Introduction
Assessments of species conservation status at the global level follow the IUCN Red List categories and criteria and are fundamental for the implementation of strategies for species conservation. A taxon that does not meet any criteria for the IUCN threat categories is categorized as Least Concern (IUCN, 2012). This classification, however, does not necessarily imply that the taxon has been assessed fully for all criteria because, in most cases, there are insufficient data available regarding threats, distribution and/or population trends. When an assessment states there is no evidence of threats this does not necessarily mean studies were carried out that provide evidence the species is not in decline or not being affected by anthropogenic activities. Often the final decision rests on the judgement of specialists based on the available data (Mace et al., Reference Mace, Collar, Gaston, Hilton-Taylor, Akçakaya and Leader-Williams2008). However, Least Concern species should be reassessed if they become exposed to threats that have not been previously recognized or investigated (McLeod, Reference McLeod2010; Sengupta & Radhakrishna, Reference Sengupta and Radhakrishna2013; Erinjery et al., Reference Erinjery, Kumar, Kumara, Mohan, Dhananjaya and Sundararaj2017; de la Torre et al., Reference de la Torre, González-Maya, Zarza, Ceballos and Medellín2018; Silva et al., Reference Silva, Endo, Silva, Santos, Sampaio and Röhe2018).
This could be the case for mammals currently categorized as Least Concern and occurring in South America, where there is considerable concern for the conservation of habitats and species in the near future. For example, the lesser anteater Tamandua tetradactyla could lose > 76% of its habitat by 2050 in Bolivia because of climate and land-cover changes (Osipova & Sangermano, Reference Osipova and Sangermano2016). Such a rate of habitat reduction fulfils the criteria for a categorization as Endangered on the IUCN Red List, but this species is currently categorized as Least Concern (Miranda et al., Reference Miranda, Fallabrino, Arteaga, Tirira, Meritt and Superina2014). The situation could be similar for other Least Concern xenarthrans (anteaters, sloths and armadillos) in South America, which are facing threats related not only to changes to their habitats but also to hunting, collision with vehicles, diseases and other anthropogenic factors that could cause large population decreases (Superina & Abba, Reference Superina and Abba2020).
Several Amazonian primate species occurring in the Arc of Deforestation (a deforestation hotspot in south-eastern Amazonia) could potentially decline in the near future as a result of habitat loss (Silva et al., Reference Silva, Endo, Silva, Santos, Sampaio and Röhe2018, Reference Silva, el Bizri, Gonçalves, Lemos, Costa-Araújo and Lima2020; Sales et al., Reference Sales, Ribeiro, Pires, Chapman and Loyola2019; Rabelo et al., Reference Rabelo, Gonçalves, Silva, Rocha, Canale, Bernardo and Boubli2020). For example, current rates of deforestation in southern Amazonia are predicted to reduce the habitat of the marmoset Mico chrysoleucos by 38–54% over 18 years (three marmoset generations; Silva et al., Reference Silva, Endo, Silva, Santos, Sampaio and Röhe2018). Although c. 20% of its range is safeguarded by protected areas and Indigenous land designations (Silva et al., Reference Silva, Endo, Silva, Santos, Sampaio and Röhe2018), these rates of habitat loss are warning signs for the conservation of M. chrysoleucos considering the threats to the Amazon rainforest under the current government administration in Brazil. Unfortunately, however, this information was not included in the current IUCN Red List assessment and the species is still categorized as Least Concern (Röhe & Mittermeier, Reference Röhe and Mittermeier2021).
A similar situation could exist for Amazonian titi monkeys (Cheracebus and Plecturocebus; sensu Byrne et al., Reference Byrne, Rylands, Carneiro, Alfaro, Bertuol and da silva2016), for which there is little information regarding threats, distribution or populations. Of the 31 species of Amazonian titi monkeys assessed for the IUCN Red List, 19 (61%) are categorized as Least Concern, with the justification that they have relatively large ranges, occur in protected areas and/or because there is no evidence of threats to their populations. Titi monkeys are mostly frugivorous, occur in a variety of forest types (which include primary and secondary forests) and are considered tolerant of forest disturbance (Michalski & Peres, Reference Michalski and Peres2005; Bicca-Marques & Heymann, Reference Biccca-Marques, Heymann, Veiga, Barnett, Ferrari and Norconk2013). Nevertheless, at least seven Plecturocebus species occur in the Arc of Deforestation (Malhi et al., Reference Malhi, Roberts, Betts, Killeen, Wenhong and Nobre2008), indicating they could potentially be threatened, and also unprotected.
Extensions of the known range of Amazonian titi monkeys have been reported in Brazil (Monção et al., Reference Monção, Selhorst and Soares-Filho2008; Quintino & Bicca-Marques, Reference Quintino and Bicca-Marques2013; Printes et al., Reference Printes, Buss, Azevedo, Ravetta and Silva2018; Rocha et al., Reference Rocha, Barnett and Spironello2019), Bolívia (Martinez & Wallace, Reference Martinez and Wallace2021) and Peru (Vermeer et al., Reference Vermeer, Tello-Alvarado, Moreno-Moreno and Guerra-Vásquez2011), with new reports of sympatry (Printes et al., Reference Printes, Buss, Azevedo, Ravetta and Silva2018; Rocha et al., Reference Rocha, Barnett and Spironello2019) showing that the geographical distribution of Amazonian titi monkeys is more complex than was previously thought. Prince Bernhard's titi monkey Plecturocebus bernhardi (Plate 1) is one such example. The range of the species was believed to be limited to the west by the Ji-Paraná River but new records on the west bank of this river indicate the distribution of this species is greater than previously thought (van Roosmalen et al., Reference van Roosmalen, van Roosmalen and Mittermeier2002; Monção et al., Reference Monção, Selhorst and Soares-Filho2008; Quintino & Bicca-Marques, Reference Quintino and Bicca-Marques2013, Silva-Diogo et al., Reference Silva-Diogo, Costa, Almeida and Fermiano2018). Plecturocebus bernhardi is categorized as Least Concern because of its relatively large range and lack of evident threats that would result in population decline (Röhe & Boubli, Reference Röhe and Boubli2018). However, data regarding the population of this species and the threats it faces are lacking. Here we present new occurrence data and the first population survey of P. bernhardi. We use predictive spatial modelling to estimate the extent of loss of its habitat and population under two future scenarios of land use (a more conservative governance scenario and a more realistic business-as-usual scenario) to reassess the conservation status of the species. This is the first study to reassess the conservation status of an Amazonian titi monkey by gathering new population data and using remote sensing information to model current and future habitat loss.

Plate 1 Prince Bernhard's titi monkey Plecturocebus bernhardi. Photo: Marcelo Santana.
Study area
The study area is in the south of the Amazon Rainforest and encompasses the Madeira–Aripuanã interfluve and both banks of the Ji-Paraná River to the Guaporé Valley (Monção et al., Reference Monção, Selhorst and Soares-Filho2008; Fig. 1, Table 1). This region includes the borders between Amazonas, Rondônia and Mato Grosso states and lies in the Arc of Deforestation, a deforestation hotspot in the Brazilian Amazon as a result of the expansion of agriculture and cattle ranching, urban encroachment, logging and infrastructure projects (Carrero et al., Reference Carrero, Fearnside, do Valle and Alves2020). The climate is tropical with a mean annual temperature of 28 °C and annual precipitation of 2,500–3,000 mm (Hayakawa & Rossetti, Reference Hayakawa and Rossetti2015). The vegetation is classified as dense lowland ombrophilous forest with seasonally flooded forests (Hayakawa & Rossetti, Reference Hayakawa and Rossetti2015).
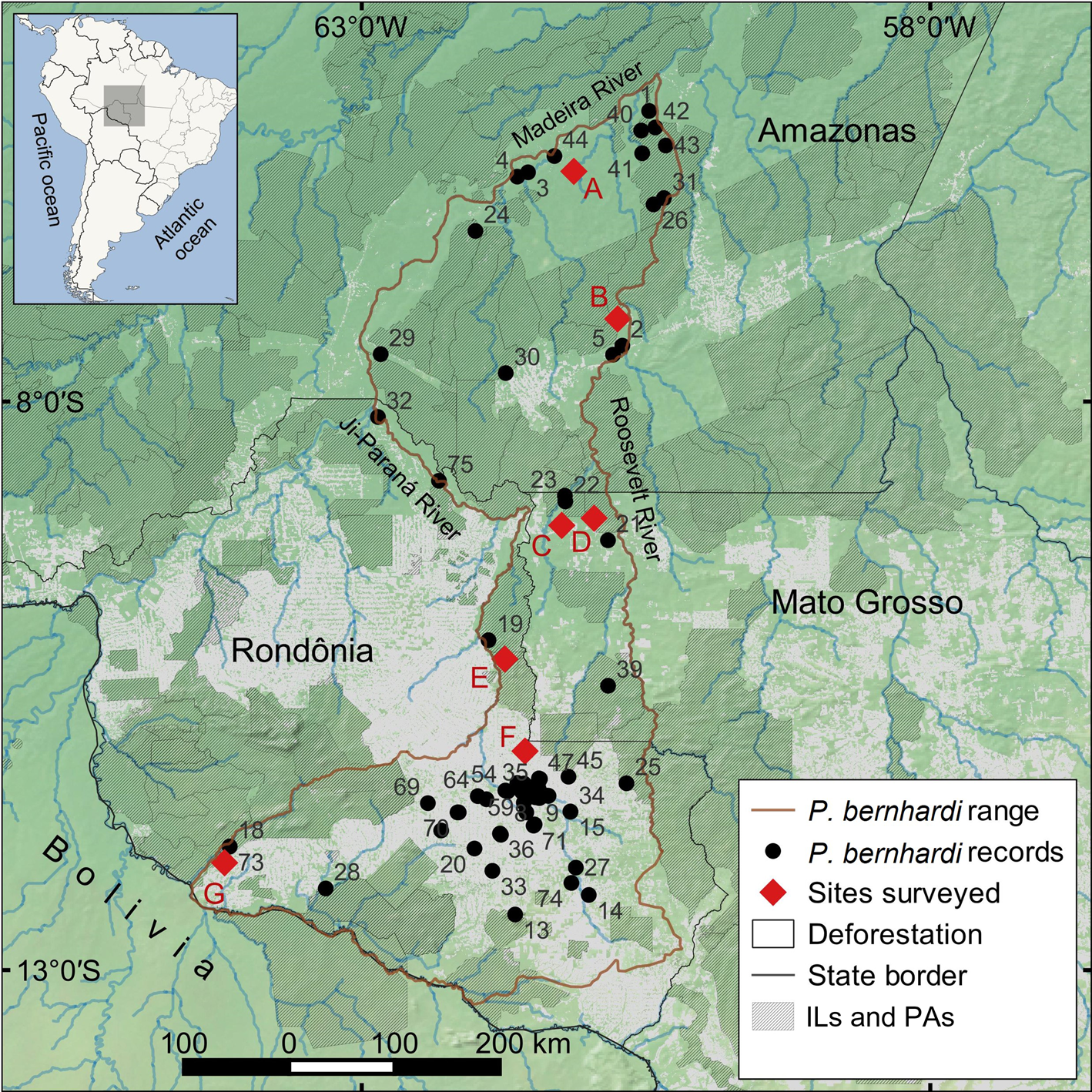
Fig. 1 The areas surveyed in this study (A-G) and the records of P. bernhardi. Numbers indicate the locality records of P. bernhardi recorded in this study and obtained from the literature (Supplementary Table 1). ILs, Indigenous lands; PAs, protected areas.
Table 1 The seven sites surveyed in the Madeira–Aripuanã interfluve and Ji-Paraná River for Prince Bernhard's titi monkey Plecturocebus bernhardi (Fig. 1).

Methods
Geographical distribution
We gathered locality records from the literature and from our primate surveys carried out during 2012–2015 at seven sites in the Madeira–Aripuanã interfluve and on both banks of upper Ji-Paraná River (Silva-Diogo et al., Reference Silva-Diogo, Costa, Almeida and Fermiano2018; Silva et al., Reference Silva, el Bizri, Gonçalves, Lemos, Costa-Araújo and Lima2020; Fig. 1, Table 1). After plotting all occurrence points using QGIS 3.20.1 (QGIS Development Team, 2021), we created a polygon with the major rivers (i.e. Roosevelt and Aripuanã Rivers to the east, Madeira River to the north and the lower Ji-Paraná River to the west) as the main barriers to the dispersal of the species within its geographical range (Amazonian rivers are considered to be barriers to the dispersal of titi monkeys; Ayres & Clutton-Brock, Reference Ayres and Clutton-Brock1992; Boubli et al., Reference Boubli, Ribas, Alfaro, Alfaro, da Silva, Pinho and Farias2015). We deducted non-forest and deforested areas from the species range to estimate the potential area of occurrence, considering that Neotropical primates are arboreal and associated with forests (Estrada et al., Reference Estrada, Garber, Rylands, Roos, Fernandez-Duque and Di Fiore2017).
Transect surveys
During January–February 2015 we carried out line transect surveys on 10 transects to estimate the population density and abundance of P. bernhardi. These transects were at two of the sites used for determining the species' geographical distribution (sites A and B in Fig. 1), in the northern portion of the study area. We positioned the transects randomly using QGIS, with each transect starting from a road or river. We oriented the transects perpendicular to road or river, to account for any gradient of environmental factors or primate density from the start of the trail to the interior of the forest. The mean length of the transects was 3.07 ± SD 0.63 km (range 2.0–4.0 km) and we placed them at least 2 km apart to guarantee spatial independence. We surveyed the transects during 07.00–11.00 in one direction and during 14.00–17.00 in the reverse direction and considered each survey during these periods of the day as independent sampling occasions. Two observers walked along the transects at a mean speed of 1.5 km/h and counted the number of individuals in each detected P. bernhardi group. We measured the perpendicular distance between the centre line of the transect and the centre of each detected group, to facilitate density estimates using distance sampling (Buckland et al., Reference Buckland, Anderson, Burnham and Lake1993). Considering walks during different periods of the day as our sampling occasions, we surveyed each transect at least eight times (mean 10, range 8–12), with at least four surveys in both morning and afternoon. We implemented a 2-day break between subsequent surveys to reduce the impact of observers on the detection rate. In total we walked 271.6 km along the transects.
We estimated the population density of P. bernhardi using Distance 7.1 (Thomas et al., Reference Thomas, Buckland, Rextad, Laake, Strindberg and Hedley2010). This analysis fits detection functions to perpendicular distances, providing the probability of detecting groups and estimating the number of individuals potentially missed by the observers. Following the method used by Silva et al. (Reference Silva, el Bizri, Gonçalves, Lemos, Costa-Araújo and Lima2020), we used a χ 2 test to determine the appropriate truncations and perpendicular distance intervals at P > 0.6. We then compared the adjustments of detection functions using the Akaike information criteria (AIC), with the models with the smallest AIC values considered as best fitting the data. We selected the model for which the density estimate had the lowest coefficient of variation (CV) when more than one function yielded a difference in AIC value (ΔAIC) < 2. We then estimated mean abundance, A, using A = D × a, whereby D is density and a is the species potential area of occurrence calculated using occurrence records (Supplementary Table 1). We calculated the 95% CI and the CV for all estimates.
Assessment of conservation status
We calculated the total habitat loss within the potential area of occurrence of P. bernhardi up to 2019. We used predictive deforestation models to assess how much habitat the species could lose over the next 24 years, which is equivalent to three titi monkey generations. This time frame of three generations is recommended for assessing population decline whether by direct observation, by measuring a decline in area of occupancy or extent of occurrence, or by measuring a decline in quality of habitat or other variables that can indicate ongoing population reduction (IUCN, 2012). We obtained data on current forest loss from MapBiomas (Reference MapBiomas2020) for 1995–2019. For predicted forest loss we considered two scenarios (after Soares-Filho et al., Reference Soares-Filho, Nepstad, Curran, Cerqueira, Garcia and Ramos2006; see also Boubli et al., Reference Boubli, Byrne, da Silva, Silva-Júnior, Araújo and Bertuol2019; Silva et al., Reference Silva, el Bizri, Gonçalves, Lemos, Costa-Araújo and Lima2020): (1) A governance scenario (i.e. considering the current deforestation trends but with a 50% cap in forest loss through the current laws being effective at preventing farmers from clearing > 50% of the forest on their properties and assuming that protected areas are managed effectively). (2) A business-as-usual scenario (i.e. with the continuation of the current deforestation trends along with poor management of protected areas).
To estimate the impact of habitat loss on the population of P. bernhardi, we used the lower bound of the CI of the estimate of abundance, which is the recommended measure of abundance for conservation status assessments (IUCN, 2012). We then used the Red List criteria (IUCN, 2012) to assess whether P. bernhardi should remain categorized as Least Concern or requires recategorization.
Results
Geographical distribution
We obtained 21 new locality records of P. bernhardi through our surveys, and 54 records from the literature (Fig. 1, Supplementary Table 1). The majority of the distribution of this species is delimited by the Aripuanã, Madeira and Ji-Paraná Rivers, but we also recorded the species on the west bank of the upper Ji-Paraná River, where its range overlaps with that of Plecturocebus brunneus (Supplementary Fig. 1), although we did not record these species in syntopy during our surveys. In the headwaters of the Ji-Paraná and Roosevelt Rivers there is a contact zone between P. bernhardi, Plecturocebus parecis and Plecturocebus cinerascens (Gusmão et al., Reference Gusmão, Messias, Carneiro, Schneider, de Alencar and Calouro2019) (Supplementary Fig. 1). We estimated that P. bernhardi potentially occurs in an area of 131,295 km2 (Fig. 1) of which 49.6% is legally protected.
Population estimate
We obtained 57 observations of P. bernhardi groups along the transects, with 161 individuals detected. The encounter rate was 0.2 groups/km (CV = 11.9). The best grouping of perpendicular distances to fit the detection curve was obtained with five intervals of 10 m each. The best detection function was uniform with one cosine adjustment term (Goodness-of-fit χ 2 = 0.67, df = 3, P = 0.88, AIC = 163.19; Fig. 2). Mean group size was 2.8 individuals/group (95% CI = 2.5–3.2, CV = 6.5). We estimated density to be 10.8 individuals/km2 (95% CI = 7.9–14.9, CV = 15.3), and 3.8 groups/km2 (95% CI = 2.9–5.1, CV = 13.8), and mean abundance to be 1,365,468 individuals (95% CI = 1,037,231–1,956,296, CV = 15.3).
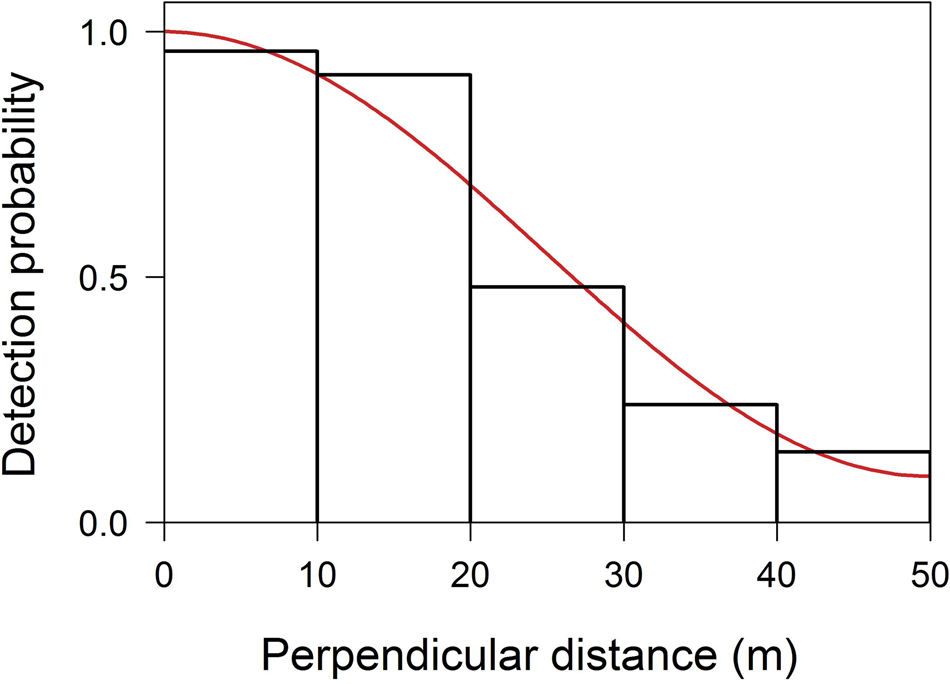
Fig. 2 Distribution of perpendicular distances of observations of P. bernhardi from the centre of transects in the Madeira–Aripuanã interfluve. The trend line indicates the best detection function fitted to the distance classes.
Reassessment of conservation status
We estimated there was a loss of 36,903 km2 of forest cover within the species' potential range during 1995–2019, 28.1% of the total original range of the species (Fig. 3). Only 0.5% of this deforestation occurred within protected areas or Indigenous lands. In our predictive models the habitat loss of the species over the next 24 years would amount to 58,365 km2 under the governance scenario and 105,289 km2 under the business-as-usual scenario (Fig. 3), 44.5 and 80.2% of the species’ potential range, respectively. We estimated predicted deforestation within protected areas and Indigenous lands would be 3.2 and 16.7% under the governance and business-as-usual scenarios, respectively. Considering the lower bound of the CI of the population abundance (1,037,231 individuals), such levels of habitat loss under the business-as-usual scenario translate into a reduction of 831,859 P. bernhardi individuals from the population over this period. Under both scenarios considered, the projected population reduction of P. bernhardi qualifies the species for categorization under a threatened category on the IUCN Red List (44.5% habitat loss indicates a categorization as Vulnerable, and 80.2% a categorization as Critically Endangered; criteria A3c, IUCN, 2012).

Fig. 3 The accumulated (1995–2019) and predicted habitat loss according to different scenarios of deforestation within the geographical range of P. bernhardi.
Discussion
Although we estimated that P. bernhardi has a minimum population size of 1,365,468 individuals within a large geographical range, the current rate of deforestation in the region will probably result in considerable decline over the next 24 years. Protected areas and Indigenous lands have been effective at maintaining low rates of deforestation within their borders, and these land categories are the only large forest fragments in the southern distribution of this species. Under the business-as-usual scenario, however, deforestation rates would increase considerably. This region is a hotspot of deforestation (Carrero et al., Reference Carrero, Fearnside, do Valle and Alves2020) and the rate of deforestation predicted by 2050 in the Amazon rainforest (Soares-Filho et al., Reference Soares-Filho, Nepstad, Curran, Cerqueira, Garcia and Ramos2006; Nobre et al., Reference Nobre, Sampaio, Borma, Castilla-Rubio, Silva and Cardoso2016; Walker et al., Reference Walker, Simmons, Arima, Galvan-Miyoshi, Antunes, Waylen and Irigaray2019) could cause reductions in the populations of several primate species (Silva et al., Reference Silva, Endo, Silva, Santos, Sampaio and Röhe2018, Reference Silva, el Bizri, Gonçalves, Lemos, Costa-Araújo and Lima2020; Rabelo et al., Reference Rabelo, Gonçalves, Silva, Rocha, Canale, Bernardo and Boubli2020). Thus the large geographical ranges of some primates in southern Amazonia should not necessarily be used to categorize a species as Least Concern, especially when most of the distribution range of a species is within an area with an actual or potential high rate of deforestation.
There have been few studies of the ecology and behaviour Amazonian titi monkeys (e.g. Palacios et al., Reference Palacios, Rodríguez and Defler1997; Bicca-Marques & Heymann, Reference Biccca-Marques, Heymann, Veiga, Barnett, Ferrari and Norconk2013; Kulp & Heymann, Reference Kulp and Heymann2015; Martinez & Wallace, Reference Martinez and Wallace2016) and inferences regarding their habitat preferences are based mainly on data from species in the Atlantic Forest (but see Wagner et al., Reference Wagner, Castro and Stevenson2009; Chagas & Ferrari, Reference Chagas and Ferrari2010). Nonetheless, the ecological flexibility of many Amazonian titi monkeys and their preference for secondary forests (Wagner et al., Reference Wagner, Castro and Stevenson2009) have been used to support their categorization as Least Concern. However, deforestation within the species' range, whether ongoing or predicted, is usually from activities that result in clear-cutting, leaving no continuous forests or trees that could be inhabited and used by any primate species. A similar situation was also found for the recently described Plecturocebus grovesi (Boubli et al., Reference Boubli, Byrne, da Silva, Silva-Júnior, Araújo and Bertuol2019) c. 230 km to the east of our study area. The species was included in the list of the 25 most threatened primates, being categorized as Critically Endangered on the IUCN Red List based on criteria A3c (i.e. population reduction projected, inferred or suspected to be met in the future; Boubli et al., Reference Boubli, Melo and Rylands2020). Questions regarding how Amazonian titi monkeys will cope with habitat fragmentation and what the genetic consequences of population reduction will be in these scenarios remain unanswered.
Based on our analysis and given the increasing rates of deforestation in the Brazilian Amazon, we recommend that P. bernhardi should be categorized at least as Vulnerable on the IUCN Red List. In addition to habitat loss, several other anthropogenic activities could further affect P. bernhardi. For example, greater contact with people in remnant forest could increase the transmission of pathogens, including diseases with high fatality rates such as yellow fever, which have been reducing populations of non-human primate species in southern Brazil significantly, including titi monkeys (de Melo Mares et al., Reference de Mello Mares, Horta, Romano, Rodrigues, Mendonça and dos Santos2020; Berthet et al., Reference Berthet, Mesbahi, Duvot, Zuberbühler, Cäsar and Bicca-Marques2021; Fernandes et al., Reference Fernandes, Cunha, Guerra, Diaz-Delgado, Ressio and Cirqueira2021). In addition, fragmentation could provide new points of access to the forest for hunters and increase the harvest of wild animals (Renó et al., Reference Renó, Novo and Escada2016). The scenario described here for P. bernhardi therefore also raises concerns for the conservation of other species in highly impacted landscape of the southern Amazon. Examples include marmosets and titi monkeys that are currently categorized as Least Concern (e.g. M. chrysoleucos, Mico intermedius, P. cinerascens) or Data Deficient (Plecturocebus miltoni, Plecturocebus vierai), but that have large portions or even the entirety of their ranges lying in the Arc of Deforestation.
Although legally protected lands are essential for the conservation of Amazonian species, their integrity and effectiveness are at risk under the policies of the current government in Brazil (Pereira et al., Reference Pereira, Ferreira, Ribeiro, Carvalho and Pereira2019, Reference Pereira, Ribeiro, Freitas and Pereira2020; Diele-Viegas et al., Reference Diele-Viegas, Pereira and Rocha2020; Paiva et al., Reference Paiva, Ruivo, Silva, Maciel, Braga and Andrade2020; Pelicice & Castello, Reference Pelicice and Castello2021). Initiatives being promoted by the government and by politicians associated with agribusiness are weakening environmental regulations for controlling deforestation, resulting in a new wave of forest loss (Magnusson et al., Reference Magnusson, Grelle, Marques, Rocha, Dias and Fontana2018; Andrade et al., Reference Andrade, Ferrante and Fearnside2021; Mataveli et al., Reference Mataveli, Chaves, Brunsell and Aragão2021; Ruaro et al., Reference Ruaro, Ferrante and Fearnside2021). Such initiatives include the proposed bills PL490/2007 and PL191/2020, allowing mining and hydropower dams within Indigenous lands, and the so called land-grabbing bills PL2633/2020 and PL510/2021, which grant an amnesty to land-grabbers and invaders that irregularly occupy exploited and deforested federal lands (Aleixo & Junior, Reference Aleixo and Junior2022). Consequently, activities that increase deforestation in the southern Amazon such as land grabbing, illegal logging, mining and fire are being facilitated against a backdrop of impunity and a lack of governance (Azevedo-Ramos et al., Reference Azevedo-RAmos, Moutinho, Arruda, Stabile, Alencar, Castro and Ribeiro2020; Cardil et al., Reference Cardil, de-Miguel, Silva, Reich, Calkin and Brancalion2020; Pereira et al., Reference Pereira, Ribeiro, Freitas and Pereira2020; das Neves et al., Reference das Neves, Blanco, Duarte, das Neves, das Neves and dos Santos2021; Mataveli et al., Reference Mataveli, Chaves, Brunsell and Aragão2021). Even a small number of misguided and irresponsible policy decisions can significantly worsen an already difficult future scenario for Amazonian primates (Estrada et al., Reference Estrada, Garber, Mittermeier, Wich, Gouveia and Dobrovolski2018; Carvalho et al., Reference Carvalho, Graham, Rebelo, Bocksberger, Meyer, Wich and Kühl2019; Sales et al., Reference Sales, Ribeiro, Pires, Chapman and Loyola2019). The increasing economic and development pressures on this biome threaten not only titi monkeys but all species currently categorized as Least Concern in this region.
Acknowledgements
Data collection and analysis were supported by the Conselho Nacional de Pesquisa e Desenvolvimento Científico e Tecnológico (process numbers: 200502/2015-8, 302140/2020-4, 300365/2021-7, 301407/2021-5, 201475/2017-0), the Conservation Leadership Programme (02111212), the Primate Action Fund (#SMA-CCO-G0000000037), Primate Conservation, Inc. (PCI#1110), the International Primatological Society, and Idea Wild. We thank the Gordon and Betty Moore Foundation (Grant Agreement to Mamirauá Institute for Sustainable Development, #5344), Isaac Theobald and Aldeísa for logistical support and Catitu and José's family for support in the field.
Author contributions
Study design: FES, HREB; data collection: FES, LPL, ACG, ODdS, JCD, MIS; data analysis: FES, LGP, CLBF, HREB; interpretation of results and writing: all authors.
Conflicts of interest
None.
Ethical standards
This research abided by the Oryx guidelines on ethical standards. There were no human subjects, experimentation with animals or collection of specimens associated with this work. This research adhered to Brazilian law governing primate research and the principles of the American Society of Primatologists for the ethical treatment of primates.






