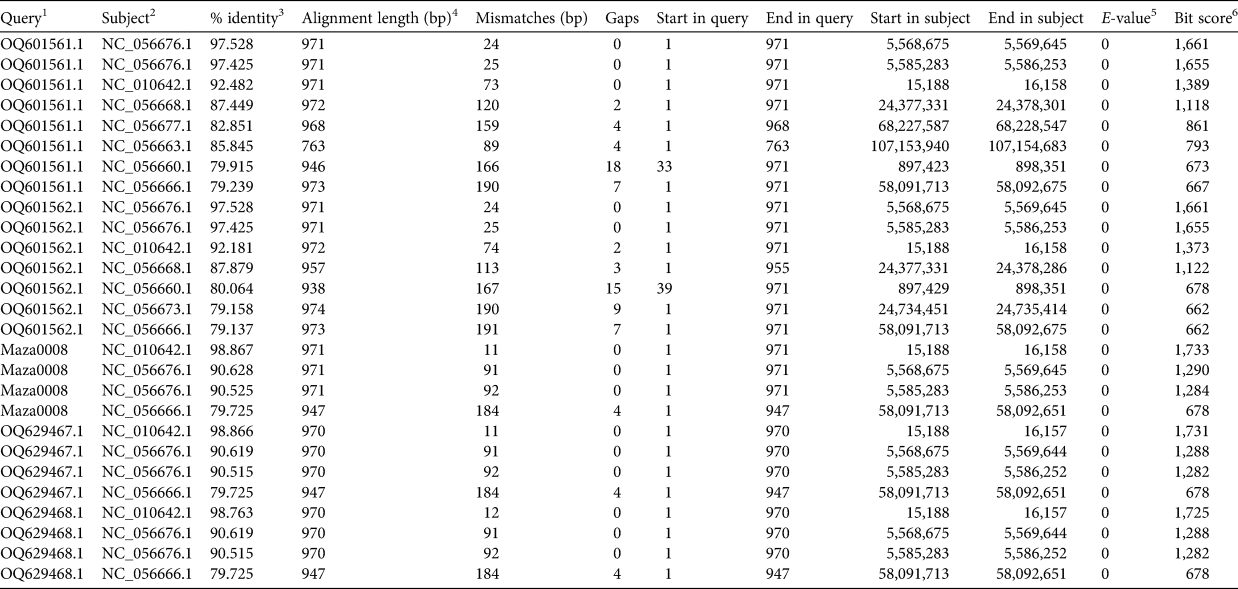
1 The queries include the putative mtDNA sequences of Wirdateti et al. (Reference Wirdateti, Yulianto, Raksasewu and Adriyanto2024) and one previously published Javan tiger mtDNA sequence (Sun et al., Reference Sun, Liu, Tiunov, Gimranov, Zhuang and Han2023).
2 The tiger reference genome.
3 The per cent of identity across the aligned sequence between Query and Subject.
4 The sequence length of the aligned sequence between Query and Subject.
5 The statistical significance of the sequence alignment between Query and Subject.
6 Evaluation of the alignment quality.
Thirdly, the high variant rate in the putative Javan tiger sequences prompted concerns related to data accuracy and quality control. There are 24 mismatches in the 971 bp sequence between the hair and the museum Javan tiger specimen, corresponding to a genetic distance of 2.473 × 10−2. The sequences are twice as divergent from one another as from the mean pairwise difference between other tiger mtDNA haplotypes, or 10 times more divergent than amongst Sunda tigers. In population genomic analyses including all tiger subspecies, only 196 variants were found across the mtDNA (15.5 kb in length with the control region removed), which is c. 12.6 variants per 1,000 bp (Liu et al., Reference Liu, Sun, Driscoll, Miquelle, Xu and Martelli2018). The genetic difference amongst the Javan, Bali Panthera tigris balica and Sumatran tigers from Sundaland is even lower, with 44 variants across the 15.5 kb mtDNA sequence, corresponding to c. 2.84 variants per 1,000 bp (Sun et al., Reference Sun, Liu, Tiunov, Gimranov, Zhuang and Han2023). For the tiger nuclear DNA, the single-nucleotide variant rate in different subspecies varies, ranging between 0.026% and 0.072%, which equates to 0.26–0.72 variants per 1,000 bp (Liu et al., Reference Liu, Sun, Driscoll, Miquelle, Xu and Martelli2018). Regardless of their mitochondrial or nuclear DNA origins, the presence of such a large number of variant sites between the putative Javan tiger sequences generated by the authors is unusual for two homologous sequences that are both from tigers, and this is indicative of data unreliability.
There are various potential reasons for these errors, but they cannot be identified based on the information provided by Wirdateti et al. (Reference Wirdateti, Yulianto, Raksasewu and Adriyanto2024). Processing DNA from single hair or museum specimens requires stringent precautions, including contamination prevention, elimination of potential inhibitors, multiple replications to exclude non-specific stochastic amplifications from trace amounts of the DNA template, and data quality measurements, amongst others. For instance, residual hair keratin could inhibit PCR and Sanger sequencing reactions, hence reducing the efficiency and quality of DNA sequence analysis (Schrader et al., Reference Schrader, Schielke, Ellerbroek and Johne2012). However, we are not able to determine from the article whether the DNA extraction and downstream experiments were handled with the precautions that are required for working with degraded genetic material, nor how such precautions might have been implemented. As few details with regard to quality control are provided, it is inappropriate to use these sequences to draw conclusions regarding the existence of the Javan tiger.
The report of the rediscovery of the Javan tiger by Wirdateti et al. (Reference Wirdateti, Yulianto, Raksasewu and Adriyanto2024) garnered widespread attention from the general public as well as amongst scientists and conservationists. We would all be thrilled to learn that the Javan tiger is not extinct and we agree with the authors that ‘[w]hether the Javan tiger actually still occurs in the wild needs to be confirmed with further genetic and field studies’ (Wirdateti et al., Reference Wirdateti, Yulianto, Raksasewu and Adriyanto2024, p. 472). However, the authors' initial conclusions based on DNA analysis of one putative tiger hair sample are more likely to be erroneous than to reflect the survival of the Javan tiger, because of the flawed experimental design employed and the lack of scientific stringency. Clear and reliable visual, physical or genetic evidence will be required to conclude that the Javan tiger still survives in Java nearly half a century since the last confirmed sighting.
Author contributions
Study design: S-JL; data analysis: Z-YS, S-JL; writing: all authors.
Acknowledgements
We thank the general public, media, scientists and the conservation community for their support of our efforts to defend scientific rigor.
Conflicts of interest
None.
Ethical standards
This research abided by the Oryx guidelines on ethical standards.
Data availability
All the data are published, available in GenBank and on Github at github.com/xinsun1/Xin_etal_2023_NEE_TigerPopGen.




