Introduction
Biodiversity conservation is essential for the maintenance of ecosystem services that guarantee survival of wildlife and the quality of human life. To achieve biodiversity conservation, stakeholders must work under a common agenda to put in place actions to protect biodiversity (Bisseleua et al., Reference Bisseleua, Missoup and Vidal2009; Tilman et al., Reference Tilman, Isbell and Cowles2014). Effective conservation decision-making is guided by scientific information on species (Mace et al., Reference Mace, Norris and Fitter2012), the currency of conservation (Rylands & Mittermeier, Reference Rylands and Mittermeier2014). Baseline data on species is therefore paramount for biodiversity conservation.
Deforestation is recognized as the major threat to terrestrial mammals (IUCN, 2019). Forest loss and fragmentation reduce the habitat of forest-dependent mammals and restrict their populations to small, isolated patches (Fahrig, Reference Fahrig2003), leading to population declines and local extinctions (Estrada et al., Reference Estrada, Garber, Rylands, Roos, Fernandez-Duque and Di Fiore2017). Accordingly, conservation science has focused on the impacts of deforestation on species distributions and abundance, and one of the main practical outcomes has been the protection of large tracts of forests (e.g. Soulé & Simberloff, Reference Soulé and Simberloff1986): the patch area paradigm in conservation.
Large tracts of forests, however, are currently rare in tropical forest hotspots (areas with a high proportion of endemic plants and extensive vegetation loss; Myers et al., Reference Myers, Mittermeier, Mittermeier, Fonseca and Kent2000) and the majority of remnants are small (< 100 ha) and embedded in human-modified landscapes (Turner & Corlett, Reference Turner and Corlett1996). In the absence of funding for large-scale reforestation, conservation of mammals in tropical forest hotspots thus needs to focus on a multitude of small forest fragments. The synergistic effects of habitat loss, degradation and fragmentation on arboreal mammals need to be examined, to inform strategies for population management and species conservation (Cassano et al., Reference Cassano, Barlow and Pardini2012; Gestich et al., Reference Gestich, Arroyo-Rodríguez, Ribeiro, Cunha and Setz2019), especially in small forest fragments.
Such information is fundamental for facilitating conservation actions beyond the creation of protected areas and for understanding how forest degradation affects arboreal mammals in fragmented landscapes, a relationship little studied (Mortelliti et al., Reference Mortelliti, Amori and Boitani2010). For mammals that depend on high quality forests, the availability of well conserved habitats may be critical (Henle et al., Reference Henle, Davies, Kleyer, Margules and Settele2004).
Here we investigate the influence of patch and landscape structure on the occurrence of the coastal black-handed titi monkey Callicebus melanochir in the Atlantic Forest hotspot (sensu Myers et al., Reference Myers, Mittermeier, Mittermeier, Fonseca and Kent2000), using this species as a model for arboreal mammals inhabiting fragmented landscapes in tropical forest hotspots. Neotropical primates are strictly forest-dependent (Cowlishaw & Dunbar, Reference Cowlishaw and Dunbar2000), directly and negatively affected by deforestation (Estrada et al., Reference Estrada, Garber, Rylands, Roos, Fernandez-Duque and Di Fiore2017), and thus are appropriate models to study the impacts of forest reduction and fragmentation on the occurrence of forest-dependent mammals.
We hypothesize that (1) patch area, quality and visibility, and landscape connectivity, affect the probability of occurrence of our model species, with patch area having the strongest influence (Fig. 1a); (2) all four patch–landscape metrics positively influence the probability of the species' occurrence (Fig. 1b); and (3) compound models (patch area plus each of the other metrics) will significantly increase model weights (Fig. 1c). Based on our findings, we discuss the usefulness of patch–landscape metrics in predicting the occurrence of titi monkeys and potentially other arboreal mammals in tropical forest hotspots and the application of such metrics for science-based conservation action.
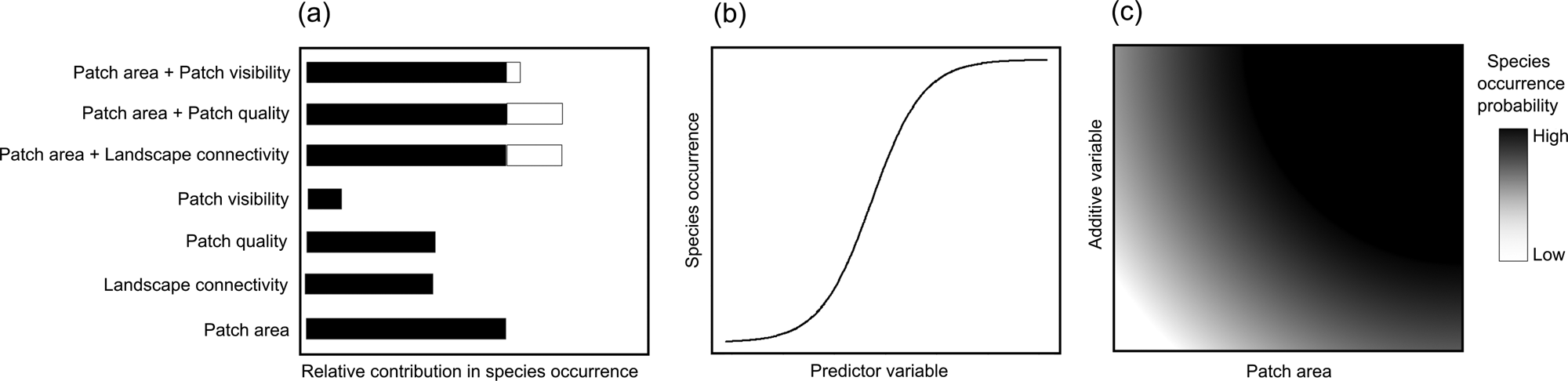
Fig. 1 Hypothesized influence of patch area, quality and visibility, and landscape connectivity, on the occurrence of the arboreal black-handed titi monkey Callicebus melanochir in a fragmented Atlantic Forest landscape (Fig. 2): (a) relative contribution of univariate (black bars) and compound models (black, patch area contribution; white, additive variable contribution) in explaining species’ occurrence; (b) expected response of the species occurrence to predictor variables in univariate models; (c) expected response of species occurrence synthesized for the three compound models having patch area and an additive variable.
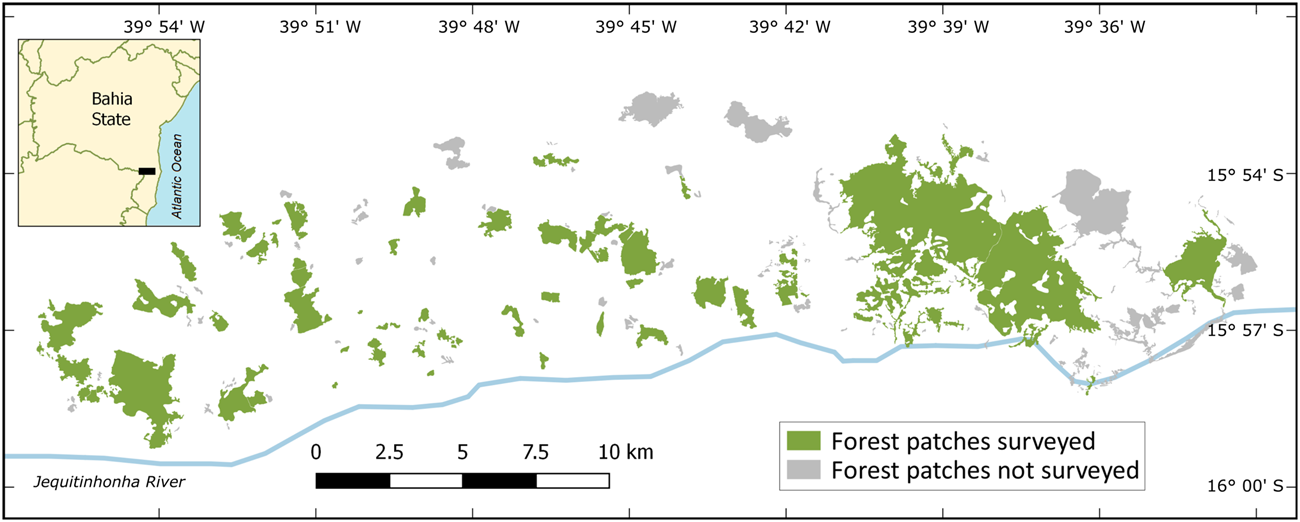
Fig. 2 Fragmented landscapes of the study area on the north margin of the Jequitinhonha River in Itapebi and Itarantim municipalities, Bahia, Brazil.
Study area
This study was conducted in fragmented landscapes of the Atlantic Forest hotspot, southern Bahia State, Brazil (Fig. 2). The study area encompasses semi-deciduous forests in a transition zone between coastal wet forests and inland deciduous forests within the Atlantic Forest domain (Thomas, Reference Thomas, Prado, Landau, Moura, Pinto, Fonseca & and Alger)2003). Anthropogenic land-use conversion since the 1970s has transformed the originally continuous wet forests of the interior of southern Bahia into small fragments embedded in a matrix of pastures for cattle ranching (Coimbra-Filho & Câmara, Reference Coimbra-Filho and Câmara1996; Landau, Reference Landau, Prado, Landau, Moura, Pinto, Fonseca and Alger)2003). The study area is therefore similar to other landscapes in the Atlantic Forest (Landau, Reference Landau, Prado, Landau, Moura, Pinto, Fonseca and Alger)2003; Ribeiro et al., Reference Ribeiro, Metgzer, Martensen, Ponzoni and Hirota2009) and other tropical forest hotspots (Turner & Corlett, Reference Turner and Corlett1996) regarding past processes and current patterns of forest loss and fragmentation.
Methods
Model species
The coastal black-handed titi monkey Callicebus melanochir (Plate 1) is endemic to the Atlantic Forest of north-east Brazil (Culot et al., Reference Culot, Pereira, Agostini, Almeida, Alves and Aximoff2019). It is an arboreal species that occasionally moves on the ground and can disperse over short distances in non-forested areas (Mason, 1986; Souza-Alves et al., Reference Souza-Alves, Mourthé, Hilário, Bicca-Marques, Regh and Gestich2019), lives in groups comprising a monogamous pair and offspring, and occupies home ranges of 22–24 ha (Müller, Reference Müller1995; Heiduck, Reference Heiduck2002). The species is categorized on the IUCN Red List as Vulnerable to extinction as a result of habitat loss and fragmentation (Veiga et al., Reference Veiga, Printes, Ferrari, Kierulff, de Oliveira and Mendes2008) but the response of individuals to such habitat modifications has not been studied.

Plate 1 A typical group of Callicebus melanochir, observed in the study area (Fig. 2). Photo: Rodrigo Costa-Araújo.
Field surveys
During March 2013–December 2014 (72 field days) surveys of the titi monkey were carried out in 38 fragments using playback censuses and interviews (following Jerusalinsky et al., Reference Jerusalinsky, Oliveira, Pereira, Santana, Bastos and Ferrari2006; Printes et al., Reference Printes, Rylands and Bicca-Marques2011). The fragments studied were randomly selected to ensure complete coverage of the variation in the area of fragments. For playback censuses, recordings of the long-calls of C. melanochir were played along pre-existing trails and forest edges using a mini amplifier connected to an MP3 player (Jerusalinsky et al., Reference Jerusalinsky, Oliveira, Pereira, Santana, Bastos and Ferrari2006; Printes et al., Reference Printes, Rylands and Bicca-Marques2011). We successfully used Callicebus barbarabrownae calls during a short pilot study prior to the survey, to detect C. melanochir and to record the species’ calls, as these were not formerly available. In the absence of standard protocols for playback surveys of titi monkeys in fragmented landscapes, we developed an approach to avoid any potential detection bias from differential propagation of sound as a result of the variable vegetation structure of forest fragments. We modulated the distance between playbacks (50–150 m) in each fragment to allow sound overlap between adjacent sampling points and improve our detection rates, controlling for sound overlap with the aid of a field assistant. At each sampling point one long-call (2 minutes 45 seconds) was played; when titi monkeys responded with vocalization only, the same long-call was played up to three subsequent times to stimulate their approach. Informal interviews were conducted with local people in the vicinity of forest fragments and with citizens in nearby communities, to collect additional data on the species' occurrence. The long-call of C. melanochir is distinctive from any other primate in the region, can be heard at a distance of several kilometres from the source. Recent reports (< 5 years) of the species' vocalization were therefore considered as accurate and included in our data.
Patch and landscape metrics
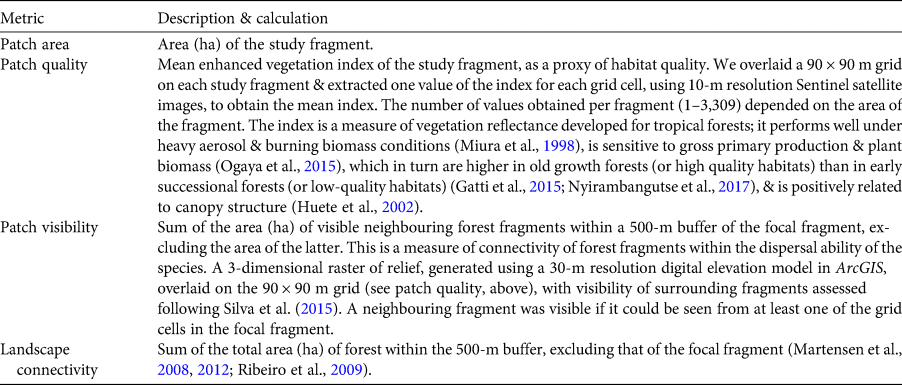
We adopted a patch–landscape perspective to investigate the responses of our model species to forest loss, degradation and fragmentation (following Arroyo-Rodríguez & Mandujano, Reference Arroyo-Rodríguez and Mandujano2009; Arroyo-Rodríguez & Fahrig, Reference Arroyo-Rodríguez and Fahrig2014), considering that the spatial scale within which populations are affected in a fragment relates to the dispersal ability of individuals (Jackson & Fahrig, Reference Jackson and Fahrig2015). We delimited forest fragments by interpreting high-resolution (< 1 m) images from the online World Imagery available through ArcGIS 10.2 (Esri, Redlands, USA) and the Open Layer plugin of QGIS 2.14 (QGIS, 2014). Fragments within 100 m of each other were considered a unique patch, assuming that titi monkeys can move across this distance in pastures (Souza-Alves et al., Reference Souza-Alves, Mourthé, Hilário, Bicca-Marques, Regh and Gestich2019). We defined the scale of the effect of forest loss and fragmentation by setting a buffer of 500 m around the edges of study fragments, assuming a conservative estimate of the maximum dispersal recorded for titi monkeys in open areas (400 m; Mason, Reference Mason and Jay1968), thus ensuring independence of the landscapes analysed. We calculated three patch-level metrics and one landscape-level metric (Supplementary Fig. 1) using the packages raster, rgeo and gtools in R 3.5.0 (Hijmans & Etten, Reference Hijmans and Etten2012; Warnes et al., Reference Warnes, Bolker and Lumley2015; Bivand et al., Reference Bivand, Rundel, Pebesma, Stuetz and Hufthammer2017). The forest fragments surveyed vary in area, quality and connectivity (Supplementary Table 1), and therefore our study area is representative of landscapes in the Atlantic Forest and other tropical forest hotspots. All four metrics (Table 1) were first tested for autocorrelation (Pearson r < 0.7; Supplementary Fig. 2).
Table 1 Patch and landscape metrics used as predictor variables in univariate and compound models of occurrence of the arboreal black-handed titi monkey Callicebus melanochir in a fragmented landscape of Atlantic Forest, Bahia State, Brazil (Fig. 2).
Data analysis
We first defined a set of univariate models considering species occurrence as a response variable and each patch–landscape metric (patch area, quality and visibility, and landscape connectivity) as a predictor variable, using linear generalized models with a binomial distribution. Because we hypothesized that patch area would have a major, but not ubiquitous, influence on the occurrence of arboreal mammals, we also defined a set of compound models combining patch area plus each of the other metrics, again with species occurrence as the response variable. We avoided models with more than two predictors because of potential difficulties with interpretation. We compared the performance of competing models within univariate and within univariate plus compound models, including also a null model representing random occurrence, using the Akaike information criterion corrected for small sample size (AICc), using the bbmle package in R. We considered univariate and compound models with ΔAICc ≤ 2.0 as equally plausible for explaining titi monkey occurrence; additionally we verified model weights to identify the best model among equally plausible models (Burnham & Anderson, Reference Burnham and Anderson2002). To identify which predictor variables are more informative within each plausible compound model, we estimated the beta coefficients of the predictor variables using the confint function of the bbmle package. Absence of zero within the estimated 95% confidence interval of a predictor variable indicates a strong effect of that variable (Gelman & Hill, Reference Gelman and Hill2007).
Results
Considering the data from playback surveys and interviews, we recorded C. melanochir in 15 of the 38 study fragments. During playback censuses we recorded groups of 2–3 individuals in areas of native forest and, for the first time, in areas of shaded cocoa crops embedded in the forest fragments.
All four patch–landscape metrics showed a positive relationship with the occurrence of our model species in generalized linear models, as we expected. The best-supported univariate model was patch area, demonstrating that occurrence of our model species is primarily driven by the area of forest (Fig. 3a, Table 2, Supplementary Table 2).
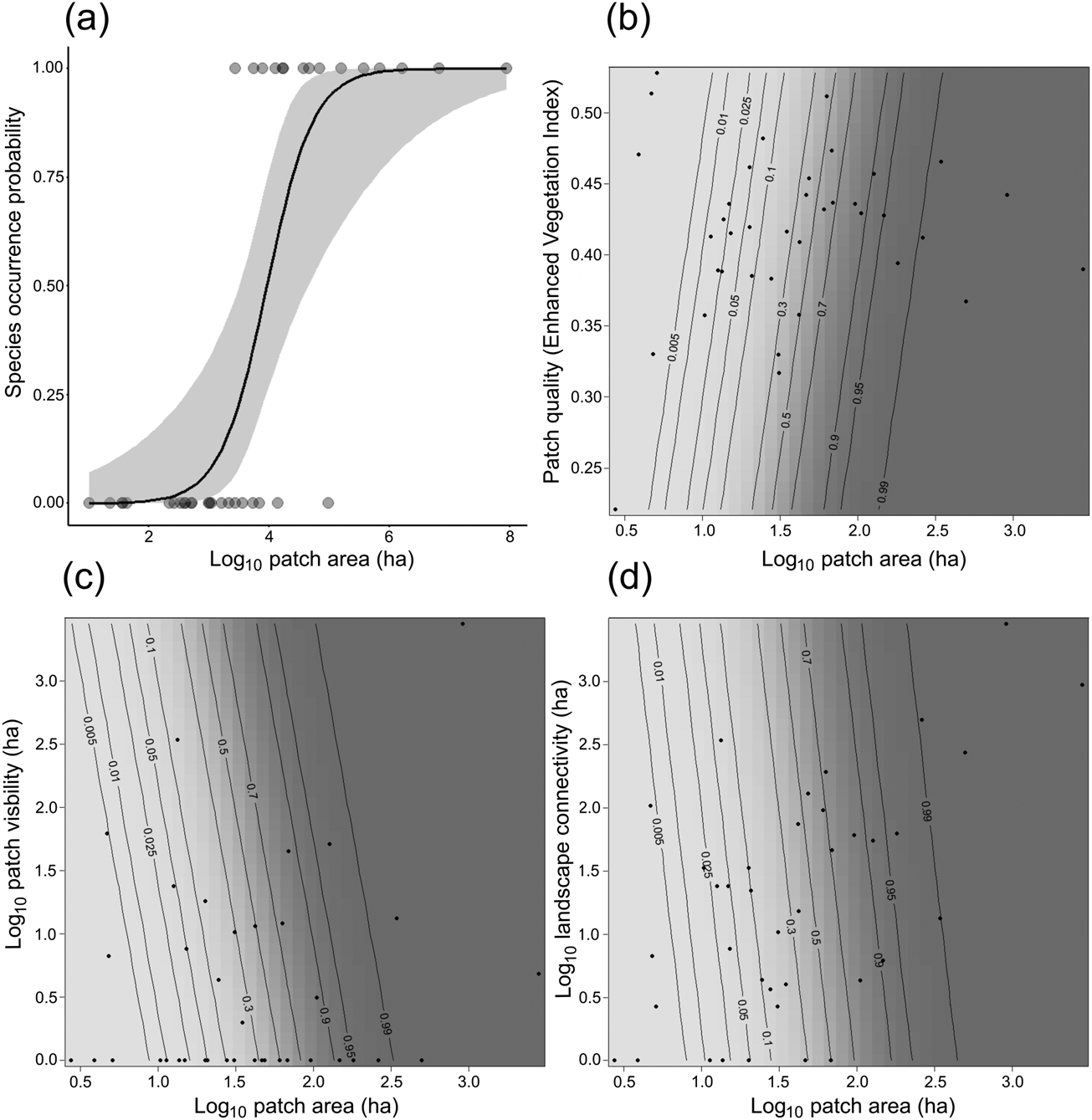
Fig. 3 Probability of occurrence of C. melanochir in fragmented landscapes in the Atlantic Forest (Fig. 2) according to predictor variables in univariate (a) and in compound models (b–d). The black lines represent the probability of species occurrence and the grey shaded area the 95% CI (a), and the response surface for the predicted species occurrence as a function of patch area and the additive variables (b–d).
Table 2 Values of the Akaike information criterion corrected for small sample size (AICc) for the relative contribution of univariate and compound models in explaining the occurrence of the arboreal C. melanochir in a fragmented landscape in the Atlantic Forest. Plausible models were identified within the set of univariate models and within the set of univariate plus compound models.
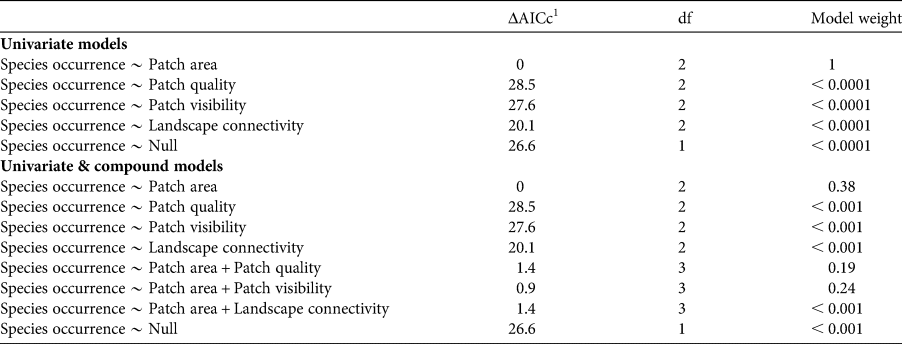
1 ΔAICc, difference between the AICc value of a given model and the model with the lowest AICc in each set.
The results from compound model selection (Table 2, Supplementary Table 2) showed an additive effect of other variables beyond the effect of patch area, demonstrating that species occurrence is also influenced by patch quality and visibility, and landscape connectivity. However, the additive variables had a weak contribution in the compound models. In Fig. 3b–d we present the response surface for the predicted occurrence of titi monkeys as a function of the compound models.
Discussion
Our findings confirm that patch area has a major effect on the occurrence of our arboreal model species C. melanochir: the larger the forest fragment, the higher is the probability of occurrence. The relationship between the area of forest fragments and the occurrence of arboreal mammals is well documented (Harcourt & Doherty, Reference Harcourt and Doherty2005; Magioli et al., Reference Magioli, Ribeiro, Ferraz and Rodrigues2015), with evidence from studies on primates (Arroyo-Rodríguez et al., Reference Arroyo-Rodríguez, Mandujano and Benítez-Malvido2008; Sharma et al., Reference Sharma, Madhusudan and Sinha2013; Silva et al., Reference Silva, Ribeiro, Hasui, Costa and Cunha2015; Carretero-Pinzón et al., Reference Carretero-Pinzón, Defler, McAlpine and Rhodes2016), carnivores (Michalski & Peres, Reference Michalski and Peres2005; Nagy-Reis et al., Reference Nagy-Reis, Nichols, Chiarello, Ribeiro and Setz2017), marsupials and rodents (Nupp & Swihart, Reference Nupp and Swihart2000; Linnell et al., Reference Linnell, Davis, Lesmeister and Swingle2017), bats (Muylaert et al., Reference Muylaert, Stevens and Ribeiro2016), and ungulates and shrews (Lawes et al., Reference Lawes, Mealin and Piper2000).
Additionally, forest quality has a positive effect on the occurrence of our model species, suggesting that forest quality is an important trait of forest fragments, balancing the effect of reduced area. Therefore, patch quality could be useful for predicting occurrence of arboreal mammals and for managing their conservation, especially in fragments of < 100 ha, which are common in tropical forest hotspots (Turner & Corlett, Reference Turner and Corlett1996; Ribeiro et al., Reference Ribeiro, Metgzer, Martensen, Ponzoni and Hirota2009).
To measure habitat quality from the perspective of a single forest-dependent species is challenging, and species-specific measurements of habitat quality can rarely be extrapolated to other taxa (Mortelliti et al., Reference Mortelliti, Amori and Boitani2010). To overcome this, we suggest the use of remotely sensed vegetation indices as proxies of forest quality for arboreal taxa in investigations of occupancy patterns and in selection of priority fragments for conservation across tropical forest hotspots. There are three advantages of this approach: (1) the enhanced and normalized difference vegetation indices are sensitive to gross primary production and plant biomass (Ogaya et al., Reference Ogaya, Barbeta, Basnou and Peñuelas2015), which are higher in old-growth forests (or high quality habitats) than in early successional forests (or low quality habitats) (Gatti et al., Reference Gatti, Castaldi, Lindsell, Coomes, Marchetti and Maesano2015; Nyirambangutse et al., Reference Nyirambangutse, Zibera, Uwizeye, Nsabimana, Bizuru and Pleijel2017); (2) as our results show, there is a positive relationship between the enhanced vegetation index and the model species occurrence; and (3) such indices can be obtained from freely available satellite images.
Connectivity of arboreal mammal populations in fragmented landscapes may be maintained by dispersal events based on opportunities for visualization of surrounding habitat patches, as indicated by the positive effect of patch visibility on the occurrence of our model species and of C. personatus (see Silva et al., Reference Silva, Ribeiro, Hasui, Costa and Cunha2015). Patch visibility offers a new way to evaluate connectivity between populations of arboreal mammals in fragmented landscapes and its importance in explaining species occurrence should be further explored using other taxa and landscapes. The use of spatial memory can also play a role in dispersal events between forest fragments but, to our knowledge, this issue has not yet been investigated.
Considering the positive effect of patch visibility, which incorporates visibility modulated by landscape relief and linear distance, on the occurrence of our model species, it is likely that such arboreal mammals disperse across pastures between visible forest fragments that are close to each other (see also Moraes et al., Reference Moraes, Ruiz-Miranda, Galetti-Jr, Niebuhr, Alexandre and Muylaert2018). Traveling over short distances between visible forest fragments, rather than dispersing over long distances may be a result of the high predation pressure arboreal mammals are exposed to during dispersal across open areas (Vuren & Armitage, Reference Vuren and Armitage1994; Sakai & Noon, Reference Sakai and Noon1997).
Callicebus melanochir may be persisting in the study area as a result of metapopulation dynamics, with the large fragments being sources and the nearby smaller fragments that are visible being sinks. As arboreal mammals, we expect that isolated populations of C. melanochir have gone extinct in the study area as a result of the Allee effect (Stephens et al., Reference Stephens, Sutherland and Freeckleton1999), genetic factors and stochastic events, considering that three titi monkey generations (Veiga et al., Reference Veiga, Bóveda-Penalba, Vermeer, Tello-Alvarado and Cornejo2011) have passed since the stabilization of forest conversion into pastures in this region in the 1990s (Coimbra-Filho & Câmara, Reference Coimbra-Filho and Câmara1996).
Our study shows that forest area is the strongest predictor of the occurrence of our model species, C. melanochir. We also found positive effects of forest quality, visibility and landscape connectivity on the species. We recommend that further research should consider the effect of forest quality on the occurrence of arboreal mammals in tropical forest hotspots and we encourage the use of remotely sensed vegetation indices as proxies of habitat quality. The adoption of the visibility metric may offer insights on the occurrence of arboreal mammals and on the connectivity of populations. In addition to forest area, the use of forest quality and visibility, and landscape connectivity, can improve detection of arboreal mammal populations and also aid in the selection of fragments and the types of actions for species conservation at local and regional scales in fragmented landscapes in tropical forest hotspots. Large remnants of tropical forest hotspots are scarce and therefore we require baseline data to support alternative conservation actions and management in small fragments.
Acknowledgements
This study was financed in part by the Coordenação Nacional de Desenvolvimento Científico e Tecnológico (140039/2018-1; 153423/2016-1; 312045/2013-1; 312292/2016-3), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (001, 527091; 20131509; PROCAD 88881.068425/2014-0, PNPD 88882.306330/2018-01), Fundação de Amparo à Pesquisa do Estado do Amazonas, Fundação de Amparo à Ciência e Tecnologia de Pernambuco (BFP-0149-2.05/19) and Fundação de Amparo à Pesquisa do Estado de São Paulo (2013/50421-2). We thank R.C. Printes for providing the recording of a C. barbarabrownae long-call and for valuable comments on surveying titi monkey, M. Lapenta, Rubi and Juni for field assistance, the people who granted access to their lands, Martin Fisher for his critique, and two anonymous reviewers for their valuable comments.
Author contributions
Conception: RC-A, ALR, MCR; data collection design, fieldwork: RC-A; mapping and quantification of landscape structure indices: ALR, FM; data analysis: ALR, MCR, FM; writing: RC-A, with input from ALR, JPS-A, TH, MCR.
Conflicts of interest
None.
Ethical standards
Fieldwork followed the code of best practice of the International Society of Primatologists, and otherwise abided by the Oryx guidelines on ethical standards.








