Introduction
Caprids are keystone species in the grassland ecosystems of the Himalayas and Central Asian mountains (Bagchi & Ritchie, Reference Bagchi and Ritchie2010; Lyngdoh et al., Reference Lyngdoh, Shrotriya, Goyal, Clements, Hayward and Habib2014; Mallon et al., Reference Mallon, Harris, Wegge, McCarthy and Mallon2016). The Siberian ibex Capra sibirica (hereafter ibex) and urial sheep Ovis vignei (hereafter urial; Nadler et al., Reference Nadler, Hoffmann and Wolff1973; Rezaei et al., Reference Rezaei, Naderi, Chintauan-Marquier, Taberlet, Virk and Naghash2010) are the two predominant wild ungulate species in the Hindu Kush mountain range in Afghanistan, and the primary prey of large wild carnivores (Simms et al., Reference Simms, Moheb, Salahudin, Ali, Ali and Wood2011; Moheb & Paley, Reference Moheb, Paley, McCarthy and Mallon2016). The ibex has a wide distribution in mountainous areas in Afghanistan, including in Wakhan District in the extreme north-east of the country (Habibi, Reference Habibi2003; Moheb et al., Reference Moheb, Mostafawi, Zahler and Fuller2018). The species is categorized globally as Near Threatened on the IUCN Red List (Reading et al., Reference Reading, Michel, Suryawanshi and Bhatnagar2020). The distribution of the urial in Afghanistan remains poorly understood but includes the central Hindu Kush range and Wakhan District, where the population could be connected with populations in northern Pakistan (Michel & Ghoddousi, Reference Michel and Ghoddousi2020). The urial prefers low-altitude and open landscapes, and major threats to its populations include human disturbance, competition with livestock, habitat fragmentation and hunting for meat (Kanderian et al., Reference Kanderian, Lawson and Zahler2011). These have led to a population decline in Afghanistan, and the species has recently been categorized as Vulnerable on the IUCN Red List (Michel & Ghoddousi, Reference Michel and Ghoddousi2020).
We conducted population surveys of the urial and ibex in the Hindu Kush range of Wakhan National Park as part of a study of the complex relationship between wild and domestic ungulates and their predators in Wakhan District. We assessed changes in the abundance of wild ungulate populations over time to inform the conservation of large carnivores and their wild prey species in the area. Here we compare and discuss the results of wild ungulate monitoring in 2015 and 2018.
Study area
We surveyed the western part of Wakhan District (hereafter Wakhan), Badakhshan Province, which was declared a national park in 2014 and is one of the most pristine mountain landscapes in Afghanistan (Fig. 1; Smallwood & Shank, Reference Smallwood, Shank, Lookingbill and Smallwood2019). Wakhan National Park consists of alpine valleys and high, snow-capped mountains shared between the Pamirs and Hindu Kush ranges, with altitudes of c. 2,510–7,490 m (Smallwood & Shank, Reference Smallwood, Shank, Lookingbill and Smallwood2019). The Hindu Kush is dominated by high, steep peaks and associated glaciers, with less steep slopes and alluvial fans in the northern part of the range. Our surveys covered parts of the northern drainages of the Hindu Kush range, with heavy snow cover and glaciers deep inside the drainages limiting access and thus constituting the boundary of the study area to the south. The Hindu Kush mountain range in Wakhan shares an international border with Pakistan to the south. The Wakhan and Pamir Rivers comprise the upper courses of the Panj River; the Wakhan River separates the Hindu Kush from the Pamir mountain range in northern Wakhan, and the Pamir and Panj Rivers constitute the northern international border with Tajikistan (Fig. 1).

Fig. 1 Location of urial Ovis vignei and Siberian ibex Capra sibirica survey sites in the Hindu Kush mountains of Wakhan National Park, Afghanistan, in 2015 and 2018.
Wakhan has a continental climate typical for the high-altitude Himalayas, with ambient temperatures remaining below c. 2.4 °C until June. Annual precipitation in the Hindu Kush is estimated to be 200–1,000 mm depending on the elevation and location (Bedunah, Reference Bedunah2009). Riparian communities dominated by willows Salix spp., dwarf-shrub deserts, steppes and alpine grasslands dominate the vegetation cover along the northern drainages of the Hindu Kush range in Wakhan.
The Wakhan Valley and Hindu Kush range are inhabited by c. 15,000 people living in c. 1,700 households in 42 villages and numerous nomadic camps (Moheb, Reference Moheb2020). Most people subsist in an agro-pastoral economy, using the meagre arable lands for grain- and legume-based agriculture. They tend in excess of 78,000 livestock, mostly sheep, goats, cattle and yaks, but also donkeys, horses and a few Bactrian camels (Moheb, Reference Moheb2020).
The snow leopard Panthera uncia and grey wolf Canis lupus are the main ungulate predators in the Hindu Kush range, but the brown bear Ursus arctos and lynx Lynx lynx also occasionally prey on ungulates. Apart from the urial and ibex, the only significant wild mammalian prey species for large carnivores in this area are the long-tailed marmot Marmota caudata and Cape hare Lepus capensis (Habibi, Reference Habibi2003).
Methods
Intensive ground surveys, although not without limitations, are the most appropriate method for surveying mountain ungulates in steep and rugged terrain (Singh & Milner-Gulland, Reference Singh and Milner-Gulland2011). We conducted double-observer ground surveys (Suryawanshi et al., Reference Suryawanshi, Bhatnagar and Mishra2012, Reference Suryawanshi, Mudappa, Khanyari, Raman, Rathore, Kumar and Patel2021) in 10 geographical areas (subunits of the survey area; Supplementary Table 1) along the Wakhan Valley. We selected these survey sites based on findings from a broader survey of the area in 2011, intending our survey to be a representative subsample of that survey. A preliminary questionnaire survey of local people in Wakhan in 2010 confirmed the findings of earlier studies (Schaller, Reference Schaller1977; Mallon, Reference Mallon1983) suggesting that persistent snow cover at higher altitudes and the appearance of grasses at lower altitudes drive wild ungulates to lower-altitude areas in the late winter and early spring. Thus, to maximize the likelihood of detecting ungulates, we conducted the surveys in April–May.
Two teams, each consisting of three people, conducted double-observer surveys during 15 April–8 May 2015 and 25 April–12 May 2018 (Fig. 1), following a previously described methodology (Suryawanshi et al., Reference Suryawanshi, Bhatnagar and Mishra2012). We covered the survey subunits in a single day to avoid duplicate counts and to meet the assumption of the population being closed during the survey. In 2015, members of the first team identified vantage points with wide visibility over the surrounding landscape. From there, they scanned the area for 10–25 min and radioed their location to the second team, before walking to the next vantage point. The second team followed the first team and surveyed the same areas after a delay of 1 hour (Suryawanshi et al., Reference Suryawanshi, Bhatnagar and Mishra2012). Each team took efforts not to influence the observations of the other team during inter-team communications. Both teams were independent in their recording of observations from the vantage points and along the routes between the vantage points. In 2018, we replicated the survey following the same routes, vantage points and protocol.
We mostly conducted surveys during 06.30–13.20, with the earliest start at 05.15 and the latest finish at 16.00, depending on the weather conditions. For nine of the 10 survey sites (Fig. 1), the observer teams walked (at a mean speed of c. 1.0 km/h) from the lower areas in the valley to the upper areas. At one site (Kuzgut-Kharich) the teams used two vehicles (one following 1 hour after the other) and searched for ungulates whilst driving (at a mean speed of c. 1.6 km/h) and while stationary at the vantage points, in the same manner as for the surveys conducted while walking. The teams recorded survey routes and vantage points using GPS devices. When ungulates were detected, we recorded herd/group composition (age, sex and number), time of the observation, direction of the movements of the animals, topographical features of the area, location on the slope and compass bearing of the sighted herd/group from the point of observation. In most cases we recorded the geographical location and compass bearing to the observed herds from a second location to calculate the exact location of the herd. This was done by triangulating from the two locations on a map. Pinpointing the exact location of the herds enabled us to estimate the distance at which we observed them and assisted in further calculations. At the end of each survey day, we compiled our data and classified the observed groups into three categories: (1) groups detected by both teams (C), (2) groups only observed by team 1 (S1), and (3) groups only observed by team 2 (S2). Reconciling the groups observed by both teams helped us confirm the assumption that groups could be individually identified based on sex and age composition, location and time.
We pooled the double-observer survey data from all 10 survey sites and estimated the number of urials and ibexes for 2015 and 2018. We used the double-observer estimation method based on mark–recapture theory (Caughley, Reference Caughley1974; Magnusson et al., Reference Magnusson, Caughley and Grigg1978), which has been validated for mountain ungulates in the Himalayas (Suryawanshi et al., Reference Suryawanshi, Bhatnagar and Mishra2012). We identified statistical differences in the urial and ibex population estimates for 2015 and 2018 using Z tests (Leki et al., Reference Leki, Thinley, Rajaratnam and Shrestha2018).
Where possible, we recorded the age and sex of all urials and ibexes observed, and calculated the sex and age ratios of each group. We did not observe new-born lambs/kids of either species during either of the surveys, and we classified nearly 1-year-old individuals as juveniles. We included animals of doubtful age class or sex in the total count but classified them as ‘unknown’. We calculated the mean herd size for urials and ibexes and tested whether animal detection in the field (herd seen by both survey teams vs only one team) was related to the herd size using χ 2 tests. Herd size did not affect detection for urials but it did for ibexes (see Results), and thus we estimated total numbers of small (≤ 20) and large (≥ 21) ibex herds separately and then summed them to estimate the total population size (the threshold for small vs large herds was based on our experience of detectability of different-sized herds).
We used the viewshed function of ArcGIS 10.3 (and later versions; Esri, Redlands, USA), which includes only the areas that can be seen (Maichak & Schuler, Reference Maichak and Schuler2018), to calculate the total area surveyed using a 30-m digital elevation model (US Geological Survey, Reference Geological Survey2019), the recorded survey routes, an observer offset of 1.7 m and a 3,800-m buffer (as determined by the maximum distance at which we detected ungulates). We converted viewshed-surveyed areas to polygons and calculated the same survey area of 288 km2 for both surveys. We determined ungulate densities using the area calculated by the viewshed function and the estimated number of individuals observed in this area.
Results
Urial median herd size was 6 in both survey years, with a range of 2–27 and 2–15 individuals in 2015 and 2018, respectively. Most groups (78%) comprised adults of both sexes, followed by male-only groups (15%) and groups of females with juveniles (7%). We observed no statistically significant changes in sex and age composition between 2015 and 2018 (χ 2 tests; P > 0.37). The overall adult sex ratio (female:male) was 100:70, juveniles comprised 23% of the population and the female:juvenile ratio was 100:53.
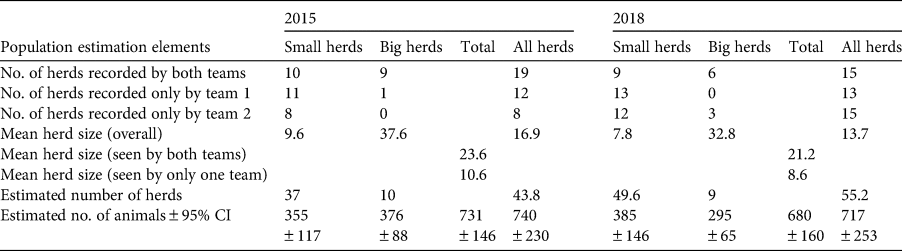
In both survey years, urial group sizes did not vary depending on whether they were observed by one or both of the survey teams (P > 0.2; Table 1). We therefore used the mean group size for each year to estimate populations. In 2015, the estimated population size and density were 208 (95% CI 130–286) individuals and 72 (95% CI 39–105) individuals/100 km2, respectively. In 2018, these had decreased to 102 (95% CI 57–147) individuals and 35 (95% CI 12–58) individuals/100 km2, representing a significant decline of c. 50% (Z = −2.2971214, P = 0.011; Fig. 2).
Table 1 Calculation of urial Ovis vignei population estimates in 2015 and 2018 from double-observer population surveys of 10 sites (total area = 288 km2) in the Wakhan Valley, Afghanistan (Fig. 2).

Overall ibex median herd sizes were 12 and 8, with a range of 2–61 and 2–53 individuals, in 2015 and 2018, respectively. Most groups (80%) were composed of adults of both sexes, followed by groups of females with juveniles (15%) and male-only groups (5%). We documented no statistically significant changes in sex and age composition between 2015 and 2018 (χ 2 tests; P > 0.30). The overall adult sex ratio (female:male) was 100:103, juveniles comprised 23% of the population and the female:juvenile ratio was 100:58.
In both survey years, ibex group sizes differed depending on whether they were seen by one or both of the survey teams (χ 2 = 7.45, P = 0.006; Table 2). We therefore used the mean sizes for small (≤ 20) vs large (≥ 21) groups for each year to calculate densities. In 2015, the estimated population size and density were 740 (95% CI 510–970), individuals and 257 (95% CI 177–337) individuals/100 km2, respectively. These were not significantly different in 2018, at 717 (95% CI 464–970) individuals and 249 (95% CI 161–337) individuals/100 km2, respectively (Z = −0.1317655, P > 0.4; Fig. 2).
Table 2 Calculation of ibex Capra sibirica population estimates in 2015 and 2018 from double-observer population surveys of 10 sites (total area = 288 km2) in the Wakhan Valley, Afghanistan (Fig. 2), for small (≤ 20 individuals) and big (≥ 21 individuals) herds.
Discussion
The estimated ibex population in our study area in Wakhan appeared stable during 2015–2018, whereas estimated urial density declined by c. 50%. As declines of ungulate populations are of concern not only with respect to the long-term viability of the species itself, but also with respect to the predators that depend on them, it is important to examine the reasons for such declines.
Our estimated density of c. 35 urials/100 km2 in Wakhan in 2018, after an apparent significant decline, is one of the lowest reported across the species’ global range. Although estimates of urial density, derived in a variety of ways across their range, have varied tremendously (Supplementary Table 2), they mostly range between < 50 and 400 individuals/100 km2; in several areas, densities of 1,000–1,500 individuals/100 km2 have also been reported (Schaller, Reference Schaller1977; Frisina et al., Reference Frisina, Awan and Woodford2007; Farhadinia et al., Reference Farhadinia, Moqanaki and Hosseini-Zavarei2014; Ghoddousi et al., Reference Ghoddousi, Hamadi, Soofi, Khorozyan, Kiabi and Waltert2016). However, these estimates are often not directly comparable because many previous assessments of urial density have been conducted in ways that were methodologically and statistically less rigorous than the double-observer survey method used in this and other studies. In addition, our precise calculation of the survey area through the viewshed analysis of geographical data may have resulted in different estimates. Urial densities in Wakhan could also be lower than elsewhere because this may be the highest-elevation population surveyed and thus be at the fringes of its suitable habitat. The altitude in the study area ranges from 2,512 to > 5,000 m, and urials were only observed at 2,988–3,991 m.
We observed mean herd sizes of 8–10 for the urial, similar to those reported elsewhere (6–10) in its range (Supplementary Table 2), except for from Ladakh, India, where mean herd sizes of 15 and 30 have been reported (the latter was influenced by a single exceptionally large group of 207 individuals; Khara et al., Reference Khara, Khanyari, Ghoshal, Rathore, Pawar, Bhatnagar and Suryawanshi2021). The female:male ratio of 100:70 was slightly higher than the range of values recorded elsewhere (100:84–100; Supplementary Table 2). The observed per cent of juveniles amongst the non-lamb population was slightly higher (23%) than in other areas (18–20%; Supplementary Table 2), perhaps suggesting relatively good reproductive success in Wakhan.
Estimates of ibex density throughout their broad geographical range vary by > 100-fold, but our estimate of c. 250 individuals/100 km2 is within the highest 15% reported elsewhere (Supplementary Table 3). Ibex herd sizes (8–12) were in the mid-range of mean herd sizes reported elsewhere (5–29; Supplementary Table 3), as was the adult female:male ratio (100:103). Our observed per cent of juveniles amongst the population (23%) was also in the mid-range of what has been reported elsewhere.
There are a number of potential causes of the apparent decline in urial numbers. There was less snow cover in 2018 compared to 2015; urials may thus have roamed over a larger area, which could have made their detection more difficult, resulting in lower population size estimates. However, survey teams were experienced at detecting wildlife in the landscape in any season and the decline was pronounced, suggesting that additional causes could have been involved. Demographic data on herd size, sex ratios and proportions of juveniles do not suggest any major demographic problems; i.e. the population decline appears not to have been caused by low reproductive rates. Competition with livestock for forage can have negative effects on mountain ungulate populations (Bagchi et al., Reference Bagchi, Mishra and Bhatnagar2004), however, livestock numbers throughout the valley were comparable in 2016 and 2018 (Z. Moheb, unpubl. data, 2016, 2018). Vegetation dynamics can alter over time for several reasons and could affect mountain ungulates; we obtained no data on vegetation dynamics for the study period and thus cannot examine whether this was a factor in the decline of the urial population. There could have been a substantial increase in predation pressure from wolves as suggested by some local livestock herders. However, no such change was evident at the landscape scale based on the results of household interviews regarding livestock depredation that were conducted in 2018 (Moheb, Reference Moheb2020). Extreme weather events could have played a significant role in urial population decline. For example, in early spring 2016 a cold wave and snowstorm killed > 20,000 livestock in Wakhan National Park (Z. Moheb, unpubl. data, 2016) and could have resulted in increased mortality in the urial population. It is also possible that urials, which are relatively mobile, could have shifted temporarily to nearby valleys and slopes that were not included in our survey; however, we obtained no data to support or reject this as a potential factor. Finally, as the urial is a species that sometimes comes closer to human settlements and uses more easily accessible terrain (Siraj-ud-Din et al., Reference Siraj-ud-Din, Minhas, Usman, Khan, Awan, Shafi and Ahmad2018) than the ibex, illegal hunting of urials could have increased in some areas (Bashari et al., Reference Bashari, Sills, Peterson and Cubbage2018). In the Dehqankhana area in particular, both urial and ibex numbers declined significantly over time (Supplementary Table 1). This area, which borders and is accessible from neighbouring Pakistan, could be more susceptible to illegal hunting. However, even if the Dehqankhana area is excluded from analysis, urial numbers elsewhere in our study area still declined by c. 40%.
The potential decline in the urial population in Wakhan is of particular concern because it is one of the largest urial populations remaining in the region (Michel, Reference Michel2010). It is also located in the transition zone of three urial subspecies. The nearest known population of urials in Tajikistan (Bukhara urial Ovis vignei bochariensis) is located more than 100 km north-west of the western border of Wakhan. Elsewhere in Afghanistan, the Afghan urial Ovis vignei cycloceros has been confirmed only in Bamyan Province, > 400 km south-west of Wakhan (Shank & Alavi, Reference Shank and Alavi2010). Interviews with hunters from Wakhan and from across the border in Pakistan have suggested a possible connection of the urial population in Wakhan with that in northern Pakistan (Ladakh urial Ovis vignei vignei; Michel, Reference Michel2010). A small population of Ladakh urials with a patchy distribution occurs throughout the Gilgit-Baltistan region (Khan et al., Reference Khan, Khan, Ahmed, Khan, Ajmal and Ali2014). Surveys based on direct sightings estimated the number of urials in the entire Baltistan region to be 172 individuals; however, the largest population in Bunji, c. 170 km aerial distance from the Wakhan population, comprises c. 109 individuals (Din et al., Reference Din, Minhas, Khan, Ali, Bibi, Ahmed and Awan2016). Considering the patchy distribution and low density of urials in nearby areas, the Wakhan population could be the largest remaining in the region.
The status of wild ungulate populations has a direct influence on their wild predators. The Siberian ibex is a key prey species for the snow leopard (Lyngdoh et al., Reference Lyngdoh, Shrotriya, Goyal, Clements, Hayward and Habib2014), and the high densities and stable population numbers recorded during 2015–2018 confirm that Wakhan is a core area for snow leopards in Afghanistan (Moheb & Paley, Reference Moheb, Paley, McCarthy and Mallon2016). By contrast, urial declines could have affected the status of the grey wolf in Wakhan National Park. Some herders have reported increased wolf numbers during the study period, which could be a result of a shift to livestock predation because of lower numbers of urials, and thus wolves being observed more frequently by herders. The complexities of carnivore–prey interactions remain poorly understood in Central Asian highlands and require further research.
Based on our findings regarding the population variations of two flagship ungulate species of Wakhan National Park, we recommend investments in continued monitoring of these populations, using the same methods as in this study to ensure comparability. This includes double-observer surveys, viewshed analysis and accounting for the varying visibility of large and small ibex herds. New tools should also be deployed, such as remote sensing for snow-cover comparison, assessment of rangeland condition and training of rangers to monitor wildlife mortality. In addition, we recommend a focus on minimizing poaching by continued development of the Spatial Monitoring and Reporting Tool (SMART; Wilfred et al., Reference Wilfred, Kayeye, Magige, Kisingo and Nahonyo2019), more effective law enforcement, increasing community awareness of poaching impacts, better involvement of the government in management planning, monitoring of local markets and extending the current protection in Wakhan to the adjacent areas in the neighbouring district of Ishkeshim in particular. Improved livestock husbandry and pasture management are needed to maintain both domestic and wild ungulate populations in the long term. Finally, further research into urial taxonomy and the relationships between populations in Wakhan, northern Pakistan and Tajikistan will help identify conservation priorities. Implementation of these recommendations will support the ecological function of the Wakhan mountain ecosystem and allow wildlife, local communities and livestock to coexist sustainably (Smallwood & Shank, Reference Smallwood, Shank, Lookingbill and Smallwood2019).
Acknowledgements
This study was supported by the UNDP GEF grant AA/Pj/PIMS: 00076820/0088001/5038 and Fondation Segré grant ‘Transboundary Conservation of Mountain Monarchs in Afghanistan and Pakistan’ awarded to the Wildlife Conservation Society Afghanistan Program. We thank Richard Paley, Qais Sahar, Zabihullah Ejlasi and Sayed Naqibullah Mostafawi at the Wildlife Conservation Society Afghanistan for their support; the Wakhi observers in the survey teams; Karmal, Ayan Big, Aziz Big and, in 2018, Shukrullah for their professionalism and data collection in the field; and Heidi Kretser (Wildlife Conservation Society) for comments on an earlier version of the manuscript.
Author contributions
Study design and fieldwork: ZM, SO; data collection: ZM, AMR, NJ; data analysis: ZM, SO, TKF; writing: ZM, SO, PIZ, TKF.
Conflicts of interest
None.
Ethical standards
This research abided by the Oryx guidelines on ethical standards. There were no human subjects, experimentation with animals and/or collection of specimens involved.







