Introduction
The proboscis monkey Nasalis larvatus is endemic to the island of Borneo. The species is a large, sexually dimorphic, arboreal colobine, living in social groups that typically consist of a single adult male and multiple females (although mixed-sex groups occasionally contain several adult males). There are also all-male groups, and males are sometimes solitary. The species is categorized as Endangered on the IUCN Red List (IUCN, 2008), and listed under Appendix I of CITES (UNEP-WCMC, 2003). In Sabah, the species is listed as Totally Protected, which prohibits hunting, trading and keeping in captivity (Sabah Wildlife Enactment 1997). Despite this level of legal protection, habitat loss and fragmentation continue to be major threats to the species across its range (Meijaard & Nijman, Reference Meijaard and Nijman2000a,Reference Meijaard and Nijmanb; Boonratana, Reference Boonratana, Marsh and Chapman2013). The proboscis monkey's lowland swamp forest habitat is threatened by logging and conversion to oil palm (Meijaard & Nijman, Reference Meijaard and Nijman2000a; Gaveau et al., Reference Gaveau, Sloan, Molidena, Yaen, Sheil and Abram2014). Because of its preference for habitats along waterways (Matsuda et al., Reference Matsuda, Tuuga and Higashi2010b, Reference Matsuda, Tuuga and Bernard2011), the loss of lowland swamp forest has had a considerable impact on this species.
Population viability analysis based on data collected during 2003–2009 assessed three proboscis monkey populations in Malaysian and Indonesian Borneo (Stark et al., Reference Stark, Nijman, Lhota, Robins and Goossens2012). It predicted that the Malaysian population (Kinabatangan region) would remain relatively stable, whereas the two Indonesian populations (Balikpapan Bay and Danau Sentarum National Park) would decrease > 50% within 50 years, with one population in Danau Sentarum National Park predicted to be extinct within 30 years (Stark et al., Reference Stark, Nijman, Lhota, Robins and Goossens2012).
We assessed changes in population abundance and group size of proboscis monkeys in the Lower Kinabatangan floodplain, in eastern Sabah in Malaysian Borneo, during 2004–2014. We also examined the results of repeated river surveys of subpopulations at three long-term study sites, Rasang, Menanggul and Abai, in the Kinabatangan basin. We suspected that the number of proboscis monkeys had declined over the 10-year period because of habitat degradation and conversion of forests to oil palm plantations (Abram et al., Reference Abram, MacMillan, Xofis, Ancrenaz, Tzanopoulos and Ong2016). Habitat loss, fragmentation and degradation affect the spatial and temporal abundance and the dispersion of food sources; we therefore also examined possible changes in group size and structure (Chapman et al., Reference Chapman, Chapman and Wrangham1995; Janson & Goldsmith, Reference Janson and Goldsmith1995; Clutton-Brock & Janson, Reference Clutton-Brock and Janson2012). We discuss the conservation implications of our results and suggest protection measures for this species.
Study area
The Lower Kinabatangan Wildlife Sanctuary is located along the Kinabatangan river in Sabah (Fig. 1). With a length of 560 km, this is the longest river in Sabah; its 16,800 km2 catchment area covers the central and south-central uplands down to the east coast of Sabah, where it meets the Sulu Sea. Designated as a wildlife sanctuary and gazetted in 2005, the Sanctuary consists of 10 fragmented forest blocks covering 270 km2, comprising seasonal and tidal swamp forest, permanent freshwater swamp, mangrove forest, and lowland dipterocarp forest (Abram et al., Reference Abram, Xofis, Tzanopoulos, MacMillan, Ancrenaz and Chung2014). In a Sabah state-wide population survey in 2004, the population of the proboscis monkey in the Lower Kinabatangan was estimated to be 1,454 (Sha et al., Reference Sha, Bernard and Nathan2008). Hunting pressure is low in the area because the communities are Muslim and people do not therefore generally hunt this species. However, this is not the case for people from outside the local communities or for other regions of Borneo (Meijaard & Nijman, Reference Meijaard and Nijman2000a).

Fig. 1 Map of Lower Kinabatangan (eastern Sabah, Malaysian Borneo), showing natural vegetation (mostly forest) found outside the protected areas (Lower Kinabatangan Wildlife Reserve and forest reserves), areas that have been lost since 2005, survey routes in 2004 and 2014, and long-term monitoring areas.
Methods
Survey of Kinabatangan river and tributaries
Surveys by boat in the late afternoon or early morning are considered the most effective method for studying proboscis monkeys because they typically sleep in riverside trees (Matsuda et al., Reference Matsuda, Tuuga and Higashi2010b), but surveys in the afternoon provide more reliable counts than at other times (Matsuda et al., Reference Matsuda, Otani, Bernard, Wong and Tuuga2016). Therefore, whereas Sha et al. (Reference Sha, Bernard and Nathan2008) combined surveys in the late afternoon (16.30–sunset) and early morning (sunrise–08.30), along the Kinabatangan river and its tributaries (hereafter ‘wide area’) we carried out surveys only in the late afternoon. We conducted 18 boat surveys covering 186.2 km on 14 field days during 4–26 September 2014 (Fig. 1). The mean distance covered per boat survey was 10.3 ± SD 3.8 km.
We surveyed each stretch of the river only once, travelling as far as possible from 16.30 to sunset, and continued the next day from where the previous survey ended (Sha et al., Reference Sha, Bernard and Nathan2008). Rivers and tributaries in close proximity to each other were usually covered in one session or on consecutive days to reduce the probability of replicating the counts. Each survey was conducted by at least one main observer and one assistant. When we discovered a group or individual proboscis monkey, we switched off the boat engine to avoid disturbing the animals and paddled closer to record their numbers, age class and sex. We recorded the locations of sightings and the survey routes using a global positioning system (GPS). It is possible that some individuals travelled between the surveys and were counted more than once. However, proboscis monkeys usually stay within a limited area near riverbanks (< 100 m) for several weeks or months (Matsuda et al., Reference Matsuda, Kubo, Tuuga and Higashi2010a), and considering the long distances covered on each survey day along continuous sections of rivers, it is unlikely that there were any duplicate sightings.
River survey in three long-term monitoring sites
In addition to the population survey along the Kinabatangan river (wide area), we carried out river surveys in late afternoon at three long-term monitoring areas. These were: (1) Rasang (hereafter ‘mainstream’), a riverine forest, survey length 13.2 km, 149 surveys carried out 3.7 ± SD 1.2 times per month during March 2007–May 2015 (40 months), (2) Menanggul (hereafter ‘tributary’), a riverine forest around a tributary of the Kinabatangan river, survey length 6.0 km, 798 surveys carried out 11.0 ± SD 5.7 times per month during May 2005–August 2014 (73 months), and (3) Abai (hereafter ‘mangrove’), a mangrove forest, survey length 13.1 km, 121 surveys carried out 3.6 ± SD 1.1 times per month during July 2010–June 2015 (34 months) (Fig. 1).
Statistical modelling of population dynamics at the long-term monitoring sites
To examine the population trend of the proboscis monkey for each subpopulation based on time-autocorrelated datasets, we developed a common Bayesian state–space model that incorporated a random-walk model to generate autocorrelated time-series sequences for all observed populations (Supplementary Material 1).
Forest loss
We digitized natural vegetation cover, which comprised forest, degraded areas, nipa palm Nypa fruticans and swamp, from Landsat 2005 and 2014 satellite imagery using ArcGIS 10.3 (Esri, Redlands, USA). To evaluate natural vegetation types for the 2014 dataset, we modified existing data from 2010/2011 by extracting data for 2014 forest areas (Abram et al., Reference Abram, Xofis, Tzanopoulos, MacMillan, Ancrenaz and Chung2014). For oil palm, we updated 2010/2011 data from Abram et al. (Reference Abram, Xofis, Tzanopoulos, MacMillan, Ancrenaz and Chung2014) using the 2014 Landsat image and divided the oil palm areas into two categories: (1) oil palm, which included areas with good canopy, cleared areas, and planted/young oil palm, and (2) unproductive areas of planted oil palm, which are typically in seasonal or tidal flood-prone areas and where the planted palms die off from extended periods of inundation (Abram et al., Reference Abram, Xofis, Tzanopoulos, MacMillan, Ancrenaz and Chung2014).
We used the river survey routes to define the start and end points of surveyed areas, and digitized the riverbanks using 2.5-m satellite imagery (SPOT-5 for 2010/2011; Planet Action, 2012) to estimate the extent of these land use and vegetation cover types within the surveyed areas. We then defined the proboscis monkey's potential range (using the buffer tool in ArcGIS) for the surveyed transect area by setting a boundary 800 m from the riverbank, the maximum distance at which proboscis monkeys have been observed before returning to the riverbanks in the Lower Kinabatangan (Matsuda et al., Reference Matsuda, Tuuga and Higashi2010b).
Land tenure of unprotected forests in 2014
We used the digitized cadastral data developed by Abram et al. (Reference Abram, Xofis, Tzanopoulos, MacMillan, Ancrenaz and Chung2014) to estimate the overlap between unprotected forests and allocated land titles for oil palm. Land titles were digitized from publicly available cadastral maps. The land title types were (1) Native title: allocated to native individuals in perpetuity for agricultural purposes and < 40 ha (Sabah Land Ordinance, 2010), (2) Country land title: alienated state land for commercial agriculture under a 99-year lease (Sabah Land Ordinance, 2010), (3) State land that has been demarcated, with boundaries but no identity code, and assumed to be under review but not alienated, and (4) State land that has not been demarcated, assumed to have no title applications and not alienated as of the last update of the available cadastral map.
Oil palm expansion in unprotected forests
To estimate the extent of unprotected forest that is unsuitable for oil palm cultivation in the proboscis monkey's potential range area for 2014, we classified all forest types within the mangrove system, seasonally flooded forest system, and limestone forest as ‘unsuitable’ for oil palm and calculated their extent.
Results
Population estimates for the Lower Kinabatangan: comparison between 2004 and 2014
The mean number of proboscis monkey groups sighted per survey was 9.1 ± SD 5.3 (range 2–19). We recorded a total of 1,960 proboscis monkeys, in 128 groups with one male and multiple females, 24 all-male groups (including solitary males), six groups with multiple males and multiple females, and six groups for which the composition could not be determined. This number was greater than that detected in 2004 (1,340 individuals; Sha et al., Reference Sha, Bernard and Nathan2008).
Mean size of groups with one male and multiple females was 13.3 ± SD 5.8 individuals (Table 1). Group size was not significantly different between the mangrove (n = 16) and riverine (n = 92) forests: 13.6 ± SD 5.3 vs 13.3 ± SD 6.6, respectively (Mann–Whitney U test: z = 0.26, P = 0.79). Mean group size for the all-male groups was 5.8 ± SD 4.0 individuals (Table 2). All-male groups were slightly larger in the riverine than in the mangrove forest, although we could not test this statistically because of the small sample size: mangrove (n = 3) and riverine (n = 13) forests, 4.7 ± SD 3.8 vs 6.1 ± SD 4.2, respectively. Mean sizes of the groups with one male and multiple females (15.7 ± SD 4.8, n = 72, range 6–28) and the all-male groups (7.6 ± SD 5.4, n = 12, range 1–17) in 2004 were generally larger than in 2014, although the differences were significant only for the groups with one male and multiple females (z = −3.44, P < 0.001) and not for the all-male groups (z = −0.58, P = 0.56).
Table 1 Composition of groups of proboscis monkeys Nasalis larvatus with one male and multiple females. Only groups with confirmed age class and sex for all individuals were included (i.e. groups containing individuals of unknown age class or sex were excluded from the calculations).

Table 2 Composition of all-male groups of proboscis monkeys. Only groups with confirmed age class and sex for all individuals were included (i.e. groups containing individuals of unknown age class or sex were excluded from the calculations).
Population estimates in long-term monitoring areas
The median and 95% CI of the estimated posterior distribution of the trend (β) parameter were 0.000 (−0.003, 0.002), 0.001 (−0.001, 0.002), and −0.001 (−0.002, 0.001) for mangrove, mainstream and tributary, respectively. We detected neither significantly increasing nor decreasing population trends (Fig. 2).
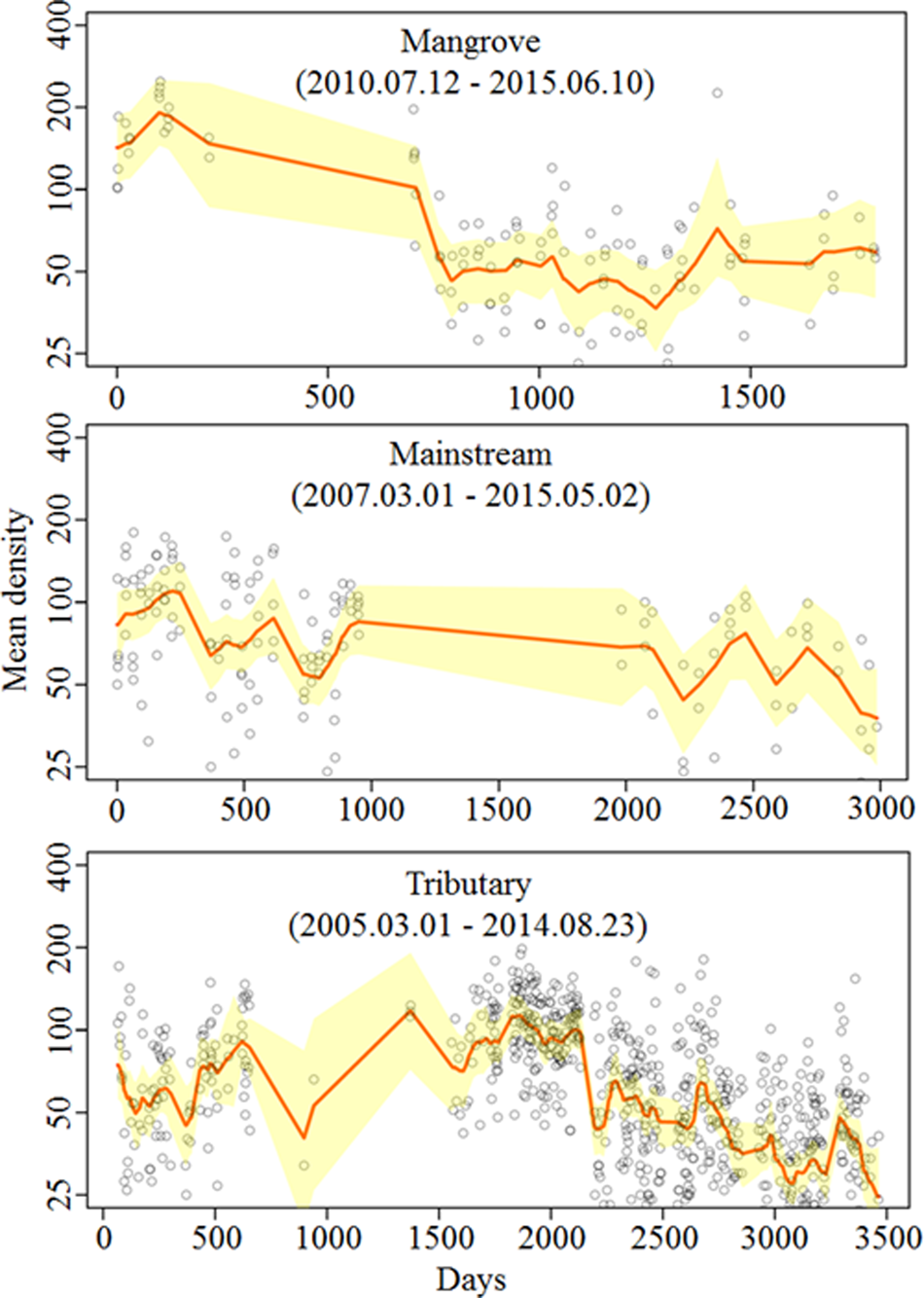
Fig. 2 Median and 95% CI of ‘trend’ (β) parameter for the mangrove, mainstream, and tributary.
Protected habitat and forest loss
During 2005–2014, 9,245 ha of forest were lost, largely to oil palm, in the Lower Kinabatangan floodplain (partially seen in Fig. 1), however, there was relatively little forest loss in the proboscis monkey's range. The estimated potential range for the proboscis monkey along the 190 km of the Lower Kinabatangan (transects with a buffer of 800 m from the riverbank) was 25,796 ha, which encompasses a variety of vegetation types and land uses. Of this, 81% (20,958 ha) was natural vegetation (mainly forest) in 2005. This had decreased to 78% (20,041 ha) in 2014, and 550 ha of the remaining forest were severely degraded. The area of natural vegetation (i.e. different types of forest of variable quality, and nipa palm areas) available as habitat for the proboscis monkey was therefore 20,041 ha in 2014.
Within the wide area transects (including a buffer of 800 m from the riverbank), 77% of the forest in the potential proboscis monkey range is protected. At the long-term monitoring sites 84% of the forest is protected in the mangrove area, 62% in the mainstream area and 74% within the tributary (Table 3).
Table 3 Summary of the protected forests in the survey areas (wide area, mangrove, mainstream, and tributary), forest loss during 2005–2014 in areas outside the protected areas, and the extent of remaining unprotected forest in 2014.

There was some forest loss in the areas outside the Wildlife Sanctuary and the forest reserves (Table 3). During 2005–2014, 12% of the forest was lost in the survey area along the Kinabatangan river and its tributaries, and in 2014, there were 4,451 ha of unprotected forest remaining. On a smaller local scale, the forest loss was 2.5% in the mangrove (278 ha remaining), 5.0% in the mainstream area (441 ha remaining) and 13% in the tributary area (248 ha remaining; Table 3).
Threatened habitat
Although there was relatively little forest loss in the potential range of the proboscis monkey, there were 4,541 ha of forest, including 128 ha of nipa palm and 613 ha of swamp, outside the protected area network (Fig. 3, Tables 3 & 4) the loss of which could affect 444 individuals or 22.7% of the total identified proboscis monkey habitat.

Fig. 3 Land use and vegetation cover outside the protected areas (Lower Kinabatangan Wildlife Sanctuary and forest reserves) in 2014 in the Lower Kinabatangan within 800 m from the riverbank along the proboscis monkey survey transect areas (surveys conducted in 2004 and 2014).
Table 4 Extent of vegetation cover (forest types) and land use (oil palm and villages) outside the Lower Kinabatangan Wildlife Sanctuary and forest reserves but within the potential range of the proboscis monkey.

At least 38% (1,481 ha) of the unprotected forest has already been allocated for oil palm cultivation (1,200 ha for estates and 281 ha for small holdings; Table 4). Eighty-five per cent (3,859 ha) of the unprotected forests were identified as unsuitable for oil palm because they are subject to seasonal or daily inundation (Table 4).
The Kinabatangan survey area included 4,830 ha of oil palm in 2014 (Table 4). There are several areas of oil palm in the potential proboscis monkey range, which are probably fragmenting the species’ habitat (Fig. 3), but 1,397 ha were unproductive because they are unsuitable for oil palm.
Discussion
The population size of the proboscis monkey in the Lower Kinabatangan was higher in 2014 (1,960 individuals) than in 2004 (1,340 individuals), which could be a result of differences in data collection: the number of monkeys counted during late afternoon surveys could be up to three times higher than counts of the same groups in the morning (Matsuda et al., Reference Matsuda, Otani, Bernard, Wong and Tuuga2016), which means the increased number of monkeys counted in 2014 may not be a sign of an actual increase in population size. River surveys in the three long-term monitoring sites suggested that these populations were stable. This is supported by a recent population viability analysis of the Kinabatangan population, which also predicted relatively stable proboscis monkey populations in this area (Stark et al., Reference Stark, Nijman, Lhota, Robins and Goossens2012).
Despite significant land-use and vegetation cover changes in the Lower Kinabatangan (Abram et al., Reference Abram, Xofis, Tzanopoulos, MacMillan, Ancrenaz and Chung2014), there was little conversion of forest to oil palm (917 ha) during 2004–2014 in the proboscis monkey habitats within 800 m of the river. The apparent population stability may be explained by the feeding ecology of the species. Feeding primarily on leaves, proboscis monkeys exploit ubiquitous food sources (Yeager, Reference Yeager1989; Matsuda et al., Reference Matsuda, Tuuga and Higashi2009; Boonratana, Reference Boonratana, Marsh and Chapman2013), which could make them resilient to small forest losses and fragmentation. However, projections of land-use allocation indicate that unprotected forests could be threatened in the near future, mainly because of the expansion of oil palm. This includes the unique lowland swamp forests that contribute to carbon sequestration and provide habitats for many other rare, endemic and threatened species (Abram et al., Reference Abram, Xofis, Tzanopoulos, MacMillan, Ancrenaz and Chung2014; Reference Abram, MacMillan, Xofis, Ancrenaz, Tzanopoulos and Ong2016).
Although the proboscis monkey population was relatively stable, group sizes in 2014 were significantly smaller than in 2004. Such a trend was also detected in one of the long-term monitoring sites (Menanggul tributary), where the mean group size was 17.0 in 1990–1991 vs 13.6 in 2005–2006 (Boonratana, Reference Boonratana, Marsh and Chapman2013). We also detected smaller group sizes at the upper Kinabatangan river area during the surveys in 2014, i.e. a mean of 6.5 ± SD 2.5 monkeys per group, with a total of 39 individuals observed (n = 6 groups observed; DJS, pers. com.), vs 2004, i.e. mean group size 11.7 ± SD 3.2, with a total of 35 individuals observed (n = 3 groups; Sha et al., Reference Sha, Bernard and Nathan2008). This suggests that localized habitat changes and fragmentation could affect the social structure, size and composition of proboscis monkey groups, although the forest loss within their range was not significant. Smaller group sizes in degraded habitats have also been noted for the black-and-white colobus Colobus guereza (Chapman et al., Reference Chapman, Wasserman, Gillespie, Newton-Fisher, Notman, Paterson and Reynolds2006), red colobus Procolobus rufomitratus (Decker & Kinnaird, Reference Decker and Kinnaird1992) and mantled howler Alouatta palliata (Clarke et al., Reference Clarke, Collins and Zucker2002).
One approach to ensuring the viability of the proboscis monkey population in the Kinabatangan would be to restore riparian areas that have been altered by human activities, primarily the establishment of oil palm plantations, of which c. 1,400 ha are unproductive because of periodic flooding. If reforested, these areas could provide habitat for the Lower Kinabatangan proboscis monkeys, but reforestation is costly because of staff requirements and the need to care for tree seedlings for 3–5 years to prevent their destruction by animals, creepers and vines. Local initiatives show that it could cost c. USD 35 million to replant the area now occupied by unproductive oil palm (c. 1,400 ha) in proboscis monkey habitats (IL, unpubl. data). Land purchase for conservation is also expensive, with a mean cost of USD 13,932 ± SD 5,662 per ha (n = 24 pieces of land, range USD 5,608–26,645 per ha; IL, unpubl. data).
We estimate that 85% (3,859 ha) of the unprotected forest within the proboscis monkey's range of our 2014 survey was unsuitable for oil palm development because of seasonal or tidal flooding. Despite this, at least 38% of the area is allocated for this purpose, which would result in the destruction of ≥ 1,480 ha of known alienated land and ≥ 3,800 ha of forested areas that are potentially unsuitable for oil palm; i.e. areas with a calculated present net value of USD 65-299 per ha per year over 25 years (Abram et al., Reference Abram, Xofis, Tzanopoulos, MacMillan, Ancrenaz and Chung2014). Protective legislation should be considered by incorporating these areas into the existing Wildlife Sanctuary or forest reserves. However, implementing such strategies is challenging. Protecting land already consigned to the establishment of oil palm is difficult because it requires a change of administrative status that needs to be supported by the State Assembly. Nevertheless, as a minimum measure, any remaining State land (i.e. non-alienated) should be identified and included in the Lower Kinabatangan Wildlife Sanctuary or forest reserves. Furthermore, efforts must be undertaken to engage with oil palm companies to ensure they effectively conserve high conservation value forest patches within their boundaries, especially as the State is gearing towards producing 100% certified sustainable palm oil through certification by the Roundtable on Sustainable Palm Oil. Within this commitment, no high conservation value forests with populations of proboscis monkeys (and other threatened species) can be converted to oil palm.
This study updates information on the population of the proboscis monkey in the Lower Kinabatangan region: we detected changes in group size, but the population was stable overall. Changes in group size could have resulted from forest fragmentation and degradation. As suitable habitat is disappearing rapidly throughout the species’ range (Meijaard & Nijman, Reference Meijaard and Nijman2000b), it is important to understand the impact of changes in forest cover on the proboscis monkey's population dynamics. Further studies are also needed to examine the effects of forest fragmentation and degradation on the abundance and distribution of the species’ food sources and sleeping sites. Our results from the Kinabatangan basin in Sabah may not be representative of all proboscis monkey populations, and Borneo-wide population surveys are urgently needed to assess the species’ wider conservation status and any ongoing threats.
Acknowledgements
We thank the Sabah Biodiversity Centre, the Sabah Wildlife Department and the Sabah Forestry Department, the Singapore Zoo, and all our colleagues, especially Asnih, Ahmad, Nazrul Bin Natsyir, Petrieadi Bin Ambo Tola, Razidi Bin Iskandar, and Sonja Luz for support. This study was mainly financed by Wildlife Reserves Singapore (Exp14/10 to IM) and partly financed by JSPS KAKENHI (#15K14605 and #21770261 to IM) and the National Geographic Society (#9254-13 to IM).
Author contributions
Project conceptualization: all authors; population surveys: IM, DJS, MA and JCMS; generating and updating spatial data: NKA; data analysis: IM, NKA and TK; writing: IM and NKA; contribution to final version: all authors.
Conflicts of interest
None.
Ethical standards
This study was conducted in compliance with the animal care regulations and laws of Malaysia.









