Non-technical Summary
Paleobiology can offer diverse insights into how climate change has affected past species and ecosystems. Timely and important areas of research focus on the potential of paleobiology to contribute to solutions for climate impacts on natural ecosystems. But how far can past responses to abrupt climate change be generalized to derive predictions for the modern and future worlds? The long timescales over which biological responses are observed in the deep-time past hamper the applicability of paleontological observations, but by how much? To address these questions, we review paleontological evidence for the impacts of geologically rapid climatic change. Fruitful avenues for future research lie in (1) characterizing the relationship between the magnitude of warming and extinction toll, (2) using physiology to bridge timescales, and (3) assessing the role of long-term climate history to predict the impact of short-term climate change. Identifying how consistent and robust paleontological signals are across timescales will help to make deep-time observations more useful for the modern world.
Introduction
We are in the midst of a coupled climate and biodiversity crisis that affects human society at a global scale (Pörtner et al. Reference Pörtner, Scholes, Arneth, Barnes, Burrows, Diamond and Duarte2023). By describing how climate and biodiversity interacted in the geological past, paleobiology can provide essential information for our potential future. As the fossil record largely predates human influence, paleobiology can identify conservation-relevant patterns on the impact of climate change on species and ecosystems (Kiessling et al. Reference Kiessling, Smith and Raja2023). In this review, we delve into research challenges and explore how a well-informed road map can effectively overcome them.
Throughout Earth's history, climate has fluctuated between icehouse and greenhouse intervals largely due to plate tectonics controlling the nature and amount of greenhouse gases in the atmosphere (Müller et al. Reference Müller, Mather, Dutkiewicz, Keller, Merdith, Gonzalez, Gorczyk and Zahirovic2022) as well as the distribution of heat governed by continental configuration. The profound impact that climate change can have on the biosphere is best seen in geologically rapid events of mean temperature change. While modern observations of climate impacts on the biosphere are dominantly focused on the often more easily observed terrestrial patterns, the marine fossil record is much richer and arguably more complete. Given that our current understanding of mass extinctions is primarily based upon time series of extinction magnitude derived from marine fossils (Raup and Sepkoski Reference Raup and Sepkoski1982; Marshall Reference Marshall2023), we here emphasize marine biological responses in the pre-Pleistocene (deep-time) fossil record. In this context, we review the evidence for past marine biological responses to climate change and identify the most promising routes of research for assessing and mitigating the ecological impacts of current and future climate change.
Current ecological impacts of climate warming are already ubiquitous, ranging from phenology (the seasonal timing of biological events) and range shifts, to changes in community structure, biodiversity, growth, and (local) extinctions (Poloczanska et al. Reference Poloczanska, Burrows, Brown, García Molinos, Halpern, Hoegh-Guldberg and Kappel2016; Cooley et al. Reference Cooley, Schoeman, Bopp, Boyd, Donner, Ghebrehiwet, Ito, Pörtner, Roberts, Tignor, Poloczanska, Mintenbeck, Alegría and Craig2022; Parmesan et al. Reference Parmesan, Morecroft, Trisurat, Adrian, Anshari, Arneth, Gao, Pörtner, Roberts, Tignor, Poloczanska, Mintenbeck, Alegría and Craig2022). Changes in phenology and geographic ranges are the most obvious ecological responses to current warming, with phenology advancing 4.4 ± 1.1 days per decade and expansion of the poleward range edges averaging 72 ± 13.5 km per decade in marine habitats (Poloczanska et al. Reference Poloczanska, Brown, Sydeman, Kiessling, Schoeman, Moore and Brander2013). However, not all ecological responses are traceable in the fossil record (Table 1), and those that are observable face the issue of causal attribution. Confirming that observed fossil biotic changes are indeed attributable to climate change is sometimes challenging, as there are often other coincidental stressors. Examples include poisoning (Vandenbroucke et al. Reference Vandenbroucke, Emsbo, Munnecke, Nuns, Duponchel, Lepot, Quijada, Paris, Servais and Kiessling2015; Broadley et al. Reference Broadley, Barry, Ballentine, Taylor and Burgess2018; Rakociński et al. Reference Rakociński, Marynowski, Pisarzowska, Bełdowski, Siedlewicz, Zatoń, Perri, Spalletta and Schönlaub2020), UV radiation (Liu et al. Reference Liu, Peng, Marshall, Lomax, Bomfleur, Kent, Fraser and Jardine2023), or shutdown of photosynthesis due to global darkness (Bond and Grasby Reference Bond and Grasby2017). By looking at pre-human worlds, we gain a signal of natural environmental stressors and avoid obstacles that confront the modern climate impact research due to mismatches in the scale of human climate forcing and the observed biological impacts (Parmesan et al. Reference Parmesan, Duarte, Poloczanska, Richardson and Singer2011). Moreover, the extensive timescale of paleontological observations can provide deeper insights than modern observations into the long-term ecological and evolutionary impacts of climate change.
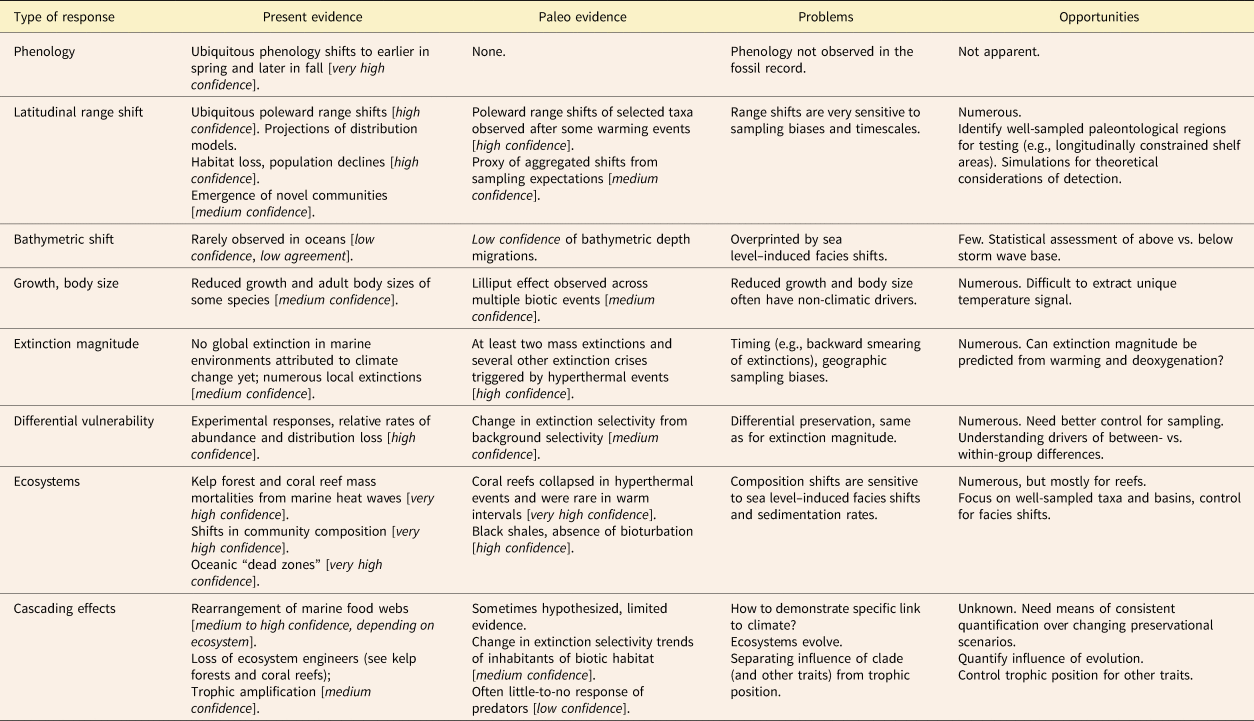
Table 1. Biologic responses to climate change, present and past. “Present evidence” column uses confidence levels as given in the IPCC WGII Sixth Assessment Report for observed evidence in modern systems (IPCC Reference Pörtner, Roberts, Tignor, Poloczanska, Mintenbeck, Alegría and Craig2022). The level of confidence is expressed using five qualifiers (“very low,” “low,” “medium,” “high,” and “very high”) and synthesizes the IPCC authors’ judgments about the validity of findings as determined through evaluation of evidence and agreement in the literature. “Paleo evidence” uses the same framework based on the references cited in this review.
The long timescales that deep-time paleontological data capture also pose challenges, particularly for application to conservation biology (Kiessling et al. Reference Kiessling, Raja, Roden, Turvey and Saupe2019). In many cases, we still lack a clear perspective on the importance of scale for assessing climate impacts. There is a rich literature on spatial scale dependency in ecological patterns (e.g., Chase and Leibold Reference Chase and Leibold2002; Keil et al. Reference Keil, Pereira, Cabral, Chase, May, Martins and Winter2018; Chase et al. Reference Chase, McGill, Thompson, Antão, Bates, Blowes and Dornelas2019; Gonzalez et al. Reference Gonzalez, Germain, Srivastava, Filotas, Dee, Gravel and Thompson2020; Huang et al. Reference Huang, Tucker, Hertel, Eyres and Albrecht2021), but much less is known about the role of temporal scale and the interaction between the two. In our context, scale dependency relates to the degree to which biological climate responses are dependent upon the time span of observation. A good example for temporal scale dependency is rate of change, wherein rates of change exhibit a power-law relationship with the time span of observation (Kemp et al. Reference Kemp, Eichenseer and Kiessling2015; Gingerich Reference Gingerich2021) such that any meaningful comparison of rates of change between the modern and deep time is prevented. For other variables, the scale dependency is less clear. For example, how well is the rank order of modern climate sensitivities among clades and ecosystems reflected in differential extinction rates in the past? To what extent does the sensitivity of reef corals to current climate change–induced heat waves (Gardner et al. Reference Gardner, Camp, Smith, Kahlke, Osman, Gendron, Hume, Pogoreutz, Voolstra and Suggett2019) correspond to increased extinction risks during ancient climate warming events? To what extent does the sensitivity of reef corals to current climate change–induced heat waves scale up to increased extinction risk at ancient climate warming events?
Here we summarize the evidence that geologically abrupt climatic changes were a dominant driver of biotic responses, and we propose research routes to reveal the impacts of deep-time climatic changes on the biosphere. We argue that our efforts will be most effective when focused on overcoming the challenges of variable spatiotemporal resolution. We highlight that promising routes are through quantifying commonalities in terms of vulnerabilities and magnitudes of change.
The Deadly Trio
Bijma et al. (Reference Bijma, Pörtner, Yesson and Rogers2013) coined the term “deadly trio” to emphasize the combined impact of warming, ocean acidification, and deoxygenation on marine ecosystems under current climate change. Each member of the deadly trio effects a range of outcomes dependent on environment, ecology, and latitude, but their strong influence on physiology means impacts tend to be interactive.
Temperature is a major driver of physiological processes (Clarke and Pörtner Reference Clarke and Pörtner2010), but oxygen supply and demand and their interactions with temperature are also crucial (Sampaio et al. Reference Sampaio, Santos, Rosa, Ferreira, Pörtner, Duarte, Levin and Rosa2021), governing metabolism and thermal tolerance (Vaquer-Sunyer and Duarte Reference Vaquer-Sunyer and Duarte2011; Reddin et al. Reference Reddin, Nätscher, Kocsis, Pörtner and Kiessling2020b). The fluctuating nature of the “oxyscape” (oxygen landscape) is thought to be a key driver of animal physiology (Fusi et al. Reference Fusi, Rigaud, Guadagnin, Barausse, Marasco, Daffonchio and Regis2023). The synergy of oxygen and temperature has far greater impacts in experiments than any other combination of temperature, oxygen, salinity, and pH (Reddin et al. Reference Reddin, Nätscher, Kocsis, Pörtner and Kiessling2020b).
Ocean acidification and other direct impacts of raised seawater CO2 have received considerable research attention in the last two decades (Kroeker et al. Reference Kroeker, Kordas, Crim, Hendriks, Ramajo, Singh, Duarte and Gattuso2013; Allison et al. Reference Allison, Cole, Hintz, Hintz, Rae and Finch2021), including the evaluation of deep-time impacts on marine life (Kiessling and Simpson Reference Kiessling and Simpson2011; Harper et al. Reference Harper, Hönisch, Zeebe, Shaffer, Haynes, Thomas and Zachos2020; Chen et al. Reference Chen, Fu, Wang, Wei, Zhang and Mansour2023). However, the impact of ocean acidification on marine life is much less consistent than often assumed (Sampaio et al. Reference Sampaio, Santos, Rosa, Ferreira, Pörtner, Duarte, Levin and Rosa2021), perhaps because pH-upregulating mechanisms exist even for relatively simple organisms such as corals (McCulloch et al. Reference McCulloch, Falter, Trotter and Montagna2012). Although evidence for ocean acidification in the end-Permian extinction has been presented (Jurikova et al. Reference Jurikova, Gutjahr, Wallmann, Flögel, Liebetrau, Posenato and Angiolini2020; Li et al. Reference Li, Wu, Shen, Wang, Chen, Algeo, Zhang and Zhang2023), the evidence for biological impacts is meager (Foster et al. Reference Foster, Hirtz, Farrell, Reistroffer, Twitchett and Martindale2022). On longer timescales (longer than 10,000 to 100,000 yr), seawater, seafloor carbonate sediments, and weathering on land buffer the effect of CO2 on pH and can even increase the seawater supersaturation of calcium carbonate (Hönisch et al. Reference Hönisch, Ridgwell, Schmidt, Thomas, Gibbs, Sluijs and Zeebe2012). Consequently, though local and short-term impacts of ocean acidification can be severe, the translation of these into observable deep-time impacts requires confirmation.
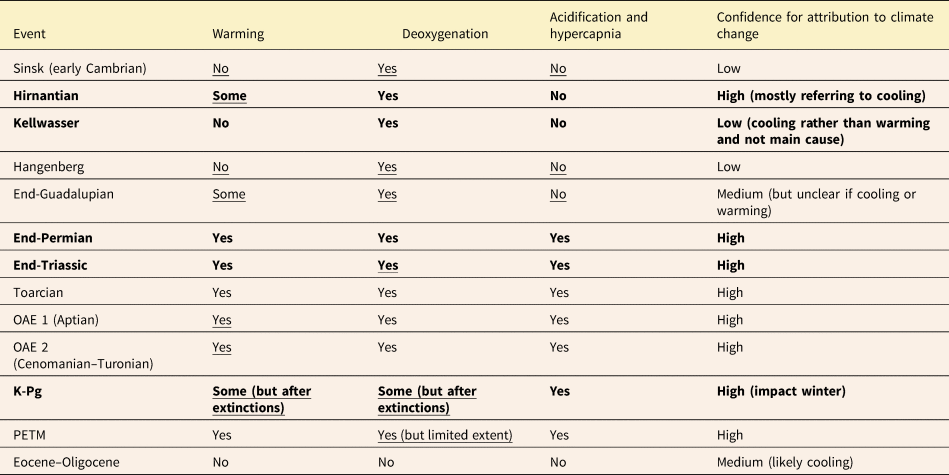
The great importance of deoxygenation in ancient biotic events is no surprise to deep-time paleobiologists, as virtually all global-scale biological events in the geological past were associated with anoxia or even euxinia (Harnik et al. Reference Harnik, Lotze, Anderson, Finkel, Finnegan, Lindberg and Liow2012; Bond and Grasby Reference Bond and Grasby2017) (Table 2). Black shales have been noted as a hallmark of Cretaceous events long before there was a broad scientific discussion of climate change (Schlanger and Jenkyns Reference Schlanger and Jenkyns1976). However, although anoxia and euxinia are facilitated by climate warming, they have numerous alternative drivers. For example, the Late Devonian events are marked by anoxia, which was likely governed by nutrient mobilization from the evolving forest ecosystems (Algeo and Scheckler Reference Algeo and Scheckler1998; Qie et al. Reference Qie, Zhang, Luo, Algeo, Chen, Xiang and Liang2023).
Table 2. Evidence of climate-related stressors across major biotic turnover events. Traditional “big five” mass extinctions are highlighted in bold. Underlined indicates evidence not yet provided in Harnik et al. (Reference Harnik, Lotze, Anderson, Finkel, Finnegan, Lindberg and Liow2012). References are listed in Supplementary Table 1. OAE, oceanic anoxic event.
Tracing Rapid Climatic Changes in Geological Time
Tracing past climatic changes with confidence is foundational to the study of their biotic impacts. Despite their shortcomings, geochemical proxies such as stable oxygen isotopes are still our main source of empirical evidence for long-term and short-term climatic changes. Such studies have been crucial to assess the magnitude of climate change across past hyperthermal events (e.g., Ruhl et al. Reference Ruhl, Bonis, Reichart, Damsté and Kürschner2011; Joachimski et al. Reference Joachimski, Lai, Shen, Jiang, Luo, Chen, Chen and Sun2012, Reference Joachimski, Alekseev, Grigoryan and Gatovsky2020; Frieling et al. Reference Frieling, Gebhardt, Huber, Adekeye, Akande, Reichart, Middelburg, Schouten and Sluijs2017; Gliwa et al. Reference Gliwa, Wiedenbeck, Schobben, Ullmann, Kiessling, Ghaderi, Struck and Korn2022; Tierney et al. Reference Tierney, Zhu, Li, Ridgwell, Hakim, Poulsen, Whiteford, Rae and Kump2022).
Paleoclimate models are another way of tracing climatic changes over time. Previous models focused on particular snapshot intervals such as the end-Cretaceous (Brugger et al. Reference Brugger, Feulner and Petri2017) or the Permian/Triassic boundary (Winguth et al. Reference Winguth, Thomas and Winguth2012, Reference Winguth, Shields and Winguth2015). A full suite of paleoclimate models across all Phanerozoic stages was first accomplished by Valdes et al. (Reference Valdes, Scotese and Lunt2021), and these are useful in providing a background against which geologically rapid climatic changes can be identified (Kiessling et al. Reference Kiessling, Smith and Raja2023) (Fig. 1). However, paleoclimate models still have issues to reliably capture some attributes of past climates such as equable latitudinal climate gradients during hothouse climates (Sagoo et al. Reference Sagoo, Valdes, Flecker and Gregoire Lauren2013) and more local features of epeiric seas (Judd et al. Reference Judd, Bhattacharya and Ivany2020). Another challenge for current climate models is the observation that climate sensitivity, the increase of surface temperature when atmospheric CO2 concentrations double, is far higher when greenhouse gas concentrations are already high (Tierney et al. Reference Tierney, Zhu, Li, Ridgwell, Hakim, Poulsen, Whiteford, Rae and Kump2022).
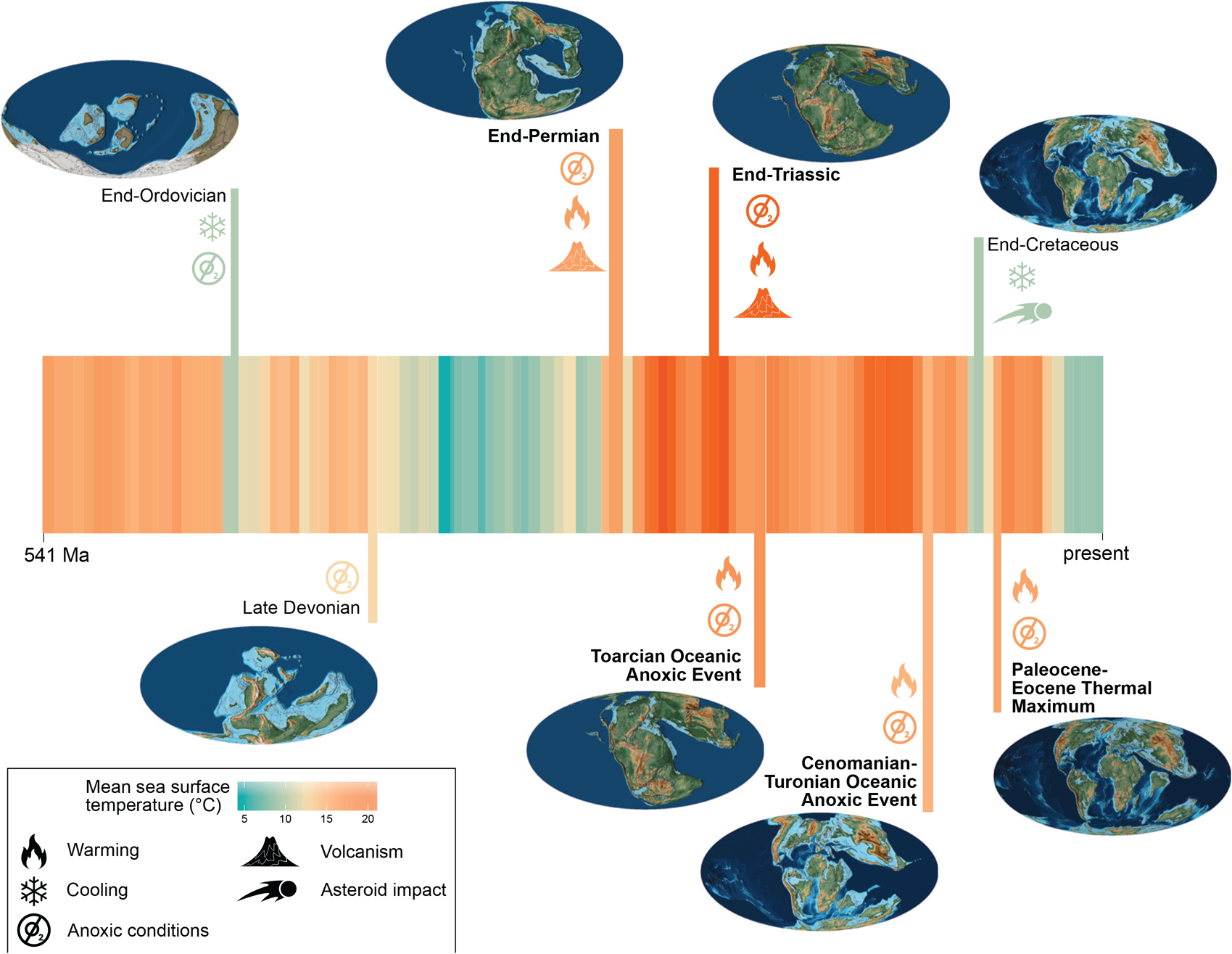
Figure 1. Climatic context of well-documented hyperthermal events across the Phanerozoic. Five extinction events (end-Ordovician, Late Devonian, end-Permian, end-Triassic, end-Cretaceous) plus three hyperthermals (Toarcian, Cenomanian–Turonian, Paleocene–Eocene thermal maximum [PETM]). Hyperthermal events are in bold. The climate stripes are based on mean global temperatures from climate models per stage (Valdes et al. Reference Valdes, Scotese and Lunt2021), and the paleographic reconstructions are from Scotese (Reference Scotese2016). Symbols refer to hypothesized ultimate (volcanism, impact) and proximate drivers (cooling, warming, deoxygenation) of extinctions.
In the face of “unknown unknowns” it is thus best to refer to proxy data for identifying ancient episodes of rapid climate change (Foster et al. Reference Foster, Hull, Lunt and Zachos2018). Such data clearly identify six hyperthermal events over the last 300 Myr that are characterized by rapid warming, deoxygenation of oceanic water, and an intensified hydrological cycle, with increased continental erosion and negative carbon isotope excursions indicating the injection of isotopically light carbon (Foster et al. Reference Foster, Hull, Lunt and Zachos2018). “Rapid” refers to both onset and duration but, importantly, is relative to geological timescales, where it can mean over tens to hundreds of thousands of years. Here, we refer to geologically rapid onset as occurring in less than 500 kyr. Besides these well-known hyperthermals, there are also other episodes of rapid climatic changes, including cooling events (Fig. 1). Some of these cooling events were associated with mass extinctions (end-Ordovician, Late Devonian, end-Cretaceous), and in the case of the end-Ordovician (Finnegan et al. Reference Finnegan, Heim, Peters and Fischer2012) and end-Cretaceous mass extinctions (Brugger et al. Reference Brugger, Feulner and Petri2017), cooling is thought to have been a primary trigger.
Among the six hyperthermal events identified in the last 300 Myr, two are exceptionally well studied: the Paleocene–Eocene thermal maximum (PETM) and the end-Permian mass extinction (EPME). These two hyperthermals showcase how similar triggers may result in vastly different responses. Both hyperthermals are thought to have been triggered by the rapid injection of greenhouse gases into the atmosphere, leading to profound warming and a global disturbance of the carbon cycle (Zachos et al. Reference Zachos, Dickens and Zeebe2008; Cui et al. Reference Cui, Li, van Soelen, Peterse and Kürschner2021). Yet, while the EPME (~252 Ma) was the most severe mass extinction of the Phanerozoic, extinction tolls across the PETM were conspicuously low (Kocsis et al. Reference Kocsis, Reddin, Alroy and Kiessling2019). Several options are available to explain the differences, ranging from the varying magnitude and rates of warming, to the different climate history, continental configuration, and buffering capacity of the Permian and Paleocene Earth systems. One immediate observation is the transient nature of the warming in the PETM (duration ca. 170 kyr; Röhl et al. Reference Röhl, Westerhold, Bralower and Zachos2007), whereas the Permian–Triassic warming lasted millions of years (Sun et al. Reference Sun, Joachimski, Wignall, Yan, Chen, Jiang, Wang and Lai2012). Explaining any vastly different outcomes of superficially similar hyperthermals is mandatory for paleobiologists aiming to contribute to current threats of biodiversity from climate change.
There is an apparent paucity of hyperthermal events in the earlier Paleozoic. Warming might have been involved in the end-Ordovician and Late Devonian mass extinctions, but they do not match Foster et al.'s (Reference Foster, Hull, Lunt and Zachos2018) definition of hyperthermals. Although the role of warming is increasingly being acknowledged in the end-Ordovician mass extinction (e.g., Bond and Grasby Reference Bond and Grasby2020), this warming was just a deglaciation of the Hirnantian glaciation, and cooling is still seen as the major trigger of the extinction (Saupe et al. Reference Saupe, Qiao, Donnadieu, Farnsworth, Kennedy-Asser, Ladant, Lunt, Pohl, Valdes and Finnegan2020). Similarly, the Late Devonian Kellwasser crisis was associated with a transient cooling episode interrupting a longer-term warming trend (Huang et al. Reference Huang, Joachimski and Gong2018). All major and minor events in the Paleozoic were associated with positive rather than negative excursions in stable carbon isotopes, which rules them out as hyperthermals sensu Foster et al. (Reference Foster, Hull, Lunt and Zachos2018). In spite of the lack of true-to-definition hyperthermal events, animals in the early Paleozoic are deemed to have been intrinsically prone to extinction because seawater temperatures were higher and continental configuration limited species’ geographic ranges (Pohl et al. Reference Pohl, Stockey, Dai, Yohler, Le Hir, Hülse, Brayard, Finnegan and Ridgwell2023).
Tracing Biological Impacts in Geological Time
Although deep-time observations are necessarily on much longer timescales than modern or historical records (Fig. 2), this is not automatically an issue if biotic responses are universal and time-scale invariant, that is, the response is similar regardless of temporal resolution and extent. For example, timescale invariance is suggested if marine extinction risk of evolutionary lineages matches the rank order of performance loss of individuals due to abiotic stressors within these lineages (Reddin et al. Reference Reddin, Nätscher, Kocsis, Pörtner and Kiessling2020b). Metabolic theory is commonly put forward to explain scale invariance. Particularly, the theory of oxygen and capacity limited thermal tolerance (OCLTT) may be applicable across organismic and evolutionary scales (Pörtner Reference Pörtner2012; Pörtner and Gutt Reference Pörtner and Gutt2016; Pörtner et al. Reference Pörtner, Bock and Mark2017). The OCLTT theory posits that molecular to whole-animal mechanisms dictate the thermal constraints on the capacity for oxygen supply to the organism in relation to oxygen demand. The synergistic effects described by OCLTT decrease individuals’ performance, which scales up over time to the vulnerability of populations and ultimately drives the species to extinction under profound climate change. OCLTT is also put forward to explain range shifts (Pörtner Reference Pörtner2021), the dwarfing of evolutionary lineages and marine communities under climate change (Calosi et al. Reference Calosi, Putnam, Twitchett and Vermandele2019), and even environmental origins of Ediacaran biota (Boag et al. Reference Boag, Stockey, Elder, Hull and Sperling2018).
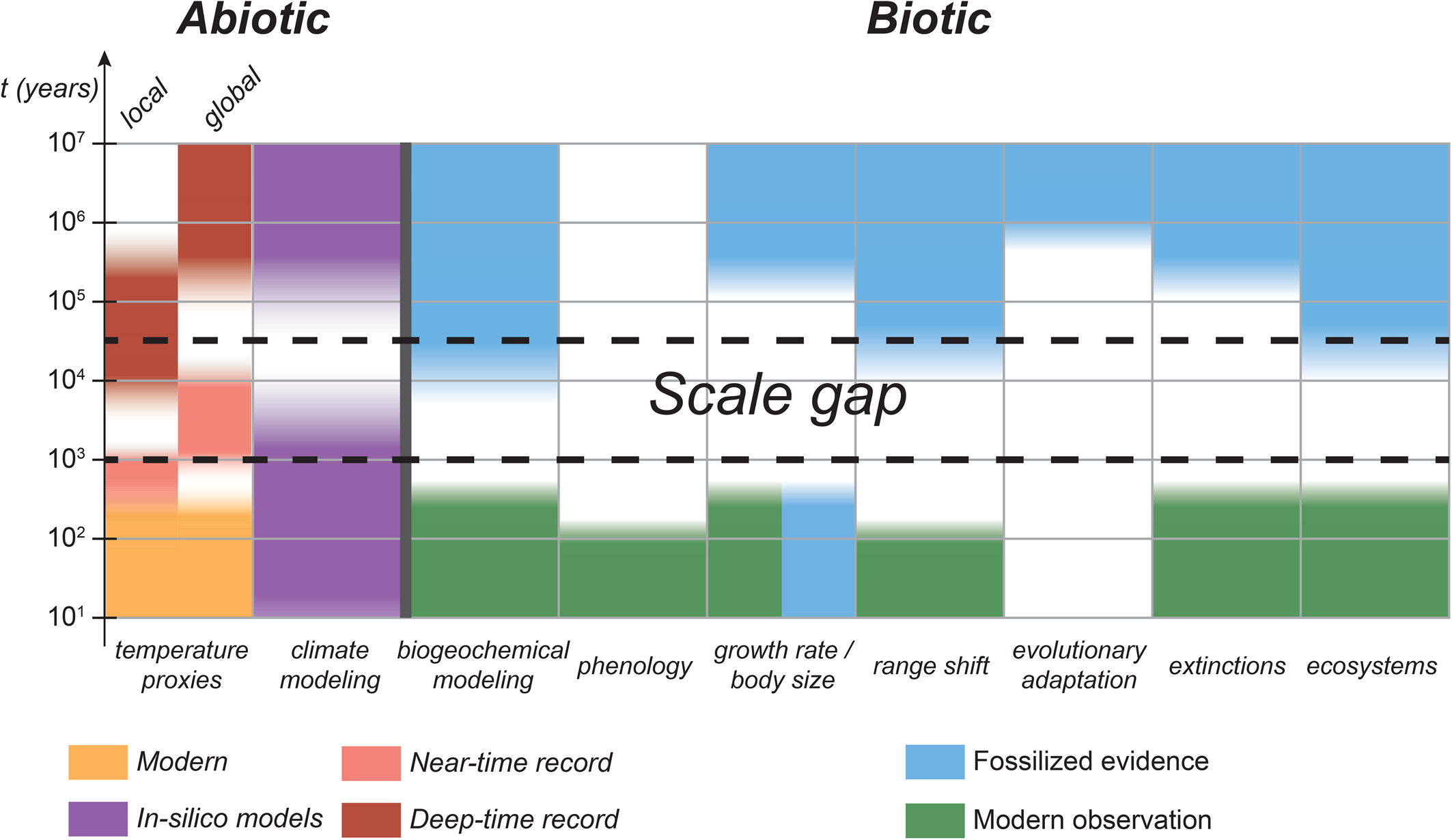
Figure 2. Timescales over which climate impacts have been studied. Timescales of less than 1 year refer to laboratory studies and mostly refer to physiological experiments. Present-day and historical observations of climate impacts refer to timescales of 100 to 102 years, whereas everything coarser than that is near time (103 to 105 years) or deep time (104 years and greater) evidence. Key references are provided in Supplementary Table 2.
To the contrary, when factors are biologically important only in local areas and on short timescales but not directly increasing extinction risk, they are instead scale variant, probably due to emergent properties in a complex system (Congreve et al. Reference Congreve, Falk and Lamsdell2017). Biotic interactions, for example, may be crucial to explain biodiversity changes at local scales and perhaps for some macroevolutionary patterns (Liow and Quental Reference Liow and Quental2024), but they are unlikely to explain the near-simultaneous extinction of many species across clades (Congreve et al. Reference Congreve, Falk and Lamsdell2017).
The degree to which biotic responses depend on particular timescales is perhaps the key question of paleontological climate impact research. Finding generalities of climate change responses, especially those that are scale invariant, will make paleontological results more relevant for the discussion of modern climate impacts. We already have a reasonable understanding of climate impacts on particular timescales (Fig. 1, Table 1), but there are still untapped opportunities for paleobiology to contribute to the field (Table 1). We briefly summarize some key paleontological evidence for the impacts of climate change on organisms, focusing on unique signals of hyperthermals.
Changes of Life-History Traits
Life-history traits such as growth rate and mode of reproduction are commonly discussed in their capacity to predict climate change impacts (Foden et al. Reference Foden, Butchart, Stuart, Vié, Akçakaya, Angulo and DeVantier2013; Pearson et al. Reference Pearson, Stanton, Shoemaker, Aiello-Lammens, Ersts, Horning and Fordham2014; MacLean and Beissinger Reference MacLean and Beissinger2017; Kubicek et al. Reference Kubicek, Breckling, Hoegh-Guldberg and Reuter2019). Here, we seek traits that are directly affected by climate change. The scope of traits to predict biological impacts is discussed in subsequent sections. The most readily preserved traits are morphological.
The reduction of adult body size under current warming is already widespread in extant species (Daufresne et al. Reference Daufresne, Lengfellner and Sommer2009; Gardner et al. Reference Gardner, Peters, Kearney, Joseph and Heinsohn2011; Forster et al. Reference Forster, Hirst and Atkinson2012; Baudron et al. Reference Baudron, Needle, Rijnsdorp and Tara Marshall2014; Calosi et al. Reference Calosi, Putnam, Twitchett and Vermandele2019). Although size reductions may only be slightly more common than size increases as a response to warming (Audzijonyte et al. Reference Audzijonyte, Richards, Stuart-Smith, Pecl, Edgar, Barrett, Payne and Blanchard2020), it is tempting to relate the widespread Lilliput effect in ancient taxa to climate warming (Piazza et al. Reference Piazza, Duarte, Renaudie and Aberhan2019, Reference Piazza, Ullmann and Aberhan2020; Rita et al. Reference Rita, Nätscher, Duarte, Weis and Baets2019). Additionally, limited oxygen supply is expected in a warming ocean due to increased oxygen demand and decreased efficiency of oxygen delivery for organisms in warmer water (Chapelle and Peck Reference Chapelle and Peck1999; Deutsch et al. Reference Deutsch, Brix, Ito, Frenzel and Thompson2011). In both the modern ocean (Audzijonyte et al. Reference Audzijonyte, Barneche, Baudron, Belmaker, Clark, Marshall, Morrongiello and van Rijn2019; Verberk et al. Reference Verberk, Atkinson, Hoefnagel, Hirst, Horne and Siepel2021) and the paleontological record (Foster et al. Reference Foster, Gliwa, Lembke, Pugh, Hofmann, Tietje, Varela, Foster, Korn and Aberhan2019), limited oxygen supply is increasingly being recognized as a culprit of reduced body size in marine invertebrates. While this makes it hard to tease apart the explicit roles of temperature versus oxygen in past crises, all hyperthermal events of the last 300 Myr are marked by a coincidence of warming and widespread anoxia. Given their synergistic impacts (see “The Deadly Trio”), a separation may only be worthwhile during hypothermal events.
While the aforementioned strong links between hyperthermals, seawater deoxygenation, and a reduction in body size exist, the pattern is not universal across the Phanerozoic. Complicating observations include an increase in the size of radiolarians across the PETM (Westacott et al. Reference Westacott, Hollis, Pascher, Dickens and Hull2022) and no size change in ostracods over the end-Permian (Nätscher et al. Reference Nätscher, Gliwa, De Baets, Ghaderi and Korn2023). In addition, a substantial Lilliput effect has also been noted in the absence of rapid warming, such as across the Cretaceous/Paleogene boundary, perhaps due to limited food supply (Smith and Jeffery Reference Smith and Jeffery1998; Aberhan et al. Reference Aberhan, Weidemeyer, Kiessling, Scasso and Medina2007). We are thus still lacking evidence for the generality of any distinct intraspecific body-size signature of hyperthermal events.
Skeletal growth specifically of reef corals appears to be impaired by warming (Cantin et al. Reference Cantin, Cohen, Karnauskas, Tarrant and McCorkle2010). However, there is a complex connection between growth rate, skeletal density, and temperature (Brachert et al. Reference Brachert, Reuter, Krüger, Böcker, Lohmann, Mertz-Kraus and Fassoulas2013), which renders it difficult to find a direct connection between temperature change and growth rate in fossil materials. Calcification rates of fossil Cenozoic corals are mostly lower than those of modern reef corals, which could be attributed to warmer climates of the past but perhaps more plausibly to a lower aragonite saturation state (Brachert et al. Reference Brachert, Corrège, Reuter, Wrozyna, Londeix, Spreter and Perrin2020). Similarly, irregular growth bands in surviving corals of the end-Triassic mass extinction have been attributed to environmental stress, but not necessarily temperature (Kiessling et al. Reference Kiessling, Roniewicz, Villier, Leonide and Struck2009). Modern studies supporting a thinning or change in the skeletal structure of bivalve shells (Mackenzie et al. Reference Mackenzie, Ormondroyd, Curling, Ball, Whiteley and Malham2014; Fitzer et al. Reference Fitzer, Torres Gabarda, Daly, Hughes, Dove, O'Connor, Potts, Scanes and Byrne2018) have yet to be demonstrated in past hyperthermals. Again, a distinct climate signal in growth and skeletal attributes is lacking across past episodes of rapid climate change. Metabolic theory suggests that body-size changes may become more variable and partly dependent on dispersal ability with warming (Deutsch et al. Reference Deutsch, Penn, Verberk, Inomura, Endress and Payne2022), which opens avenues for future research.
Distribution Shifts
Poleward range shifts are among the clearest responses of current climate warming (Sunday et al. Reference Sunday, Bates and Dulvy2012; Pecl et al. Reference Pecl, Araújo, Bell, Blanchard, Bonebrake, Chen and Clark2017; Kumagai et al. Reference Kumagai, García Molinos, Yamano, Takao, Fujii and Yamanaka2018). Ranges shift rapidly, especially in the ocean (Poloczanska et al. Reference Poloczanska, Brown, Sydeman, Kiessling, Schoeman, Moore and Brander2013; Lenoir et al. Reference Lenoir, Bertrand, Comte, Bourgeaud, Hattab, Murienne and Grenouillet2020), and track climate velocities well (Pinsky et al. Reference Pinsky, Worm, Fogarty, Sarmiento and Levin2013, Reference Pinsky, Selden and Kitchel2020), especially when considering dispersibility (Sunday et al. Reference Sunday, Pecl, Frusher, Hobday, Hill, Holbrook and Edgar2015). The rapid response of distributions to climate change challenges their detection in deep time (Yasuhara and Deutsch Reference Yasuhara and Deutsch2022). A short warming pulse such as the PETM likely leaves behind only limited numbers of fossils in higher latitudes, before subsequent cooling would shift ranges back equatorward. This would limit the chances of detecting transient range shifts in the fossil record. With this in mind, it is surprising that the PETM is especially rich in documented range shifts (Bowen et al. Reference Bowen, Clyde, Koch, Ting, Alroy, Tsubamoto, Wang and Wang2002; Wing et al. Reference Wing, Harrington, Smith, Bloch, Boyer and Freeman2005), although well-documented marine examples are limited to microplankton (Speijer et al. Reference Speijer, Scheibner, Stassen and Morsi2012). Equatorward range shifts with cooling have also been recorded (Kelley and Raymond Reference Kelley and Raymond1991), but there is only a single demonstration of latitudinal range shifts in accord with long-term temperature change across the Phanerozoic. This study (Reddin et al. Reference Reddin, Kocsis and Kiessling2018, Reference Reddin, Kocsis and Kiessling2020a), after correcting for sampling, found that range shifts roughly followed temperature changes derived from stable oxygen isotopes ever since the Ordovician.
Rather than tracing surviving taxa between time bins, quantifying latitudinal diversity gradients (LDG) may reveal the interplay between evolutionary dynamics and range shifts (Jablonski et al. Reference Jablonski, Roy and Valentine2006; Raja and Kiessling Reference Raja and Kiessling2021). The observation of diversity peaks away from the tropics, especially during greenhouse intervals, is suggestive of a climate signal (Mannion et al. Reference Mannion, Upchurch, Benson and Goswami2014; Brodie and Mannion Reference Brodie and Mannion2023), as tropical temperatures often approached metazoan limits (Storch et al. Reference Storch, Menzel, Frickenhaus and Pörtner2014) and the optimal temperature for aquatic phyla is centered around 20°C (Boag et al. Reference Boag, Gearty and Stockey2021; Costello et al. Reference Costello, Corkrey, Bates, Burrows, Chaudhary, Edgar, Stuart-Smith, Yasuhara and Wei2023). Overheated tropics seem to limit biodiversity due to both regional extirpations and raised relative extinction rates (Reddin et al. Reference Reddin, Kocsis and Kiessling2019). Latitudinal diversity shifts match well with range shifts on short timescales because here distributional changes alone, rather than speciation and extinction, are responsible for the LDG (Kiessling et al. Reference Kiessling, Simpson, Beck, Mewis and Pandolfi2012; Yasuhara et al. Reference Yasuhara, Wei, Kucera, Costello, Tittensor, Kiessling and Bonebrake2020; Chaudhary et al. Reference Chaudhary, Richardson, Schoeman and Costello2021). The flat LDG in the aftermath of the EPME is a mixed signal of high tropical extinction and poleward range shifts (Song et al. Reference Song, Huang, Jia, Dai, Wignall and Dunhill2020).
Besides poleward range shifts, depth migration might appear to be a promising route to escape the heat of shallow waters (Yasuhara and Deutsch Reference Yasuhara and Deutsch2023). Two factors limit migration of shallow-water species into greater water depths: (1) limited food supply in more light-limited, deeper settings and (2) limited oxygen availability in deeper waters approaching the oxygen minimum zone (Breitburg et al. Reference Breitburg, Levin, Oschlies, Grégoire, Chavez, Conley and Garçon2018). Accordingly, modern depth shifts are more pronounced in midlatitudes (Chaikin and Belmaker Reference Chaikin and Belmaker2023) and late Pleistocene to Holocene depth shifts have been observed in deep-sea ostracods for which the aforementioned limitations do not apply (Yasuhara et al. Reference Yasuhara, Cronin, deMenocal, Okahashi and Linsley2008). Perhaps due to the association of anoxia with ancient hyperthermal events, patterns of fossil depth shifts are not documented across past hyperthermals.
Extinction
Extinction is a defining theme for paleontology and perhaps the greatest asset of the discipline for deep-time climate impact research. Confirmed extinctions due to current climate change are rare (IPCC Reference Pörtner, Roberts, Tignor, Poloczanska, Mintenbeck, Alegría and Craig2022). Although future projections using species distribution models reach 14–32% of macroscopic species within 50 yr under even intermediate warming scenarios (Wiens and Zelinka Reference Wiens and Zelinka2024), the scope of species distribution models to predict extinction risk is currently problematic (Zurell et al. Reference Zurell, Fritz, Rönnfeldt and Steinbauer2023). The wealth of empirical evidence for climate-related extinction, properly scaled, provides a unique opportunity for paleontological data to validate such models and presumably the future under high warming scenarios.
For marine extinctions, the past drivers were likely the same deadly trio threatening modern marine life under climate change: warming, deoxygenation, and acidification (Payne and Clapham Reference Payne and Clapham2012). Groups that are most vulnerable to climate-related stressors on the timescale of days to weeks tend to have higher extinction rates, especially during hyperthermal events, than groups with greater resilience (Reddin et al. Reference Reddin, Nätscher, Kocsis, Pörtner and Kiessling2020b). Laboratory experiments suggest that synergies among stressors particularly pertain to combined warming and hypoxia, while other combinations of stressors such as warming and acidification do not lead to a greater effect size than individual stressors (Reddin et al. Reference Reddin, Nätscher, Kocsis, Pörtner and Kiessling2020b). Metabolic expectations combined with Earth system modeling also suggested that the combination of warming and deoxygenation can cause sufficient habitat loss to drive mass extinction (Deutsch et al. Reference Deutsch, Ferrel, Seibel, Pörtner and Huey2015; Penn et al. Reference Penn, Deutsch, Payne and Sperling2018).
Seeking life-history traits of raised vulnerability has a long history in paleobiology, particularly across major extinction events (e.g., Jablonski and Raup Reference Jablonski and Raup1995; Aberhan and Baumiller Reference Aberhan and Baumiller2003; Kiessling et al. Reference Kiessling, Aberhan, Brenneis and Wagner2007; Clapham and Payne Reference Clapham and Payne2011; Payne et al. Reference Payne, Bush, Heim, Knope and McCauley2016; Clapham Reference Clapham2017; Foster et al. Reference Foster, Allen, Kitzmann, Münchmeyer, Rettelbach, Witts, Whittle, Larina, Clapham and Dunhill2023). However, there are few studies to assess the specific signal of climate change (Ezard et al. Reference Ezard, Aze, Pearson and Purvis2011; Woodhouse et al. Reference Woodhouse, Swain, Fagan, Fraass and Lowery2023). Reddin et al. (Reference Reddin, Kocsis, Aberhan and Kiessling2021) compared a large suite of traits alongside taxonomic patterns across the six hyperthermal events identified by Foster et al. (Reference Foster, Hull, Lunt and Zachos2018) and found that photosymbiosis, deposit feeding, having actively burrowing or swimming adult life stages, an affinity for reef habitat, or aragonitic relative to low-Mg calcite skeletal composition consistently enhanced the extinction vulnerability of marine genera. Vulnerability here was defined relative to the genus's background extinction selectivity (sensu Raup and Boyajian Reference Raup and Boyajian1988). A meta-analysis showed that acidification responses of organisms in modern experiments were particularly severe for those with greater fossilization potential, suggesting that ancient acidification impacts should be particularly clear in the suite of organisms preserved as fossils (Reddin et al. Reference Reddin, Nätscher, Kocsis, Pörtner and Kiessling2020b). Surprisingly, ancient mass extinctions, including those that were triggered by hyperthermals, did not selectively remove larger-bodied forms (Payne et al. Reference Payne, Bush, Heim, Knope and McCauley2016). However, hyperthermals appear to increase the relative extinction risk of larger-bodied genera compared with non-hyperthermal times (Reddin et al. Reference Reddin, Kocsis, Aberhan and Kiessling2021).
A robust prediction for the expected extinction toll based on a certain magnitude of global temperature change would be a leap forward. Song et al. (Reference Song, Kemp, Tian, Chu, Song and Dai2021) argued that 5.2°C of temperature change, warming or cooling, is a critical threshold for a mass extinction to occur, whereas Kaiho (Reference Kaiho2022) suggested 7°C of absolute temperature change as a critical value. Given that these results were achieved at rather coarse stratigraphic resolutions, revisiting the basic results at finer temporal resolutions would certainly be beneficial (Spalding and Hull Reference Spalding and Hull2021). Song et al. (Reference Song, Kemp, Tian, Chu, Song and Dai2021) also reported a rate of climate change of >10°C/Myr as critical for a mass extinction event. As we discuss later, referring to rates is generally problematic when timescales are different (see “The Crux with Rates of Change”). Although the critical rates may be informative on the stage-level stratigraphic resolutions in Song et al. (Reference Song, Kemp, Tian, Chu, Song and Dai2021), they have no relevance for current rates of change.
A potentially important and fitting subject for paleobiological scrutiny is the influence and mechanism of evolutionary legacies, which shape the vulnerability of organisms to climate change (Bennett et al. Reference Bennett, Sunday, Calosi, Villalobos, Martínez, Molina-Venegas and Araújo2021). The longer-term climatic history a clade has experienced influences its response to short-term climate change (Mathes et al. Reference Mathes, Kiessling and Steinbauer2021a,Reference Mathes, van Dijk, Kiessling and Steinbauerb). For example, abrupt warming is more deleterious when built upon a long-term warming trend than when the short-term warming follows a long-term cooling (Mathes et al. Reference Mathes, van Dijk, Kiessling and Steinbauer2021b). Coinciding directions of recent and legacy climatic change appear to consistently amplify the extinction response to recent climatic change, either in the case of warming or cooling. Evolutionary legacy may also be behind the observation that tropical species live close to their upper thermal limits, such that even mild temperature increases can lead to large regional species losses (Hodapp et al. Reference Hodapp, Roca, Fiorentino, Garilao, Kaschner, Kesner-Reyes and Schneider2023). More work on the relative influence of climate legacy on extinction risk is clearly a promising avenue for future research.
Ecosystems
Assessing the response of entire ecosystems to climate changes is perhaps more important than evaluating the responses of individual species. Clearly, the cumulative pressures on individual species must lead to altered ecosystem resilience (Malhi et al. Reference Malhi, Franklin, Seddon, Solan, Turner, Field and Knowlton2020). Modern marine ecosystems are sometimes said to be already exposed to pressures not encountered in millions of years (Hoegh-Guldberg and Bruno Reference Hoegh-Guldberg and Bruno2010), but this statement is not sufficiently scrutinized.
The main changes observed in modern marine ecosystems due to climate change include reduced primary productivity in most regions, altered food web dynamics, and reduced abundance of habitat-forming species (Hoegh-Guldberg and Bruno Reference Hoegh-Guldberg and Bruno2010; Cooley et al. Reference Cooley, Schoeman, Bopp, Boyd, Donner, Ghebrehiwet, Ito, Pörtner, Roberts, Tignor, Poloczanska, Mintenbeck, Alegría and Craig2022). These patterns can also be observed in response to past climatic changes.
Changes in past primary productivity can be traced with geochemical methods, which are beyond the scope of this review. However, there is increasing evidence for reduced primary productivity across the end-Permian (Algeo et al. Reference Algeo, Henderson, Tong, Feng, Yin and Tyson2013; Shen et al. Reference Shen, Schoepfer, Feng, Zhou, Yu, Song, Wei and Algeo2015; Grasby et al. Reference Grasby, Beauchamp and Knies2016) and end-Triassic crises (Schoepfer et al. Reference Schoepfer, Algeo, Ward, Williford and Haggart2016; Fujisaki et al. Reference Fujisaki, Fukami, Matsui, Sato, Sawaki and Suzuki2020) in line with projections under future climate change (Cooley et al. Reference Cooley, Schoeman, Bopp, Boyd, Donner, Ghebrehiwet, Ito, Pörtner, Roberts, Tignor, Poloczanska, Mintenbeck, Alegría and Craig2022). As a consequence of extinctions and reduced productivity, altered food web structures are to be expected (Roopnarine Reference Roopnarine2006) and have been noted across major environmental disturbances (e.g., Aberhan and Kiessling Reference Aberhan and Kiessling2015; Huang et al. Reference Huang, Chen, Roopnarine, Benton, Zhao, Feng and Li2023). Such changes are also evident in bioturbation intensity, which is commonly reduced after climate-induced crises (Calosi et al. Reference Calosi, Putnam, Twitchett and Vermandele2019). Increases in extinction risk of deposit feeders during hyperthermals relative to other times (Reddin et al. Reference Reddin, Kocsis, Aberhan and Kiessling2021) may occur because rapidly warming and periodically anoxic seafloor conditions sway resource competition among deposit feeders away from metazoans and toward prokaryotes (Crichton et al. Reference Crichton, Wilson, Ridgwell, Boscolo-Galazzo, John, Wade and Pearson2023).
Reduced abundance of habitat-forming species is a key response to climate change in present and past marine ecosystems. Together with kelp forests, coral reefs are known as the marine ecosystem most vulnerable to modern climate change (Cooley et al. Reference Cooley, Schoeman, Bopp, Boyd, Donner, Ghebrehiwet, Ito, Pörtner, Roberts, Tignor, Poloczanska, Mintenbeck, Alegría and Craig2022). Coral reefs also have an excellent geological record tracing back at least to the Ordovician (Kröger et al. Reference Kröger, Desrochers and Ernst2017). Although there is no significant cross-correlation between mean seawater temperature and fossil reef abundance (Kiessling Reference Kiessling, Kiessling, Flügel and Golonka2002, Reference Kiessling2009), it is nevertheless striking that times of global warmth such as the middle and Late Cretaceous and the early Paleogene were marked by depressed reef building (Pandolfi and Kiessling Reference Pandolfi and Kiessling2014). Likewise, the biotic composition of reefs seems to be linked to seawater temperature. For example, the long-term change from microbial-sponge-dominated reefs to coral-dominated reefs in the Triassic may be linked to cooling (Kiessling Reference Kiessling2010), whereas the Cretaceous demise of coral reefs and increase of rudist biostromes may be linked to warming (Pandolfi and Kiessling Reference Pandolfi and Kiessling2014). In this way, the reef builder's abundance and altered composition is a consistent signature of climate response. The study of habitat-forming species, their interactions, and ecosystem service from deep time to modern offers unique insights into ecosystem responses to climate change.
Adaptation to Climate Changes
The scope of modern marine organisms for local adaptation in a warming world is strongly debated (Hoffmann and Sgrò Reference Hoffmann and Sgrò2011; Araújo et al. Reference Araújo, Ferri-Yáñez, Bozinovic, Marquet, Valladares and Chown2013; Munday et al. Reference Munday, Warner, Monro, Pandolfi and Marshall2013; Kurman et al. Reference Kurman, Gómez, Georgian, Lunden and Cordes2017; Torda et al. Reference Torda, Donelson, Aranda, Barshis, Bay, Berumen and Bourne2017; Bairos-Novak et al. Reference Bairos-Novak, Hoogenboom, van Oppen and Connolly2021; Lachs et al. Reference Lachs, Donner, Mumby, Bythell, Humanes, East and Guest2023). Although the evolvability of many organismic traits is high, the adaptation potential to warming climates appears to be low (Araújo et al. Reference Araújo, Ferri-Yáñez, Bozinovic, Marquet, Valladares and Chown2013). Thermal limits have evolved mostly to become narrower with the evolution of increasingly complex domains of life (Storch et al. Reference Storch, Menzel, Frickenhaus and Pörtner2014). The fossil record supports that adaptation options to climate change are limited, with increasing evidence of niche conservatism within evolutionary lineages. Specifically, the thermal niches of species appear to be conserved over thousands to millions of years in foraminifers and mollusks, respectively (Yasuhara et al. Reference Yasuhara, Hunt, Dowsett, Robinson and Stoll2012; Saupe et al. Reference Saupe, Hendricks, Portell, Dowsett, Haywood, Hunter and Lieberman2014; Antell et al. Reference Antell, Fenton, Valdes and Saupe2021). Similarly, the message of the deep-time coral reef record is clear: coral reefs have limited scope for adaptation to climate warming, regardless of the inferred rate of change. Times of prolonged warming were equally bad for coral reef development, as were times of geologically rapid warming (Pandolfi and Kiessling Reference Pandolfi and Kiessling2014).
Rates of climate change are often in focus for the assessment of the adaptation potential (Peck et al. Reference Peck, Clark, Morley, Massey and Rossetti2009; Quintero and Wiens Reference Quintero and Wiens2013; Jezkova and Wiens Reference Jezkova and Wiens2016; Fordham et al. Reference Fordham, Jackson, Brown, Huntley, Brook, Dahl-Jensen and Gilbert2020; Williams et al. Reference Williams, Ordonez and Svenning2021). Simulations support that rates of climate change are critically important for speciation and extinction (Qiao et al. Reference Qiao, Saupe, Soberon, Peterson and Myers2016), but we argue that empirical rate measurements in deep time have no direct relevance for current climate impact research, especially not when they are compared across vastly different time spans such as this century against evolutionary rates measured over millions of years (Quintero and Wiens Reference Quintero and Wiens2013).
The Crux with Rates of Change
Rates of natural processes are virtually impossible to compare across timescales. Most natural processes advance more like a car on a long-distance trip rather than a rocket in space; similar to how the average speed of a car decreases as the distance increases due to bends in the road and pauses as the driver gets distracted, or needs to sleep, the progression of these processes slows down over time. Similarly, evolutionary rates, rates of climate change, and some measures of extinction rates are critically dependent upon the timescale of observation (Blois and Hadly Reference Blois and Hadly2009; Gingerich Reference Gingerich2009, Reference Gingerich2019, Reference Gingerich2021; Kemp et al. Reference Kemp, Eichenseer and Kiessling2015). Because none of these processes are monotonic, the true rate can change over time and, like a detoured car, even reverse direction (Fig. 3). Amid a longer-term warming trend, there might be intervals of stasis and even cooling. Similarly, there might be times of evolutionary stasis amid a directional long-term change. Overall, these vagaries produce a negative power-law relationship between time span of observation and observed rate of change. Without considering this temporal scaling, any comparison of historical and prehistorical rates of change is meaningless (Gingerich Reference Gingerich2019; Watkins Reference Watkins2023). It is mathematically straightforward to normalize rates to any arbitrary time span, but it is better to compare rates only when either the numerator (magnitude of change) or denominator (time span of change) is the same (Gingerich Reference Gingerich2019). Achieving a finer temporal resolution across hyperthermal events may also help to assess the basic pattern of true rate trajectories.
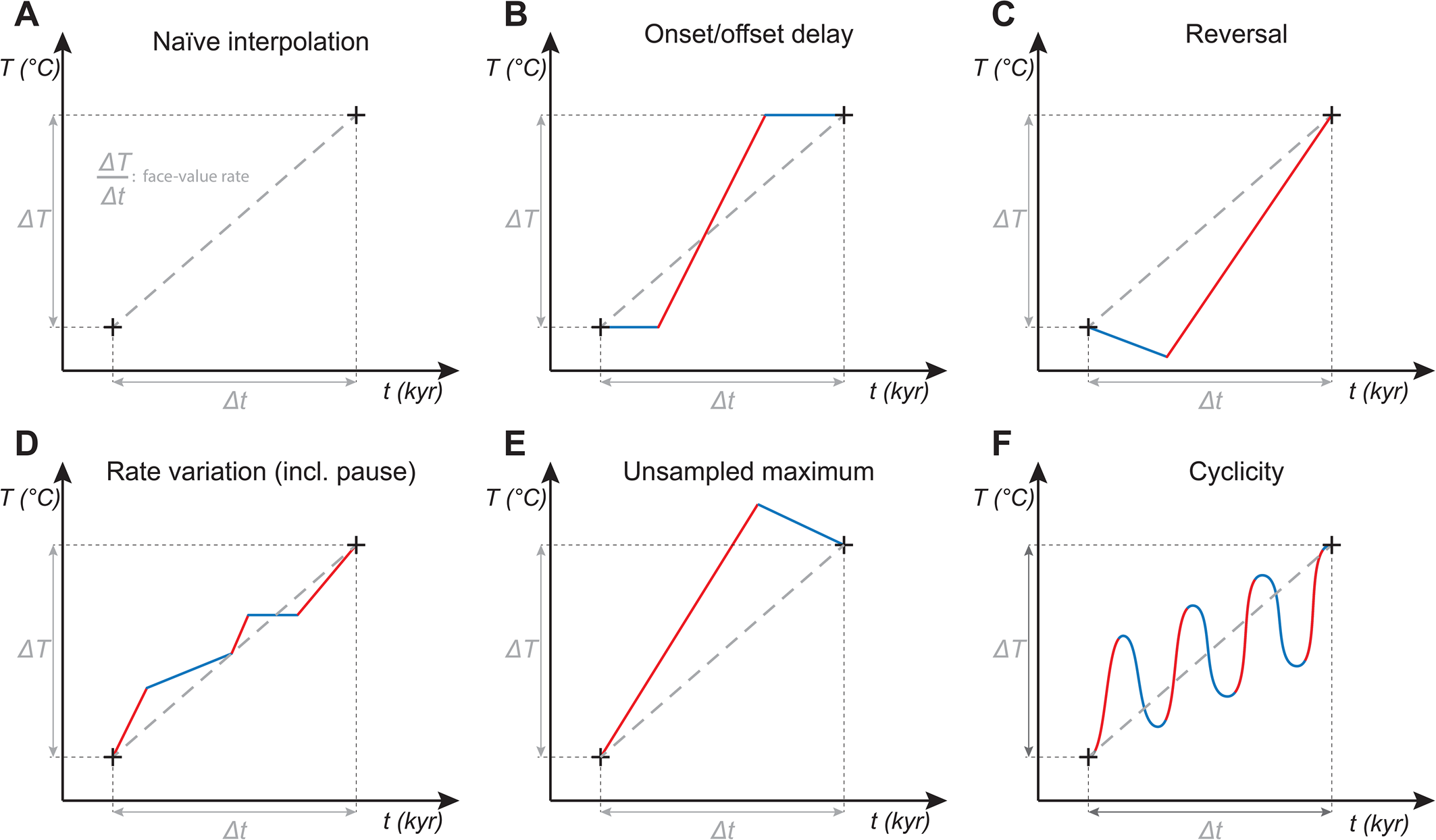
Figure 3. Potential patterns of temperature rise (ΔT) within a longer (e.g., geologically resolvable) time interval (Δt) of observation. Taking the rates at face value implies a naïve, linear interpolation (A). True temperature changes are likely to be much more complex and made up as a composite of patterns (B–F), all involving greater rates (steeper slopes, red color) at some times within Δt than the naïve rate. Increasing the temporal resolution is unlikely to capture the true maximum rate within Δt, but it might inform us of the underlying process(es). Within each panel, all trajectories move from the lower left to the upper right cross.
Research Challenges and a Way Forward
There are promising routes for paleobiology to better address and translate into conservation strategies under climate change. As discussed previously (Kiessling et al. Reference Kiessling, Smith and Raja2023), there are a number of simple measures to make paleontological findings more policy relevant. Foremost, reporting of paleobiological results needs to be improved by being clearer about effect sizes, uncertainties, and time spans over which effects are observed. Second, research needs to be more targeted toward projecting climate change impacts. The search for commonalities among mass extinctions was once fruitful for revealing macroevolutionary principles, but now a critical comparison among past hyperthermal events may be more rewarding (Reddin et al. Reference Reddin, Aberhan, Dimitrijević, Dowding, Kocsis, Mathes, Nätscher, Patzkowsky and Kiessling2023).
We identify three outstanding scientific questions: (1) Context dependency: why are responses to similar climatic changes sometimes very different? (2) Scale dependency: which responses, if any, are scale-invariant and how can we quantify scale-dependence? (3) Applicability: how can we translate deep-time paleontological findings into conservation strategies?
The first question is perhaps the most pressing. There are differences in extinction magnitude that cannot be simply attributed to differences in climate forcing. There seems to be a stronger impact of hyperthermals that occurred during Pangean times than later on, which has been attributed to a lack of chemical buffering in the pre-Jurassic oceans (Zeebe and Westbroek Reference Zeebe and Westbroek2003; Wignall Reference Wignall2016). This hypothesis makes the implicit assumption that disturbances of the carbon cycles, for example, through ocean acidification, were directly responsible for the extinctions. Following Sampaio et al. (Reference Sampaio, Santos, Rosa, Ferreira, Pörtner, Duarte, Levin and Rosa2021) and Reddin et al. (Reference Reddin, Nätscher, Kocsis, Pörtner and Kiessling2020b), we challenge this assumption, noting that the interplay of deoxygenation and temperature rise was likely a major extinction trigger. Recent evidence suggests that the extent of anoxia across the PETM was much more limited than during Mesozoic anoxic events (Clarkson et al. Reference Clarkson, Lenton, Andersen, Bagard, Dickson and Vance2021) and the ocean was generally less prone to anoxia after the Turonian (Smith and Stockley Reference Smith and Stockley2005; Kiessling and Kocsis Reference Kiessling and Kocsis2015). Climate legacies (Mathes et al. Reference Mathes, van Dijk, Kiessling and Steinbauer2021b) and the shorter duration of the warming might also contribute to the lower impact of post-Cretaceous warmings.
The second question (scale dependency) is directly linked to the third (applicability). Scale dependency needs to be quantified as much as possible with both empirical paleontological work and modeling. How predictable are the impacts of climate change across timescales? For example, sponges are sometimes conceived as the winners of climate change relative to corals (Bell et al. Reference Bell, Bennett, Rovellini and Webster2018), but the evidence is equivocal in the modern ocean (Lesser and Slattery Reference Lesser and Slattery2020). The fossil record tells us that sponges were consistently less vulnerable during past hyperthermal events than corals when differing sampling completeness is taken into account (Reddin et al. Reference Reddin, Kocsis, Aberhan and Kiessling2021), but more work is clearly needed to specify which groups of sponges might be the future reef builders. We may benefit from ecological approaches to measure scale dependence in biodiversity–ecosystem functioning relationships (Gonzalez et al. Reference Gonzalez, Germain, Srivastava, Filotas, Dee, Gravel and Thompson2020).
The fossil record can also help reveal how a climate-induced mass extinction unfolds. Gradually increasing warming leading to gradual body-size reductions and pulsed extinctions culminating in a more or less abrupt extinction have been documented for the EPME (Kiessling et al. Reference Kiessling, Schobben, Ghaderi, Hairapetian, Leda and Korn2018; Gliwa et al. Reference Gliwa, Wiedenbeck, Schobben, Ullmann, Kiessling, Ghaderi, Struck and Korn2022). However, we still need a higher temporal resolution of both geochemical and paleontological data at global scales, including a consideration of sampling biases at all hyperthermal events. Simulations based on metabolic theory are promising to bridge gaps in space, time, and genealogical as well as ecological hierarchies (Penn and Deutsch Reference Penn and Deutsch2022). For instance, poleward range shifts may be a precursor to raised tropical and polar extinctions (the former being scale dependent and the latter potentially scale invariant), as endemic species are funneled into habitat area bottlenecks (Reddin et al. Reference Reddin, Aberhan, Raja and Kocsis2022). Finally, assessing the impacts of ancient climatic events also requires robust estimates of background variability and a comparison with events that were likely not primarily driven by climate change. The latter are rare, as even the impact-driven end-Cretaceous mass extinction had severe climatic consequences, albeit with cooling dominating (Vellekoop et al. Reference Vellekoop, Esmeray-Senlet, Miller, Browning, Sluijs, van de Schootbrugge, Sinninghe Damsté and Brinkhuis2016). In addition, background variability is probably insufficiently characterized due to the research focus of extreme events. A future quest will thus be to contrast hyperthermal extinction drivers against non-hyperthermal drivers.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (KI 806/15-2, KI 806/17-1, AB 109/11-1, Ko 5382/2-1), and by the Paleosynthesis Project through the Volkswagen Stiftung (Az 96 796). The work is embedded in the Research Unit TERSANE (FOR 2332: Temperature-related Stressors as a Unifying Principle in Ancient Extinctions). We thank reviewers P. Monarrez and M. Yasuhara as well as editor M. Patzkowsky for their valuable comments.
Competing Interest
The authors declare no competing interests.
Data Availability Statement
Supplementary materials are available from the Zenodo Repository: https://zenodo.org/doi/10.5281/zenodo.13283783.







