Non-technical Summary
The Paleozoic evolution of a complex terrestrial biota has been among the most important events in Earth history. Here, we address how to integrate information from fossil and extant life highlighting four fundamental patterns: (1) The soil fauna shows remarkable continuity over 400 million years through to the present. (2) The evolution of animal ecologies closely tracks the opportunities provided by plant evolution via flight and burrowing as plants evolved the canopy stature and rooting depth of trees. (3) The skewed nutrient ratios of plants are a challenge that can be avoided by means of small body size, but that then places limits on the maximum body sizes seen before the evolution of insect and vertebrate herbivores. (4) The most recent 150 million years suggest a second pulse of animal terrestrializations, perhaps related to the evolution of flowering plants, but this linkage is questionable.
Introduction
The Perils of a Part-Time Focus
The advent of a complex land biota of multicellular plants, animals, and fungi has been as important for shaping the geobiological trajectory of our planet as the evolution of oxygenic photosynthesis, the Cambrian explosion of animal life, and various mass extinctions. And yet, terrestrialization lacks a dedicated group of researchers equivalent to those focused on other fundamental events in Earth history; we all visit part-time from our own disciplines. That may mean being a paleobotanist, paleoentomologist, vertebrate paleontologist, or a member of one of the similarly distinct communities of scientists studying the living members of the biota and their phylogenetic relationships. It could mean being a geochemist, sedimentologist, or Earth system modeler intersecting potential environmental implications of terrestrial life. For decades, studies of the Cambrian explosion have integrated marine geochemical data with sedimentological and ichnological data pertaining to animal behavior on and in the ocean floor, with biological data bridging fossil occurrences, divergence dates estimated from molecular clocks, developmental biology, and ecological inferences about the relationships among animals at different trophic levels (Erwin and Valentine Reference Erwin and Valentine2013). However, similarly integrative studies pertaining to terrestrialization are few.
Consequently, the study of terrestrialization lacks a shared language and even a shared infrastructure of facts and dates. Paleontology provides a clear pattern where available: forests first appear in the Devonian, winged insects and land-based tetrapod vertebrates in the Carboniferous. However, those patterns are surrounded by voids. Paleontology cannot tell us when slime molds or pot worms or fungi first originated. Time-calibrated molecular phylogenies of modern life, ancestral-state reconstruction, and comparative genomics provide a means to fill these gaps. Nonetheless, contrasting molecular phylogenetic methodologies often produce discordant results, and discrepancies also exist when age estimates intersect lineages with robust fossil records (Schachat et al. Reference Schachat, Goldstein, DeSalle, Bobo, Boyce, Payne and Labandeira2023a). Such temporal discrepancies are not unique to terrestrial life, but the problem is compounded on land because of both the greater patchiness of the terrestrial fossil record and, indeed, the greater patchiness of the smaller and more fragmented communities of researchers interested in terrestrialization. Ultimately, paleontological and neontological end-members each contribute to the problem.
Paleontologists can be overly enthusiastic when attributing phylogenetic affinity to ambiguous fossils, resulting in what may be a stem-group ancestor of a modern group being assigned to a specific sublineage within that modern crown. If those misattributions are adopted at face value and included as fossil calibrations of molecular clock analyses, then estimates are necessarily pushed further back in time (Lucking et al. Reference Lucking, Huhndorf, Pfister, Plata and Lumbsch2009; Schachat et al. Reference Schachat, Goldstein, DeSalle, Bobo, Boyce, Payne and Labandeira2023a). A commendable recent effort has been the purging of fossils from the rolls of potential calibration points when lacking preservation of unambiguous synapomorphies of the lineage in question (Parham et al. Reference Parham, Donoghue, Bell, Calway, Head, Holroyd, Inoue, Irmis, Joyce and Ksepka2012; Wolfe et al. Reference Wolfe, Daley, Legg and Edgecombe2016).
On the molecular phylogenetics side, methods have improved dramatically with the advent of likelihood-based methods, relaxed clocks, and Bayesian approaches that include diverse tree and node constraint priors (Thorne et al. Reference Thorne, Kishino and Painter1998; Sanderson Reference Sanderson2002; Yang and Rannala Reference Yang and Rannala2005; Drummond et al. Reference Drummond, Ho, Phillips and Rambaut2006; Drummond and Rambaut Reference Drummond and Rambaut2007; Heath et al. Reference Heath, Huelsenbeck and Stadler2014). Further improvement can come through an increasing dialogue with paleontologists rather than a unidirectional handoff of fossil calibrations. The many decisions involved in tree and node calibration priors are themselves hypotheses for which the fossil record might be seen as a useful test. Sometimes those considerations become the focus (Near et al. Reference Near, Bolnick and Wainwright2005; Marshall Reference Marshall2008, Reference Marshall2019; Dornburg et al. Reference Dornburg, Beaulieu, Oliver and Near2011). More frequently, however, molecular clock dates that are early relative to the fossil evidence are treated as an accurate history newly revealed. The fossil record is then blamed for the discrepancy instead of being leveraged as a potential source for evaluation. The absence of a dialogue between neontologists and paleontologists ultimately limits efforts to build a synthetic understanding of change through time: with any phylogenetic analysis being unlikely to be the first or the last estimate of a lineage’s age, the readership is left with the task of choosing which analysis to follow, whether based on most recent date of publication or closest correspondence to expectations.
Integrating Phylogenies with a Geological Perspective (Exemplified with Insect Flight)
Most of life does not fossilize. Thus, a focus by paleontologists on only the fossil record, such as it is, would be an abdication of most of the history of life as not being in the domain of paleontologists. And if terrestrial paleoecology means just vascular plants, vertebrates, and insects, then that is a similar abdication of the evolution of terrestrial ecosystems. At the least, paleontologists need to incorporate molecular phylogenetic information as one of several lines of evidence subject to contextual interpretation alongside other lines of evidence like geochemistry and sedimentology.
Luckily, geology already provides a conceptual framework for considering phylogenetic information via absolute versus relative time; a time-calibrated phylogeny provides an estimate of both. Whether or not absolute age estimates are trusted, the successive relationships encoded in the topology of a phylogeny represent a relative timescale just as much as the relative timescales provided by bio-, magneto-, or isotope stratigraphy (Brown and Smith Reference Brown and Smith2018; Budd and Mann Reference Budd and Mann2020, Reference Budd and Mann2023; Pennell Reference Pennell2023). Indeed, the relative timescale afforded by a cladogram provides a powerful mechanism to compare with the fossil record to ascertain whether absolute dates derived from the time calibration of that same phylogeny should be considered suspect (Schachat et al. Reference Schachat, Goldstein, DeSalle, Bobo, Boyce, Payne and Labandeira2023a). If early molecular clock–based age estimates for a lineage are correct despite sometimes predating the first fossil occurrence by hundreds of millions of years, then cladogenesis long preceded those first appearances and both later and earlier derived sublineages would be equally available for sampling. Thus, the sequence of first appearances should be effectively randomized (Fig. 1A). However, a close correspondence between the relative timescale of the topology and the sequential first appearances in the fossil record would instead suggest the absence of an extended period during which the clade existed but escaped preservation, and that it is the fossil record that should be trusted over molecular clock dates (Fig. 1B).
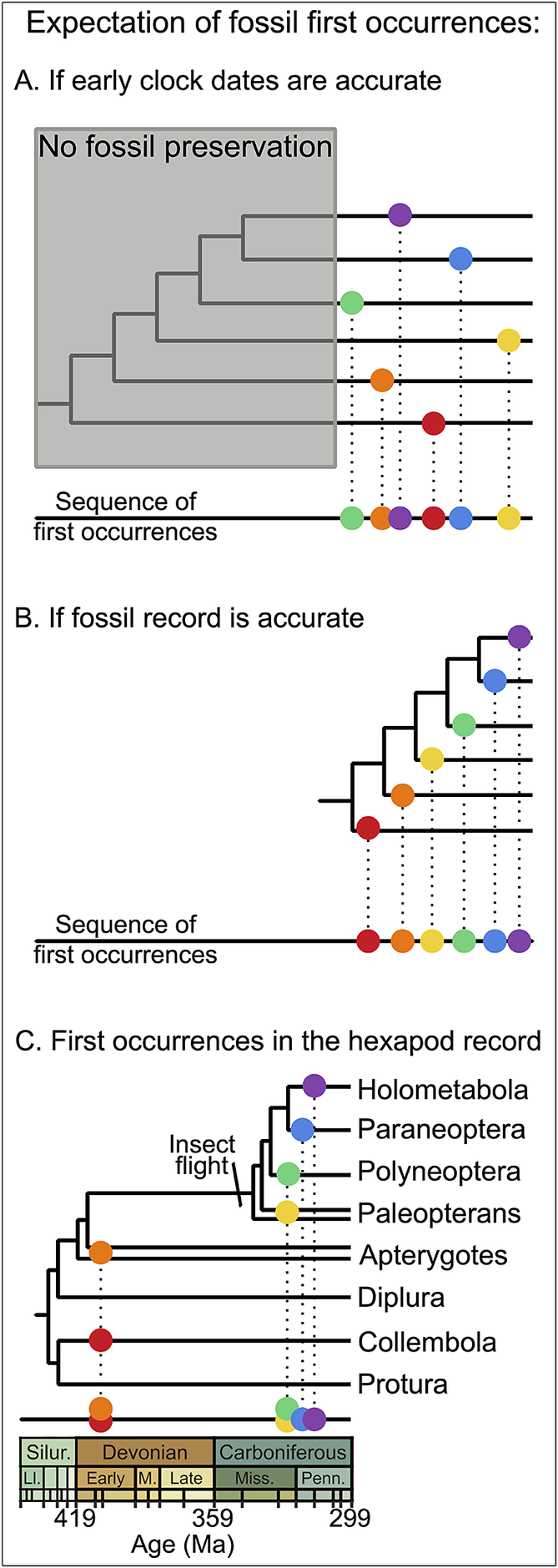
Figure 1. Molecular clock dates and expectations for the fossil record. A, If early clock dates are accurate and the early history of a clade is not recorded (gray box), then the sequence of first appearances is expected to be random with respect to the phylogenetic topology once preservation commences, because many sublineages will be newly available for sampling simultaneously. B, If the fossil record accurately reflects clade ages, then the sequence of first appearances should conform to the phylogenetic topology. C, In practice, the fossil record for a variety of lineages does tend to reproduce the phylogenetic sequence, as demonstrated here with hexapod first occurrences (Fayers and Trewin Reference Fayers and Trewin2005; Dunlop and Garwood Reference Dunlop and Garwood2017; Schachat et al. Reference Schachat, Labandeira, Saltzman, Cramer, Payne and Boyce2018, Reference Schachat, Goldstein, DeSalle, Bobo, Boyce, Payne and Labandeira2023a).
Among the most important evolutionary transformations, the early evolution of hexapods carrying through to the appearance of insect flight is a concrete example of how these issues can be approached. The fossil record supports the evolution of flight as being of immediate and profound impact: hexapods were initially a minor component of early terrestrial systems compared with arachnids and myriapods, with just three or four Devonian fossils and none at all known from a 60 Myr gap spanning the later Devonian and earlier Carboniferous, but winged insects immediately dominate the terrestrial arthropod record with their first appearance in the mid-Carboniferous (Schachat et al. Reference Schachat, Labandeira, Saltzman, Cramer, Payne and Boyce2018). Despite the clarity of this pattern, a dozen molecular clock estimates have argued for a cryptic evolution of insect flight as early as the Silurian (Schachat et al. Reference Schachat, Goldstein, DeSalle, Bobo, Boyce, Payne and Labandeira2023a). Taking to the air may seem an entirely separate concern from terrestrialization but becomes an unavoidable part of the discussion when it is repeatedly projected to be older than the fossil record of a simple land flora of even a few centimeters height.
With disarticulated wings being the bread and butter of paleoentomology, the suggestion that somehow not a single wing fossil exists from the first 100 Myr of their history is an awkward one. Rather than relying on a general discomfort, however, flight before the Carboniferous can be quantitatively rejected (Schachat et al. Reference Schachat, Goldstein, DeSalle, Bobo, Boyce, Payne and Labandeira2023a). Those 100 Myr of cryptic prehistory would be remarkably improbable given the close correspondence between the relative timing of fossil first occurrences and the phylogenetic branching order (Fig. 1C): apterygotes appeared before paleopterans and early neopterans, which came before paraneopterans and holometabolans, with similar sequences subsequently developing within each sublineage.
For generations of students of evolutionary history, a savvy stance has been to emphasize the fossil record’s deficiencies. Even Darwin did it to explain the Precambrian (Darwin Reference Darwin1859). When we do have it, however, the record is pretty good, and that should be a relief. Insects gliding from tree canopies has long been recognized as a plausible scenario for flight evolution (Snodgrass Reference Snodgrass1935; Hamilton Reference Hamilton1971; Hasenfuss Reference Hasenfuss2002). The early clock dates for flight evolution, coupled with a complete lack of a tree canopy before the Middle Devonian, have required the suggestion that the 20–50 cm height of Early Devonian plants might have been an adequate drop to foster gliding (e.g., Grimaldi and Engel Reference Grimaldi and Engel2005: fig. 6.2a). Instead, the fossil record closely corresponds to the phylogenetic topology and agrees surprisingly well with expectations for the evolution of flight: after the first appearance of forest canopies in the Middle Devonian (Stein et al. Reference Stein, Berry, Hernick and Mannolini2012, Reference Stein, Berry, Morris, Hernick, Mannolini, Ver Straeten, Landing, Marshall, Wellman and Beerling2020; Davies et al. Reference Davies, McMahon and Berry2024), canopy leaf damage consistent with insect-sized herbivores first appeared in the early Mississippian(Iannuzzi and Labandeira Reference Iannuzzi and Labandeira2008; Gorman Reference Gorman2013) but remained uncommon until after the mid-Carboniferous first appearance of winged insects—after which herbivory became systemic along with the explosive diversification of winged insects, newly ubiquitous and dominant over terrestrial ecosystems (Labandeira Reference Labandeira1997, Reference Labandeira1998; Schachat et al. Reference Schachat, Labandeira, Saltzman, Cramer, Payne and Boyce2018).
While the quality of the insect fossil record should be recognized, age estimates from time-calibrated phylogenies are all we are ever likely to have for other important threads of the terrestrial biota, ranging from soil algae to slime molds to tardigrades (Fiz-Palacios et al. Reference Fiz-Palacios, Romeralo, Ahmadzadeh, Weststrand, Ahlberg and Baldauf2013; Nelsen et al. Reference Nelsen, Lücking, Boyce, Lumbsch and Ree2020b; Howard et al. Reference Howard, Giacomelli, Lozano-Fernandez, Edgecombe, Fleming, Kristensen, Ma, Olesen, Sørensen and Thomsen2022). We need those clock dates, even if they should be treated with skepticism and the confidence intervals involved can be large. In other intermediate cases, a fossil record may be absent, but a secure phylogenetic topology is sufficient, as the existence of lineages lacking a fossil record can be confirmed via fossiliferous relatives. For example, Early Devonian fossils of a collembolan and an insect require the presence of dipluran and proturan lineages.
Here, we provide a synthetic accounting of the evolution of the terrestrial biota through geologic time, making use of the fossil record as available while also acknowledging that a large fraction of the biota can be understood only through a phylogenetic perspective. The approach taken for the integration of disparate sources of evolutionary history—fossils and phylogenies—is, first, to rely on the fossil record for dates where it is of sufficient quality to replicate the sequence of first occurrences as congruent with phylogenetic analyses. Second, if a lineage lacks fossils but has close sibling lineages that do have an adequate fossil record, then those sibling records are used to establish the stem-group age of the lineage in question. Third, if an adequate fossil record is unavailable, even after consideration of sibling groups, then the time-calibration of molecular phylogenies can be used as a broad age estimate to ensure that important lineages are not ignored when considering the history of terrestrial systems. However, even where molecular clocks must be relied upon, they can be considered in the context of environmental constraints from Earth history for which absolute dates can be known, such as Snowball Earth. This exercise provides a shared historical infrastructure that then allows recognition of fundamental evolutionary patterns and comparisons with those seen elsewhere in the history of life, including the marine record.
The Fossil and Phylogenetic Records
Unicellular Microbial Life
Sporadic evidence for microbial activity on land trails back throughout the Proterozoic into the Archean (Horodyski and Knauth Reference Horodyski and Knauth1994; Gutzmer and Beukes Reference Gutzmer and Beukes1998; Watanabe et al. Reference Watanabe, Martini and Ohmoto2000; Prave Reference Prave2002; Strother et al. Reference Strother, Battison, Brasier and Wellman2011; Brasier et al. Reference Brasier, Culwick, Battison, Callow and Brasier2017). An Archean terrestrial biota can be expected to have involved a diversity of prokaryotic metabolisms, but productivity among available nonoxygenic photoautotrophs would have been limited by the slow flux of electron donors (e.g., hydrogen, iron, sulfur) needed for photosynthetic carbon fixation as sourced from rock weathering and volcanic outgassing (Canfield et al. Reference Canfield, Rosing and Bjerrum2006; Ward et al. Reference Ward, Rasmussen and Fischer2019; Crockford et al. Reference Crockford, On, Ward, Milo and Halevy2023).
Perhaps the single most transformative event for the land surface has been the evolution of cyanobacteria early in the Paleoproterozoic and the subsequent increase in atmospheric oxygen (Fischer et al. Reference Fischer, Hemp and Johnson2016; Shih et al. Reference Shih, Hemp, Ward, Matzke and Fischer2017). Cyanobacteria split water as an electron source for carbon fixation, thereby increasing the potential for terrestrial productivity by breaking the dependence on tectonics and volcanic outgassing rates for electron donors. The oxygen-based establishment of an atmospheric ozone shield would have curtailed ultraviolet stresses on terrestrial life (Kasting and Donahue Reference Kasting and Donahue1980; Zachar and Boza Reference Zachar and Boza2020) but may not have been as essential a prerequisite as originally thought (Cockell and Raven Reference Cockell and Raven2007). Changes in planetary albedo from the increasing microbial pigmentation of land surfaces should then have had profound effects on climate, however poorly constrained these albedo changes may be at present (Boyce and Lee Reference Boyce and Lee2017). Finally, although full oxygenation of the marine water column may not have been achieved until the Paleozoic (Sperling et al. Reference Sperling, Wolock, Morgan, Gill, Kunzmann, Halverson, Macdonald, Knoll and Johnston2015), the impact of oxygen on the land surface would have been immediate as it was directly exposed to the atmosphere. Pyrite, siderite, biotite, and other formerly stable minerals were now subject to oxidative dissolution, thereby changing patterns of weathering, erosion, elemental cycling, and marine runoff (Bachan and Kump Reference Bachan and Kump2015; Fischer et al. Reference Fischer, Hemp and Johnson2016; Goodfellow et al. Reference Goodfellow, Hilley, Webb, Sklar, Moon and Olson2016).
The oldest (~1.05 Ga) terrestrial fossils of unicellular eukaryotes are lacustrine (Wellman and Strother Reference Wellman and Strother2015), but similar life should be expected of surrounding soils, given how porous environmental boundaries can be at a microbial scale (e.g., Geisen et al. Reference Geisen, Mitchell, Adl, Bonkowski, Dunthorn, Ekelund and Fernández2018). Over the Neoproterozoic, green algae (Chlorophyta) were likely sources of eukaryotic photosynthesis in terrestrial soils. Extant diversity of Chlorophyta is largely confined to three classes, two of which—Trebouxiophyceae and Chlorophyceae—overwhelmingly occupy freshwater and terrestrial habitats (Leliaert et al. Reference Leliaert, Smith, Moreau, Herron, Verbruggen, Delwiche and De Clerck2012). Both lineages are estimated to have Neoproterozoic crowns (Del Cortona et al. Reference Del Cortona, Jackson, Bucchini, Van Bel, D’hondt, Škaloud, Delwiche, Knoll, Raven and Verbruggen2020; Nelsen et al. Reference Nelsen, Lücking, Boyce, Lumbsch and Ree2020b). A third terrestrial chlorophyte clade, Trentepohliales, is embedded in the otherwise marine Ulvophyceae. Stem ages have been used to suggest a Neoproterozoic origin of Trentepohliales (Lutzoni et al. Reference Lutzoni, Nowak, Alfaro, Reeb, Miadlikowska, Krug and Arnold2018), but analyses with adequate taxon sampling to resolve crown diversification place it much younger, no earlier than the Paleozoic (Del Cortona et al. Reference Del Cortona, Jackson, Bucchini, Van Bel, D’hondt, Škaloud, Delwiche, Knoll, Raven and Verbruggen2020; Nelsen et al. Reference Nelsen, Lücking, Boyce, Lumbsch and Ree2020b).
All metabolisms beyond fermentative heterotrophy are fundamentally prokaryotic, but eukaryotes do still make unique contributions to the system. Their size—particularly when multicellular—enables eukaryotes to modify their physical environment and to bridge physiologically relevant gradients, such as between illumination from above and soil water from below (Boyce et al. Reference Boyce, Fan and Zwieniecki2017; Fan et al. Reference Fan, Miguez-Macho, Jobbágy, Jackson and Otero-Casal2017), or between disjunct concentrations in the environment of different essential nutrients (Filley et al. Reference Filley, Blanchette, Simpson and Fogel2001). As a result, multicellular eukaryotes became an important frontier for prokaryotic conquest in the Phanerozoic with the symbiotic occupation of guts, roots, and thalli (e.g., Nelsen et al. Reference Nelsen, Lücking, Boyce, Lumbsch and Ree2020a).
A final characteristic unique to eukaryotes is a complex cytoskeleton enabling phagotrophy. Prokaryotes and those eukaryotes with cell walls (e.g., plants, fungi) engage in osmotrophic absorption of nutrients in solution. Those eukaryote unicells that lack cell walls (e.g., amoebae, ciliates, choanoflagellates) can ingest particles via phagocytosis, and the muscular action of multicellular animals can scale up feeding whether eventual cellular phagocytosis is involved or not. Given its ancient eukaryotic heritage (Cavalier-Smith Reference Cavalier-Smith2002; Zachar and Boza Reference Zachar and Boza2020), phagotrophy should be considered an early likelihood among unicellular soil eukaryotes. However, the earliest direct requirement for terrestrial phagotrophy would be molecular phylogenetic estimates of divergence times of ca. 700 Ma (95% confidence intervals: 293–859 Ma) for major slime mold lineages (Fiz-Palacios et al. Reference Fiz-Palacios, Romeralo, Ahmadzadeh, Weststrand, Ahlberg and Baldauf2013) with spore dispersal mechanisms that are functional only in a subaerial context.
Phylogenetic ancestral-state reconstruction has been used to argue that both cyanobacteria and the eukaryotic Archaeplastida (red, green, and glaucophyte algae) did not transition to land secondarily from the marine realm but, rather, had their primary origin in freshwater environments and were on land from the start (Sánchez-Baracaldo Reference Sánchez-Baracaldo2015; Sánchez-Baracaldo et al. Reference Sánchez-Baracaldo, Raven, Pisani and Knoll2017). Although nothing rules out such a possibility, the phylogenetic evidence is suspect, given that these lineages originated before the extreme evolutionary filter of Snowball Earth. While animal life could have persisted in the brine beneath the sea ice (Simpson Reference Simpson2021), photosynthetic life requires light at a time when the entire surface of the planet would have been a freshwater environment (Hoffman et al. Reference Hoffman, Abbot, Ashkenazy, Benn, Brocks, Cohen and Cox2017). Even if open marine pockets existed during the glaciation due to hydrothermal activity or oceanic circulation patterns, saltwater-based photosynthesis would have been extinguished during the terminal Snowball thaw when a kilometer-thick lid of hot stagnant fresh water would have capped the Earth’s oceans for 50 kyr (Yang et al. Reference Yang, Jansen, Macdonald and Abbot2017). Thus, if Cryogenian survival of photosynthesis was restricted to freshwater lineages, the reconstruction of a freshwater ancestral state for lineages that originated earlier in the Proterozoic should be seen as an artifact of later events (Fig. 2). Notably, a marine origin has not been questioned for the other major photosynthetic lineage among eukaryotes, but those Stramenopiles (e.g., diatoms, kelps, chrysophytes, etc., as well as nonphotosynthetic oomycetes) are reconstructed to have evolved through the Phanerozoic (Brown and Sorhannus Reference Brown and Sorhannus2010; Matari and Blair Reference Matari and Blair2014)—postdating the Snowball Earth culling of marine photosynthesis.
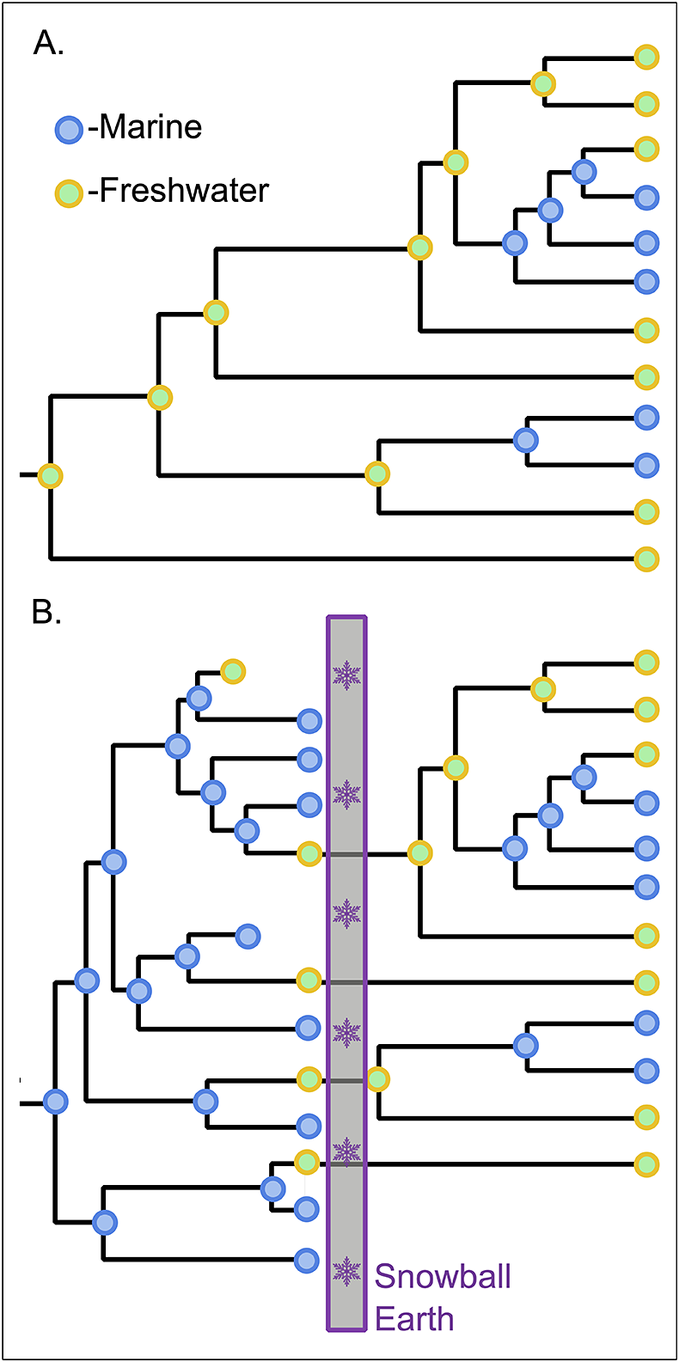
Figure 2. Potential impact of Snowball Earth on interpretation of the early evolution of photosynthesis. A, Inference of ancestral ecologies from the modern phylogenetic distribution of habitats has been shown to be consistent with a freshwater origin of photosynthesis for both cyanobacteria and Archaeplastida eukaryotes. Phylogeny simplified from the Archaeplastida fraction of the phylogeny in Sánchez-Baracaldo et al. (Reference Sánchez-Baracaldo, Raven, Pisani and Knoll2017). B, Hypothetical example of how a marine origin could have been obscured by the Cryogenian Snowball Earth event as an extinction filter favoring the survival of only freshwater photosynthesis. The modern expression of this scenario would result in an inferred character state history and tree topology identical to that in A.
Finally, to state clearly: no geologic evidence exists for anything other than microbial systems on land before the Paleozoic. Simple algal filaments may or may not have existed, but no complex multicellular eukaryotes were present.
Fungi (and Other Filamentous Osmotrophs)
Early fungal evolution is bookended by two constraints: the Devonian Rhynie Chert as a minimum and the reality of a Cryogenian Snowball Earth. Fungal hyphae are relatively abundant as an interstitial component of the plant fossil record, but hyphae alone can rarely be identified to any meaningful specificity (e.g., Nelsen et al. Reference Nelsen, DiMichele, Peters and Boyce2016: fig. 2). Dispersed fungal spores can also be common and can offer substantial phenotypic variation; however, convergent evolution of spore form makes assignment of fossils to extant clades a challenge in its early stages (Berbee et al. Reference Berbee, Le Renard and Carmean2015). With detailed preservation of reproductive structures and other diagnostic features first available at Rhynie (Fig. 3), the first unambiguous appearances of several phyla accumulate at this one locality, including Chytridiomycota, Blastocladiomycota, Mucoromycota, and Ascomycota (Taylor et al. Reference Taylor, Klavins, Krings, Taylor, Kerp and Hass2004a, Reference Taylor, Hass, Kerp, Krings and Hanlin2005, Reference Taylor, Krings and Kerp2006, Reference Taylor, Krings and Taylor2014; Krings et al. Reference Krings, Taylor, Hass, Kerp, Dotzler and Hermsen2007b; Strullu-Derrien et al. Reference Strullu-Derrien, Kenrick, Pressel, Duckett, Rioult and Strullu2014, Reference Strullu-Derrien, Goral, Longcore, Olesen, Kenrick and Edgecombe2016, Reference Strullu-Derrien, Spencer, Goral, Dee, Honegger, Kenrick, Longcore and Berbee2017, Reference Strullu‐Derrien, Selosse, Kenrick and Martin2018; Berbee et al. Reference Berbee, James and Strullu-Derrien2017; Honegger et al. Reference Honegger, Edwards, Axe and Strullu-Derrien2017).
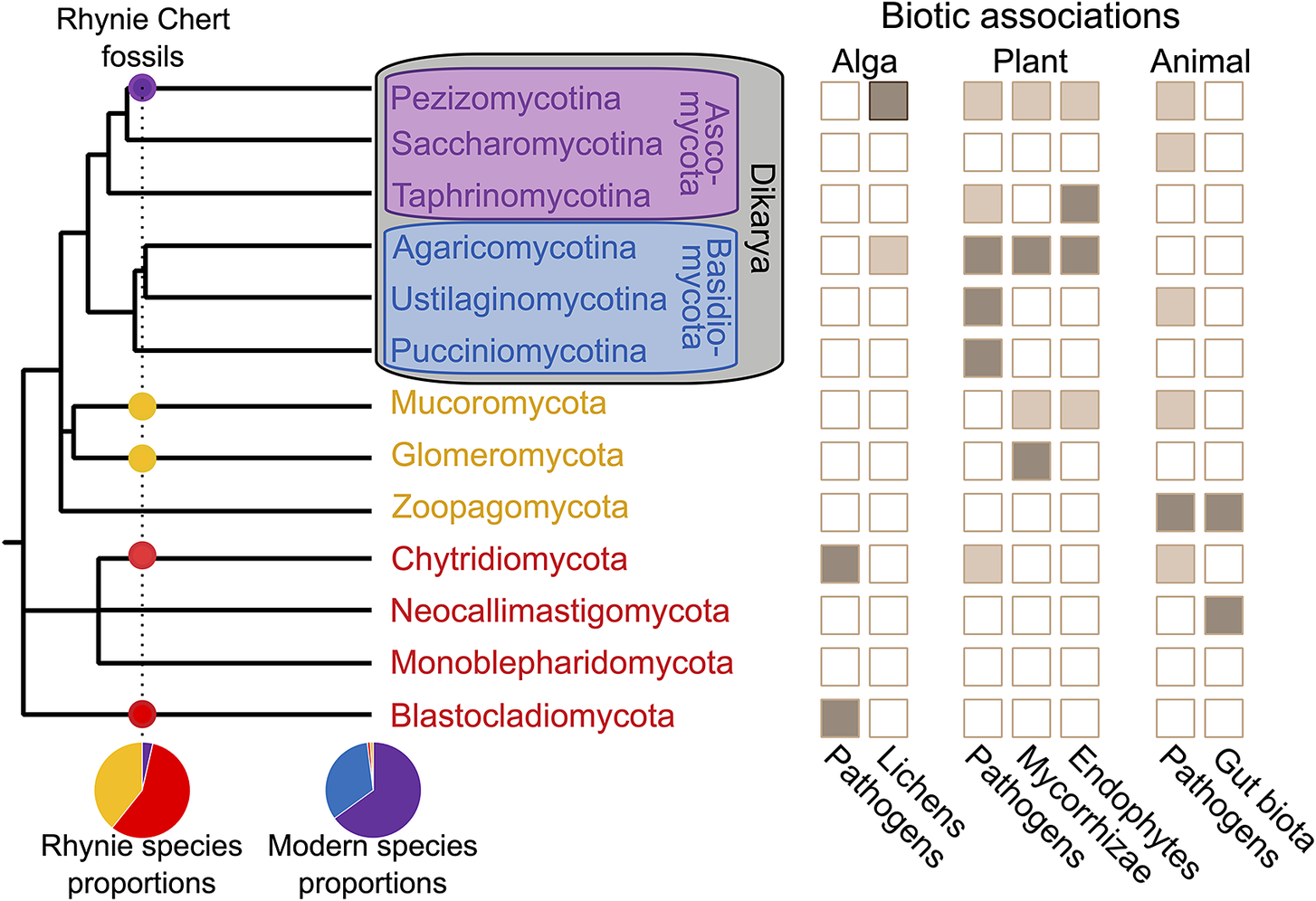
Figure 3. Fungal phylogeny, distribution of ecologies, and proportional representation of different lineages in the Lower Devonian Rhynie Chert versus the modern world. Topology represents a minimal list of major fungal phyla (following Li et al. Reference Li, Steenwyk, Chang, Wang, James, Stajich, Spatafora, Groenewald, Dunn and Hittinger2021). Several species-poor lineages are omitted, e.g., the plant parasites of the minor Dikarya phylum Entorrhizomycota, and other lineages that are sometimes elevated to the phylum level are subsumed into other groups, e.g., the insect pathogens of Entomophthorales are sometimes placed in their own phylum as Entomophthoromycota but are, here, included with the Zoopagomycota. Ecologies indicated if present in a lineage (light brown) or an abundant trait that may be a defining characteristic of one or more major sublineages within a phylum (dark brown) (Naranjo-Ortiz and Gabaldón Reference Naranjo-Ortiz and Gabaldón2019). Proportional representation among Rhynie fossils of species from major lineages based on Krings et al. (Reference Krings, Harper and Taylor2018).
Because all Rhynie first appearances are contemporaneous, the single most-derived lineage within the phylogenetic topology as sampled for the analysis would set the pace for time calibration of the fungal phylogeny, and exact placement of that one lineage could dramatically impact overall interpretation. For example, placement of the Rhynie ascomycete Palaeopyrenomycites in a specific extant class in contrast to the stem of the ascomycete subphylum Pezizomycotina changes age estimates for the fungi by 500 Myr (Taylor and Berbee Reference Taylor and Berbee2006; Lucking et al. Reference Lucking, Huhndorf, Pfister, Plata and Lumbsch2009; Berbee and Taylor Reference Berbee and Taylor2010). Such decisions underlie estimations of crown-group fungi originating between 950 and 810 Ma (median: 876 Ma), filamentous fungi between 780 and 690 Ma (median: 736 Ma), and the Ascomycota and Basidiomycota of Dikarya between 680 and 615 Ma (median: 649 Ma)—all before or during the Cryogenian (Lutzoni et al. Reference Lutzoni, Nowak, Alfaro, Reeb, Miadlikowska, Krug and Arnold2018).
Is a Cryogenian fungal radiation a reasonable expectation, given the evidence of global Snowball glaciation? Early-diverging fungal lineages are ancestrally unicellular and aquatic in fresh water and soil-water films; consequently, no obvious barriers would have existed to prevent such organisms from persisting through a Snowball (Berbee et al. Reference Berbee, James and Strullu-Derrien2017), whether in the near-surface meltwater of sheltered cryoconite microcosms or in the sediments of the Cryogenian equivalents of the dry valleys of modern Antarctica (Hoffman et al. Reference Hoffman, Abbot, Ashkenazy, Benn, Brocks, Cohen and Cox2017; Naranjo‐Ortiz and Gabaldón Reference Naranjo-Ortiz and Gabaldón2019). However, it is widely recognized that much of the diversity of the filamentous fungi is closely associated with the land plants (Fig. 3). With no evidence of Precambrian land plants, this argument implicitly assumes many fungal clades whose diversification is linked to plants would have originated long prior and repeatedly evolved to use land plants as a substrate 200–300 Myr after these fungal lineages originated. This becomes problematic when large coherent lineages of pathogens like the smuts (Ustilaginomycetes) are estimated to be 150 Myr older than their flowering plant hosts (Lutzoni et al. Reference Lutzoni, Nowak, Alfaro, Reeb, Miadlikowska, Krug and Arnold2018). Thus, the fossil record of pathogen hosts—whether angiosperms for the smuts or winged insects for crown Entomophthorales—might be a useful age constraint for molecular clock dating analyses. When considering a Neoproterozoic establishment of the filamentous fungi including Dikarya, the Rhynie Chert again provides essential context. Even if we do not have useful fungal preservation before Rhynie, the composition of the fungal biota of Rhynie is informative (Fig. 3). In the modern world, the ascomycetes and basidiomycetes of Dikarya make up 98% of all fungal diversity (Voigt et al. Reference Voigt, James, Kirk, A. Santiago, Waldman, Griffith, Fu, Radek, Strassert and Wurzbacher2021). At Rhynie, however, no basidiomycetes and only two or three ascomycetes have been described; thus, perhaps 90% of the fungal species diversity at Rhynie belongs to early-diverging lineages that today collectively encompass only 2% of all fungal species (Krings et al. Reference Krings, Harper and Taylor2018). As a hydrothermal wetland, the Rhynie Chert may be expected to have preserved a biased sample of the fungal biota; however, modern wetland environments are still dominated by Dikarya, whether hydrothermal or not (Zhan et al. Reference Zhan, Liu, Wang, Wang, Xia, Wang, Cui, Xiao and Wang2021; Bazzicalupo et al. Reference Bazzicalupo, Erlandson, Branine, Ratz, Ruffing, Nguyen and Branco2022). Reconciling the fossil record at Rhynie with molecular clock–based ages of Dikarya—which predate the Rhynie Chert by more than 200 Myr—would require that any presence of Dikarya through that span was only as one more of the various lineages of filamentous fungi and with nothing like their modern overwhelming abundance and ecological diversity. With so much of modern fungal biology tied specifically to Dikarya, the fungal biota of the Paleozoic should be recognized as highly distinct with many modern ecologies still absent (Taylor et al. Reference Taylor, Klavins, Krings, Taylor, Kerp and Hass2004a).
Despite these discrepancies, time-calibrated phylogenies have strong corrective value for the interpretation of putative fungal fossils. Filamentous fossils as old as the Mesoproterozoic have been described as fungi (Butterfield Reference Butterfield2005; Berbee et al. Reference Berbee, Strullu-Derrien, Delaux, Strother, Kenrick, Selosse and Taylor2020). While some may just be diagenetic artifacts, others are exquisite. However, most are far too old to be accommodated in the fungal phylogeny and its timeline. And that phylogeny does not exist in isolation: all life is related and confirmation of derived filamentous fungi deep in the Proterozoic would likely pull animals and other forms of multicellular life further back into the Proterozoic. No uniquely fungal characteristics are present in these fossils to require warping the phylogeny to accommodate them when filamentous forms have evolved repeatedly among eukaryotes (e.g., Miao et al. Reference Miao, Yin, Knoll, Qu and Zhu2024). Indeed, even prokaryotic filaments of actinobacteria and the extracellular sheaths of cyanobacteria could be confused for fungal hyphae (Krings et al. Reference Krings, Kerp, Hass, Taylor and Dotzler2007a). Nor is this problem unique to putative fungi. Filamentous Palaeovaucheria as old as the Mesoproterozoic has been related to modern xanthophyte algae (Butterfield Reference Butterfield2004), but subsequently available molecular clock–based analyses instead indicate such an affinity would be implausible before the Mesozoic (Brown and Sorhannus Reference Brown and Sorhannus2010). Because a filament is one of the fundamental geometries of life that has been converged upon repeatedly, some fossil filaments may be unrelated to extant examples of the form.
Time calibration as a filter for viable interpretations of fossil affinities carries over into the Phanerozoic at a finer phylogenetic resolution. “Lichens” can be an ubiquitous but vague expectation for what came before land plants (e.g., Berner Reference Berner1992), but time calibration of the important modern lichen lineages provides ages younger than the vascular plants, with many appearing only during the Mesozoic and the oldest unlikely before the Carboniferous (Nelsen et al. Reference Nelsen, Lücking, Boyce, Lumbsch and Ree2020b). Various problematic thalloid fossils of the Silurian and Devonian have been loosely compared to lichens (Stein et al. Reference Stein, Harmon and Hueber1993; Jahren et al. Reference Jahren, Porter and Kuglitsch2003; Fletcher et al. Reference Fletcher, Beerling and Chaloner2004; Taylor et al. Reference Taylor, Free, Boyce, Helgemo and Ochoada2004b; Tomescu and Rothwell Reference Tomescu, Rothwell, Greb and DiMichele2006; Honegger et al. Reference Honegger, Edwards and Axe2012) but must represent extinct approximations of their overall form that may or may not be related to fungi. Similarly, the giant trunks of the filamentous heterotroph Prototaxites (Boyce et al. Reference Boyce, Hotton, Fogel, Cody, Hazen, Knoll and Hueber2007) have been compared to specific lineages among the derived mushroom-forming Agaricomycetes of the Basidiomycota (Hueber Reference Hueber2001). Although a reasonable suggestion when first made, subsequent improvements in the resolution and age of the fungal phylogeny (Varga et al. Reference Varga, Krizsán, Földi, Dima, Sánchez-García, Sánchez-Ramírez and Szöllősi2019) indicate that such mushroom-clade comparisons would be irrelevant for Prototaxites, it being already present as early as the Silurian. Consequently, if Prototaxites was a fungus—whether basidiomycete, ascomycete, or other (Hueber Reference Hueber2001; Honegger et al. Reference Honegger, Edwards, Axe and Strullu-Derrien2017)—it would have to represent an extinct convergent origin of a tissue structure as complex as that observed in some modern Dikarya (Nelsen and Boyce Reference Nelsen and Boyce2022). Mushrooms are not as old as the dirt; rather, they are younger than dinosaurs and crown-group mammals (Close et al. Reference Close, Friedman, Lloyd and Benson2015).
And yet, reciprocally, the fossil record can also cast light on the phylogenetic context. A few fungus-like fossils have been described from Rhynie, including oomycete Stramenopiles (Krings et al. Reference Krings, Taylor and Dotzler2011, Reference Krings, Harper and Taylor2018). This interpretation might be viewed as problematic, as the Phanerozoic radiation of Stramenopiles has already been mentioned as being surprisingly young in contrast to other fundamental lineages of Eukaryota that represent Proterozoic radiations. However, while estimates of the Oomycota crown of filamentous pathogens and soil saprobes suggest a Jurassic diversification (Brown and Sorhannus Reference Brown and Sorhannus2010; Matari and Blair Reference Matari and Blair2014), these same analyses yield stem-age estimates that trail back to the Devonian and can accommodate these Rhynie fossils. Thus, while the assumption of biological and ecological continuity between the earliest stem ancestry and the modern crown of a lineage might usually represent a risk, here, such continuity appears to be borne out.
Animals
Animals present unique challenges for reconstructing patterns of terrestrialization, given the dozens of separate lineages arising independently from an aquatic ancestry. Indeed, nematodes alone may encompass dozens of terrestrializations, given how many higher-level nematode lineages are found indiscriminately on both land and sea (Kiontke and Fitch Reference Kiontke and Fitch2013). Those nematodes also highlight a second challenge already mentioned: attention has been focused almost exclusively on the minority of lineages having a robust fossil record, thereby ignoring those lineages with little chance of fossil preservation before Cretaceous amber. As a concrete example: across three influential books covering terrestrialization and the assembly of early terrestrial ecosystems (Little Reference Little1990; Behrensmeyer et al. Reference Behrensmeyer, Damuth, DiMichele, Potts, Sues and Wing1992; Clack Reference Clack2012), just four mentions in total are made of soil mesofauna like mites and collembolans. Microfauna like nematodes and tardigrades receive no mention at all.
An incomplete narrative that excludes the small is easy to formulate. If focus is on the large and robust lineages, much emphasis devolves onto the physical act of leaving the water, particularly given the clear evidence that all Devonian and many Carboniferous tetrapods were fully aquatic (Coates et al. Reference Coates, Ruta and Friedman2008; Clack Reference Clack2012; Schoch Reference Schoch2014), as well as stubborn arguments that some early scorpions might have been so (Poschmann et al. Reference Poschmann, Dunlop, Kamenz and Scholtz2008; Kühl et al. Reference Kühl, Bergmann, Dunlop, Garwood and Rust2012; Howard et al. Reference Howard, Edgecombe, Legg, Pisani and Lozano-Fernandez2019, Reference Howard, Puttick, Edgecombe and Lozano-Fernandez2020). Consistent with this focus, the earlier subaerial trace fossil record engenders debate over whether tracks are likely to have been of terrestrial interlopers that were otherwise aquatic (Johnson et al. Reference Johnson, Briggs, Suthren, Wright and Tunnicliff1994; Briggs et al. Reference Briggs, Suthren and Wright2019; Shillito and Davies Reference Shillito and Davies2019). And even this early trace fossil record recapitulates some of the same biases as other aspects of the fossil record, as many land animal lineages are too small to leave tracks.
Such narratives constructed exclusively on the fossil record present a real problem. The preserved Silurian fauna (Fig. 4) consists of millipede and centipede myriapods and scorpion and trigonotarbid arachnids (Jeram et al. Reference Jeram, Selden and Edwards1990; Shear and Edgecombe Reference Shear and Edgecombe2010). Although scorpions happen to provide the oldest terrestrial body fossils, they should be viewed more as a culmination of soil food webs rather than an initiation; much must have happened already to sustain such large predators. As advocated earlier, phylogenies can provide essential context regarding the hidden complexity of early ecosystems by providing a relative timescale for the appearance of distinct lineages.

Figure 4. Stratigraphic distribution of fossil first occurrences in arachnids and myriapods. In both cases, assessment of the correspondence between phylogeny and fossil first occurrences is hampered by limitations of phylogenetic understanding, but expectations are met in a prominent sublineage where relationships are understood. A, Understanding of the arachnid phylogeny remains in a state of flux, except that all lineages bearing book lungs are well supported to be closely related within the Tetrapulmonata and more inclusive Arachnopulmonata clades (Sharma et al. Reference Sharma, Kaluziak, Perez-Porro, Gonzalez, Hormiga, Wheeler and Giribet2014; Howard et al. Reference Howard, Puttick, Edgecombe and Lozano-Fernandez2020; Ballesteros et al. Reference Ballesteros, Santibáñez-López, Baker, Benavides, Cunha, Gainett, Ontano, Setton, Arango and Gavish-Regev2022). Within the Arachnopulmonata, a clear sequential pattern of first occurrences is developed (Dunlop and Penney Reference Dunlop and Penney2012). Open circles indicate stem-group ancestors to a lineage, with darker shading indicating increasing proximity to the modern crown. Extinct trigontarbid and uraraneid lineages are included as stem-group ancestors of Tetrapulmonata and spiders, respectively. Open symbol labeled with “?” indicates mesofossil cuticle with characteristics unique to modern amblypygids but too fragmentary to secure affinities. B, Among myriapods, a stratigraphic sequence of first occurrences is developed in centipedes and also may be true of millipedes, but assessment of millipedes is hampered by an incomplete understanding of how various fossils are related as stem-group ancestors to the modern lineages (Shear and Edgecombe Reference Shear and Edgecombe2010; Wolfe et al. Reference Wolfe, Daley, Legg and Edgecombe2016; Brookfield et al. Reference Brookfield, Catlos and Suarez2021). Asterisks indicate lineages now extinct.
Alongside centipedes and millipedes, the pauropod and symphylan lineages of the Myriapoda must date back to the Silurian as well (Shear and Edgecombe Reference Shear and Edgecombe2010). Without fossils, their Silurian biology cannot be addressed directly, but both lineages are now diminutive members of the soil fauna. Although lacking fossil preservation, the basal polyxenid bristly millipedes of Penicillata are no larger than pauropods and symphylans. Basal centipedes are fast-moving surface scutigeromorphs dependent on gracile legs, as first seen in the latest Silurian, followed by forms dependent on body undulations, including lithobiomorphs that must have been distinct in the Devonian and scolopendromorphs first seen in the Carboniferous (Fig. 4). Derived deep-burrowing lineages of millipedes and centipedes appeared later in the Paleozoic (Shear and Edgecombe Reference Shear and Edgecombe2010).
Arachnid phylogeny remains contentious (Sharma et al. Reference Sharma, Kaluziak, Perez-Porro, Gonzalez, Hormiga, Wheeler and Giribet2014; Howard et al. Reference Howard, Puttick, Edgecombe and Lozano-Fernandez2020; Ballesteros et al. Reference Ballesteros, Santibáñez-López, Baker, Benavides, Cunha, Gainett, Ontano, Setton, Arango and Gavish-Regev2022), but what clarity we do have is that all lineages possessing book lungs form the Arachnopulmonata clade (Fig. 4). Other than solifugids, arachnids of substantial size (i.e., >1 cm body length) are all arachnopulmonates: scorpions, trigontarbids, spiders, amblypygids, and uropygids. Thus, regardless of the true phylogenetic topology, any sibling lineages to the Arachnopulmonata that would have been distinct in the Silurian would have been drawn from lineages that are now smaller soil fauna components, including palpigrads, mites, ticks, and pseudoscorpions or at least no larger than a centimeter, as in harvestmen and ricinuleids. Although attention-grabbing, decimeter-scale scorpion fossils are not the whole story; indeed, the body length of the first Silurian trigonotarbid fossil is only 1 mm (Jeram et al. Reference Jeram, Selden and Edwards1990). The Lower Devonian Rhynie Chert provides the first direct preservation of mites and harvestmen (Dunlop and Garwood Reference Dunlop and Garwood2017). The Middle Devonian of Gilboa preserves pseudoscorpion arachnids. All other modern arachnid orders have at least stem-group representation among Carboniferous fossils, with the exceptions of palpigrads, schizomids, and ticks, each a lineage with small body sizes and scant preservation potential (Dunlop and Penney Reference Dunlop and Penney2012). Regardless of what the true arachnid phylogeny may be, these three missing lineages can be presumed also to have been present by the Carboniferous, because all potential sibling lineages were distinguishable by then.
For hexapods, the Rhynie Chert records both a collembolan and what appears to be a glancing section through a basal apterygote insect (Fayers and Trewin Reference Fayers and Trewin2005; Dunlop and Garwood Reference Dunlop and Garwood2017). Thus, all four hexapod lineages of collembolans, proturans, diplurans, and insects must have arisen by the Early Devonian (Fig. 1C). An additional Rhynie specimen preserving a pair of mandibles had long been interpreted as an insect but has been recently recognized to be a likely centipede (Haug and Haug Reference Haug and Haug2017). The two other potential hexapod fossils of the later Devonian are both of archaeognathan insects: a macerated cuticle of a pair of compound eyes from Gilboa (Shear et al. Reference Shear, Bonamo, Grierson, Rolfe, Smith and Norton1984) and a body fossil that has been questioned as too complete not to be a modern contaminant (Labandeira et al. Reference Labandeira, Beall and Hueber1988; Jeram et al. Reference Jeram, Selden and Edwards1990), although resolution of the latter issue would require further investigation. This Devonian material is broadly consistent with detritivory, although the presence of herbivory cannot be ruled out, for example, among collembolans. Herbivory and predation among larger insects figured prominently in their explosive diversification later in the Carboniferous.
Beyond the three major lineages of arachnids, myriapods, and hexapods, all terrestrial arthropods appear to be later additions to the system. The cosmopolitan distribution of modern oniscidean isopods has been argued to reflect a Pangean legacy (Broly et al. Reference Broly, Deville and Maillet2013), but that would only require a Jurassic origin before Pangea’s breakup. Molecular clock studies, which already differed by a factor of three in their age estimates, are now further complicated by the suggestion that Oniscidea might not be monophyletic (Lins et al. Reference Lins, Ho and Lo2017; Dimitriou et al. Reference Dimitriou, Taiti, Schmalfuss and Sfenthourakis2018, Reference Dimitriou, Taiti and Sfenthourakis2019). Ambiguities aside, oniscideans are preserved directly in Early Cretaceous amber (Broly et al. Reference Broly, Deville and Maillet2013, Reference Broly, Maillet and Ross2015; Poinar Reference Poinar2018). Among decapods, several distinct lineages of brachyuran true crabs, as well as anomuran hermit crabs, and astacid crayfish are terrestrial; none are older than the Cretaceous (Bracken-Grissom et al. Reference Bracken-Grissom, Cannon, Cabezas, Feldmann, Schweitzer, Ahyong, Felder, Lemaitre and Crandall2013; Luque et al. Reference Luque, Feldmann, Vernygora, Schweitzer, Cameron, Kerr, Vega, Duque, Strange and Palmer2019, Reference Luque, Bracken-Grissom, Ortega-Hernández and Wolfe2023; Wolfe et al. Reference Wolfe, Breinholt, Crandall, Lemmon, Lemmon, Timm, Siddall and Bracken-Grissom2019, Reference Wolfe, Ballou, Luque, Watson-Zink, Ahyong, Barido-Sottani and Chan2023; Watson-Zink Reference Watson-Zink2021). Talitrid amphipods date back to the late Paleogene (Copilaş-Ciocianu et al. Reference Copilaş-Ciocianu, Borko and Fišer2020). Various copepods, ostracods, and branchiopod species that might be deemed terrestrial tend to be nested within genera that are otherwise aquatic (Sousa et al. Reference Sousa, Elmoor-Loureiro and Panarelli2017; Marin and Tiunov Reference Marin and Tiunov2023), thereby suggesting a recent origin without ruling out previous, now-extinct iterations of terrestriality among these lineages.
Among non-arthropod ecdysozoans, tardigrades, nematodes, and onychophorans all would have joined the terrestrial fauna in the Paleozoic. Terrestrial tardigrade fossils are unavailable before amber, but time-calibrated phylogenies suggest their presence by the mid-Paleozoic (Howard et al. Reference Howard, Giacomelli, Lozano-Fernandez, Edgecombe, Fleming, Kristensen, Ma, Olesen, Sørensen and Thomsen2022). Remarkably, direct Paleozoic fossil evidence is available for both terrestrial nematodes and onychophorans. Herbivorous nematodes are found directly within their plant hosts at Rhynie (Poinar et al. Reference Poinar, Kerp and Hass2008). A variety of “lobopodian” fossils stretching back to the Cambrian might be ancestral variously to any or all of the Panarthropoda phyla of Arthopoda, Tardigrada, and Onychophora, but a Carboniferous specimen has what appears to be slime spigots (Garwood et al. Reference Garwood, Edgecombe, Charbonnier, Chabard, Sotty and Giribet2016), securing both a relation to total-group onychophorans and that the lineage was already on land, as projectile glue could not be functional in an aquatic context.
Modern lineages of Spiralia within the terrestrial biota appear to be later additions. Among mollusks, two lineages of land snails are known from the Carboniferous (Solem and Yochelson Reference Solem and Yochelson1979), but their original assignments to modern land snail clades are now recognized as erroneous (Kano et al. Reference Kano, Chiba and Kase2002; Dayrat et al. Reference Dayrat, Conrad, Balayan, White, Albrecht, Golding, Gomes, Harasewych and de Frias Martins2011). The modern lineages of land snails and slugs are all Cretaceous and younger based on both fossil and phylogenetic evidence. There are many of them—more than 30 when considering the smaller endemic lineages that continue to be found (Vermeij and Watson-Zink Reference Vermeij and Watson-Zink2022). However, the Styllomatophora have had the most geographically widespread success and are by far the most diverse, with more than 20,000 species. Among the three lineages of clitellate annelids with terrestrial representation, only the tiny enchytraeids may have been present by the later Permian based on time-calibrated phylogenies; origins of earthworms and leeches are Mesozoic (Erséus et al. Reference Erséus, Williams, Horn, Halanych, Santos, James, des Châtelliers and Anderson2020). A leech-like cocoon preserved in the Triassic (Bomfleur et al. Reference Bomfleur, Kerp, Taylor, Moestrup and Taylor2012) may represent an extinct terrestrialization, given that modern terrestrial leeches are expected to be younger. Consistent with molecular phylogenetic estimates, trace fossil evidence of earthworms appeared over the Jurassic (Genise et al. Reference Genise, Bedatou, Bellosi, Sarzetti, Sánchez, Krause, Mángano and Buatois2016). Terrestrial bdelloid rotifers are preserved only in amber, but phylogenetic estimates suggest the bdelloid crown may itself be Cenozoic (Poinar and Ricci Reference Poinar and Ricci1992; Tang et al. Reference Tang, Obertegger, Fontaneto and Barraclough2014). Land planarians and nemerteans have neither fossil preservation nor much in the way of phylogenetic age constraints (Sola et al. Reference Sola, Sluys, Gritzalis and Riutort2013; Benítez-Álvarez et al. Reference Benítez-Álvarez, Leal-Zanchet, Oceguera-Figueroa, Ferreira, de Medeiros Bento, Braccini, Sluys and Riutort2020).
Only appearing over the Carboniferous, terrestrial tetrapod vertebrates were a late addition to a system that was already complex (Coates et al. Reference Coates, Ruta and Friedman2008; Clack Reference Clack2012; Schoch Reference Schoch2014). Even once on land, their presence was only as adults, with juveniles remaining aquatic. Only with late Carboniferous amniotes did fully terrestrial tetrapods exist, while diverse aquatic forms persisted in freshwater environments. Terrestriality is independently derived among the modern lissamphibian lineage with an ancestry extending back to Carboniferous temnospondyls. Although some omnivory verging on herbivory was present among latest Carboniferous amniotes and their close relatives, tetrapods were overwhelmingly predatory, whether that meant consumption of fish, invertebrates, or other tetrapods. The modern prevalence of tetrapod herbivory was not established until the late Permian (Reisz and Sues Reference Reisz, Sues and Sues2000).
Land Plants
Plants progressively altered all aspects of the environment, including atmospheric composition, climate, terrestrial sedimentation and fluvial transport, weathering and soil formation, organic matter deposition, and the carbon cycle (Berner Reference Berner1992, Reference Berner2003, Reference Berner2006; Davies and Gibling Reference Davies and Gibling2010, Reference Davies and Gibling2013; Boyce and Lee Reference Boyce and Lee2017; Lenton et al. Reference Lenton, Daines and Mills2018; Ibarra et al. Reference Ibarra, Rugenstein, Bachan, Baresch, Lau, Thomas, Lee, Boyce and Chamberlain2019; D’Antonio et al. Reference D’Antonio, Ibarra and Boyce2020; Ielpi et al. Reference Ielpi, Lapôtre, Gibling and Boyce2022; Boyce et al. Reference Boyce2023). Given that plants dominate terrestrial biomass and play a fundamental role in shaping environments, the plant fossil record is unique in its provision of an anchor for the evolution of other groups as well as of the environment itself. When did different fungi evolve relative to their host plants? How precipitously did CO2 concentrations decline with the evolution of deep rooting? When did insect flight evolve relative to the Devonian evolution of trees? How have plants impacted the formation of floodplains and meandering of rivers? Angiosperm evolution also becomes a crucial marker for other components of the biota starting in the Cretaceous with potential relevance to later terrestrializations.
Luckily, the plant fossil record may also be uniquely suited to provide an accurate answer regarding the timing of many important early events: land plants may be the largest of individual organisms across most landscapes but will also source the most abundant and widespread of terrestrial microfossils via wind-dispersed pollen and spores (Traverse Reference Traverse2007). The distinctive spore tetrads of basal land plants and tricolpate pollen of eudicot angiosperms are abundant biostratigraphic markers important enough to be incorporated into phylogenetic time calibration as maximum ages for the lineage, not just minimums (Magallón and Sanderson Reference Magallón and Sanderson2005; Lutzoni et al. Reference Lutzoni, Nowak, Alfaro, Reeb, Miadlikowska, Krug and Arnold2018; Morris et al. Reference Morris, Puttick, Clark, Edwards, Kenrick, Pressel, Wellman, Yang, Schneider and Donoghue2018; Ramírez-Barahona et al. Reference Ramírez-Barahona, Sauquet and Magallón2020). Not all sediments preserve organic matter (Strömberg Reference Strömberg2004), but those that do are likely to preserve spores and pollen.
In addition to having a strong palynological record, early land plants are widely understood to have evolved in the wet, lowland depositional settings where preservational potential of plant fossils is highest. Various seed plant groups—most notably the angiosperms—have occasionally been suggested by some to have had a long fossil-free prehistory due to a hypothetical origin in uplands or drylands with little chance of preservation (Axelrod Reference Axelrod1952; Doyle and Hickey Reference Doyle, Hickey and Beck1976). That logic does not apply to early land plant evolution, because the earliest nonvascular land plants, derived from aquatic algae, would have had no capacity to move water on their own and instead would have been dependent on its passive availability. Desiccation tolerance in vegetative tissues is found in many nonvascular plants and soil algae (and is ubiquitous for spores and other propagules), but still requires hydration with adequate frequency and duration to maintain a positive photosynthetic carbon balance (Proctor et al. Reference Proctor, Oliver, Wood, Alpert, Stark, Cleavitt and Mishler2007; Oliver et al. Reference Oliver, Farrant, Hilhorst, Mundree, Williams and Bewley2020). Both mosses and liverworts can be dominant components of modern desert soil crusts (Seppelt et al. Reference Seppelt, Downing, Deane-Coe, Zhang, Zhang, Weber, Büdel and Belnap2016) but with species that are phylogenetically nested many nodes away from any land plant common ancestry. Modern marvels of physiological efficiency among land plants allow for desert survival, but initial evolution of desiccation tolerance in vegetative tissues should be expected adjacent to waterbodies in lowland floodplains (Raven Reference Raven1995)—common sites of sediment deposition and fossil preservation (Hotton et al. Reference Hotton, Hueber, Griffing, Bridge, Gensel and Edwards2001; Edwards and Richardson Reference Edwards and Richardson2004). Access to and transport of ground water increased in Devonian vascular plants with the evolution of the interrelated traits of deep rooting, wood, and trees (Boyce et al. Reference Boyce, Fan and Zwieniecki2017), but hints of upland, dry-adapted, homoiohydric plants do not appear before the Carboniferous, as best documented by fluvial transport of upland material as charcoal fragments to lowland deposition and by upland sediments occasionally captured directly within sinkholes and caves (Leary and Pfefferkorn Reference Leary and Pfefferkorn1977; Bateman and Scott Reference Bateman and Scott1990; DiMichele and Aronson Reference DiMichele and Aronson1992; Plotnick et al. Reference Plotnick, Kenig, Scott, Glasspool, Eble and Lang2009). Thereafter, the spread of vascular plants to increasingly arid and distal habitats becomes an important aspect of terrestrial evolution and geobiology, but is difficult to constrain (Boyce and Leslie Reference Boyce and Leslie2012). However long that spread may have taken, the early evolution of land plants could not have been sequestered out of sight up a mountain or in a desert.
Land plant spores are globally distributed in sediments starting in the Middle Ordovician (Steemans et al. Reference Steemans, Hérissé, Melvin, Miller, Paris, Verniers and Wellman2009). Not all ages will be represented with appropriate rocks at any individual locality. As a consequence, the ages of the earliest spores have drifted back further into the Middle Ordovician as geographic and temporal sampling has increased globally, but the change in age has been minimal—just a few million years (Strother et al. Reference Strother, Al-Hajri and Traverse1996; Rubinstein et al. Reference Rubinstein, Gerrienne, de la Puente, Astini and Steemans2010). While ambiguous palynomorphs from the late Cambrian have been suggested to be land plant related (Baldwin et al. Reference Baldwin, Strother, Beck, Rose and McIlroy2004; Taylor and Strother Reference Taylor and Strother2009), these differ substantially from embryophyte spores in size, arrangement, and wall structure (Steemans et al. Reference Steemans, Petus, Breuer, Mauller-Mendlowicz, Gerrienne and Talent2012). Even their proponents argue only for an early stem-group relationship to the embryophytes, with comparisons drawn to charophyte algae.
Early spore diversity was complex—many forms are now extinct, and relationships could be surprising when spores have been found in situ on the parent plant (Edwards et al. Reference Edwards, Morris, Axe, Duckett, Pressel and Kenrick2022)—but Late Ordovician palynofloras did include the first trilete spores most closely associated with the polysporangiophyte lineage that includes the vascular plants and their stem-group ancestors (Steemans et al. Reference Steemans, Hérissé, Melvin, Miller, Paris, Verniers and Wellman2009). However, that stem-group ancestry is increasingly recognized to have involved physiologies more like modern bryophytes (Boyce Reference Boyce2008; Edwards et al. Reference Edwards, Morris, Axe, Duckett, Pressel and Kenrick2022; Tomescu Reference Tomescu2022). Tiny axes too thin to accommodate photosynthetic tissues, determinate branching patterns, a lack of rhizoids, and presence of potential transfer cells all indicate the diploid sporophyte generation was dependent on the haploid gametophyte generation for both photosynthesis and substrate interaction, as in modern mosses, hornworts, and liverworts (Boyce Reference Boyce2008; Edwards et al. Reference Edwards, Morris, Axe, Duckett, Pressel and Kenrick2022). Silurian through Early Devonian fossils then show the mosaic accumulation of traits that became universal in the sporophyte generation of crown-group vascular plants: branching, thicker axes capable of their own photosynthesis, roots or rhizoids, indeterminate growth, and the water-conducting tracheids that give vascular plants their name (Shute and Edwards Reference Shute and Edwards1989; Kenrick and Crane Reference Kenrick and Crane1997; Edwards et al. Reference Edwards, Banks, Ciurca and Laub2004; Boyce Reference Boyce2008; Libertín et al. Reference Libertín, Kvaček, Bek, Žárský and Štorch2018).
Crown-group vascular plants first appeared in the late Silurian with stem-group lycopsid ancestors, herbaceous plants with complex primary vasculature, a fringe of adventitious roots along the prostrate indeterminately growing rhizomes, and the beginnings of small leaves on upright axes less than a meter tall (Kenrick and Crane Reference Kenrick and Crane1997; Taylor et al. Reference Taylor, Taylor and Krings2009). Contemporaneous plants from the other lineage of vascular plants that later included ferns, horsetails, and the seed plants were of even simpler construction (Knoll et al. Reference Knoll, Niklas, Gensel and Tiffney1984). Thus, innovations like roots, leaves, and wood all evolved independently across these lineages as they diverged from simple common ancestors over the Devonian and onward (Niklas Reference Niklas1997; Kenrick Reference Kenrick, Waisel, Eshel and Kafkafi2002; Boyce Reference Boyce, Zwieniecki and Holbrook2005, Reference Boyce2010; Tomescu Reference Tomescu2008; Kenrick and Strullu-Derrien Reference Kenrick and Strullu-Derrien2014). Trees evolved independently four or five times across these vascular plant lineages during the later Devonian. One of those arborescent groups, the archaeopterid progymnosperms, were stem-group relatives of the seed plants (Beck Reference Beck1960; Meyer-Berthaud et al. Reference Meyer-Berthaud, Scheckler and Wendt1999). However, the first seed plants of the Late Devonian were small shrubs, so that Carboniferous seed plants represent multiple additional evolutions of tree forms (Rothwell Reference Rothwell1989; Decombeix et al. Reference Decombeix, Meyer-Berthaud and Galtier2011). Similarly, both sphenopsids (Schweitzer Reference Schweitzer1967; Rößler et al. Reference Rößler, Feng and Noll2012) and several distinct fern lineages (DiMichele and Phillips Reference DiMichele and Phillips2002; Hueber and Galtier Reference Hueber and Galtier2002; Stein et al. Reference Stein, Mannolini, Hernick, Landing and Berry2007; Meyer-Berthaud et al. Reference Meyer-Berthaud, Soria and Decombeix2010) also included multiple evolutions of arborescence across the later Paleozoic.
As discussed (Fig. 1), a cryptic early diversification should yield a randomized sequence of the first appearances of major lineages in the fossil record (Schachat et al. Reference Schachat, Goldstein, DeSalle, Bobo, Boyce, Payne and Labandeira2023a); the progression of the land plant fossil record presents a consistency with the phylogeny that casts doubt upon much earlier dates (Fig. 5). This branching order is reflected in the sequential appearance of plant clades beginning with the first potential crown-group land plants in the Middle Ordovician. This is followed by the appearance of the vascular plant stem-group in the Late Ordovician, and subsequent progressive innovations in that stem-group leading to crown-group vascular plants in the late Silurian with stem-group lycopsids. Other major lineages of vascular plants appear over the Devonian, including stem-group relatives of the seed plants in the Middle Devonian with the progymnosperms and culminating with seeds in the Late Devonian. Over this time, plant heights progressed incrementally from millimeters to tens of meters, and axial vasculature progressed from threads a few cells thick to abundantly woody tree trunks. The consistency of these patterns strongly suggests that no chunks of petrified wood await discovery in Ordovician or Silurian rocks (much less the Proterozoic: Su et al. Reference Su, Yang, Shi, Ma, Zhou, Hedges and Zhong2021).
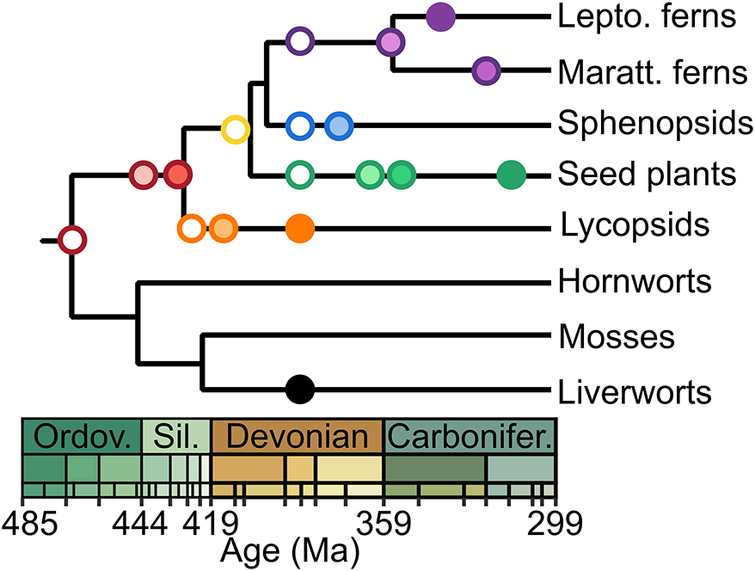
Figure 5. Stratigraphic distribution of fossil first occurrences in the land plant record. Symbols follow the usage in Fig. 4. Progymnosperms and early seed plants (e.g., Devonian Elkinsia and early Carboniferous lyginopterids) included as stem-group ancestors to crown-group seed plants. Stem-group ancestry to the modern ferns includes Devonian Pseudosporochnales and Zygopteridales, as well as Carboniferous Psaroniaceae for the Marattiales. Stem-group ancestry of sphenopsids includes Devonian Ibyka and Calamitales. The relationship between vascular plants and the three bryophyte lineages remains unsettled (Lutzoni et al. Reference Lutzoni, Nowak, Alfaro, Reeb, Miadlikowska, Krug and Arnold2018; Bell et al. Reference Bell, Lin, Gerelle, Joya, Chang, Taylor, Rothfels, Larsson, Villarreal and Li2020), but the phylogenetic topology employed here is currently most favored (Su et al. Reference Su, Yang, Shi, Ma, Zhou, Hedges and Zhong2021). The phylogenetic and stratigraphic placement of fossils (Kenrick and Crane Reference Kenrick and Crane1997; Taylor et al. Reference Taylor, Taylor and Krings2009; Rubinstein et al. Reference Rubinstein, Gerrienne, de la Puente, Astini and Steemans2010; Libertín et al. Reference Libertín, Kvaček, Bek, Žárský and Štorch2018) strongly supports trust of the fossil record over the much earlier dates that can be found in molecular clock studies (e.g., Su et al. Reference Su, Yang, Shi, Ma, Zhou, Hedges and Zhong2021).
And yet, molecular phylogenetics has also been essential. The high degree of morphological convergence outlined earlier, with the repeated evolution of similar organs and tissues, means that the use of plant morphology to discern broad phylogenetic relationships is fraught with risk. Although complicated by the high number of extinct lineages, our understanding of relationships within important living lineages, such as leptosporangiate ferns, seed plants, and flowering seed plants, is highly dependent on molecular phylogenetic inference (APG 2016; PPG 1 2016). It can then be noted that the fossil record of each of those important extant lineages closely corresponds to an unfurling of the molecular topology (Taylor et al. Reference Taylor, Taylor and Krings2009; Soltis et al. Reference Soltis, Soltis, Endress, Chase, Manchester, Judd, Majure and Mavrodiev2018)—indicating again that the evolutionary timing suggested by the fossil record is accurate.
Discussion
Most of the biota does not readily fossilize most of the time. That is true for entire large clades of enormous consequence, like nematodes. Even the lineages that do have robust fossil records will lack taxonomic saturation comparable to that employed in many diversification rate analyses as well as lack the preservation of specific traits (such as behavior, anatomy, ecology, and physiology) that can instead be inferred through the use of ancestral-state reconstruction and comparative genomics (Givnish et al. Reference Givnish, Barfuss, Van Ee, Riina, Schulte, Horres, Gonsiska, Jabaily, Crayn and Smith2014, Reference Givnish, Spalink, Ames, Lyon, Hunter, Zuluaga, Iles, Clements, Arroyo and Leebens-Mack2015; Chang et al. Reference Chang, Wang, Sekimoto, Aerts, Choi, Clum, LaButti, Lindquist, Ngan and Ohm2015; Dee et al. Reference Dee, Mollicone, Longcore, Roberson and Berbee2015; Torruella et al. Reference Torruella, De Mendoza, Grau-Bové, Antó, Chaplin, Campo, Eme, Perez-Cordon, Whipps and Nichols2015; Lutzoni et al. Reference Lutzoni, Nowak, Alfaro, Reeb, Miadlikowska, Krug and Arnold2018; Nelsen et al. Reference Nelsen, Ree and Moreau2018, Reference Nelsen, Lücking, Boyce, Lumbsch and Ree2020a,Reference Nelsen, Lücking, Boyce, Lumbsch and Reeb, Reference Nelsen, Leavitt, Heller, Muggia and Lumbsch2021, Reference Nelsen, Moreau, Boyce and Ree2023; Testo et al. Reference Testo, Field and Barrington2018; Varga et al. Reference Varga, Krizsán, Földi, Dima, Sánchez-García, Sánchez-Ramírez and Szöllősi2019; Sánchez-García et al. Reference Sánchez-García, Ryberg, Khan, Varga, Nagy and Hibbett2020; Boyce et al. Reference Boyce, Ibarra, Nelsen and D’Antonio2023; Stephens et al. Reference Stephens, Gallagher, Dun, Cornwell and Sauquet2023). As a stand-alone representation of evolutionary history, the fossil record is insufficient. However, the fossil record that we do have provides a coherent and repeatable sequence of events that can be tied directly into the absolute timescale provided by the rock record—that is irreplaceable.
On balance, the geological record can uniquely provide a historical framework via the absolute dating of events and fossil occurrences, and neontological approaches leveraging molecular phylogenetic data from extant taxa can then enable consideration of the gaps in that framework. This perspective might be employed throughout evolutionary history, as already seen with the discourse regarding the diversification of metazoans in the Cambrian explosion. In the context of terrestrialization, this perspective raises four issues for further consideration.
A Cryptic Continuity to the Terrestrial Fauna
The integration of fossil and molecular phylogenetic evidence brings focus to the evolution of complex life on land being the evolution of the soil biota. By the Devonian, the soil fauna contained stem-group and, in many cases, crown-group nematodes, tardigrades, mites, pseudoscorpions, harvestmen, pauropods, symphylans, millipedes, centipedes, proturans, diplurans, collembolans, and apterygote insects. That list is remarkably consistent with our modern soil faunas, and it is striking how little the soil fauna appears to have changed since our first Devonian glimpses of it (Fig. 6). In contrast to a tetrapod fauna that turned over six times in just 100 Myr during the early, middle, and late Permian and Early, Middle, and Late Triassic (Benton Reference Benton2014), the soil fauna has largely been in place for 400 Myr. To be sure, there have been important additions over later time, such as ants, beetle larvae, and earthworms, but those are additions without any known subtractions.
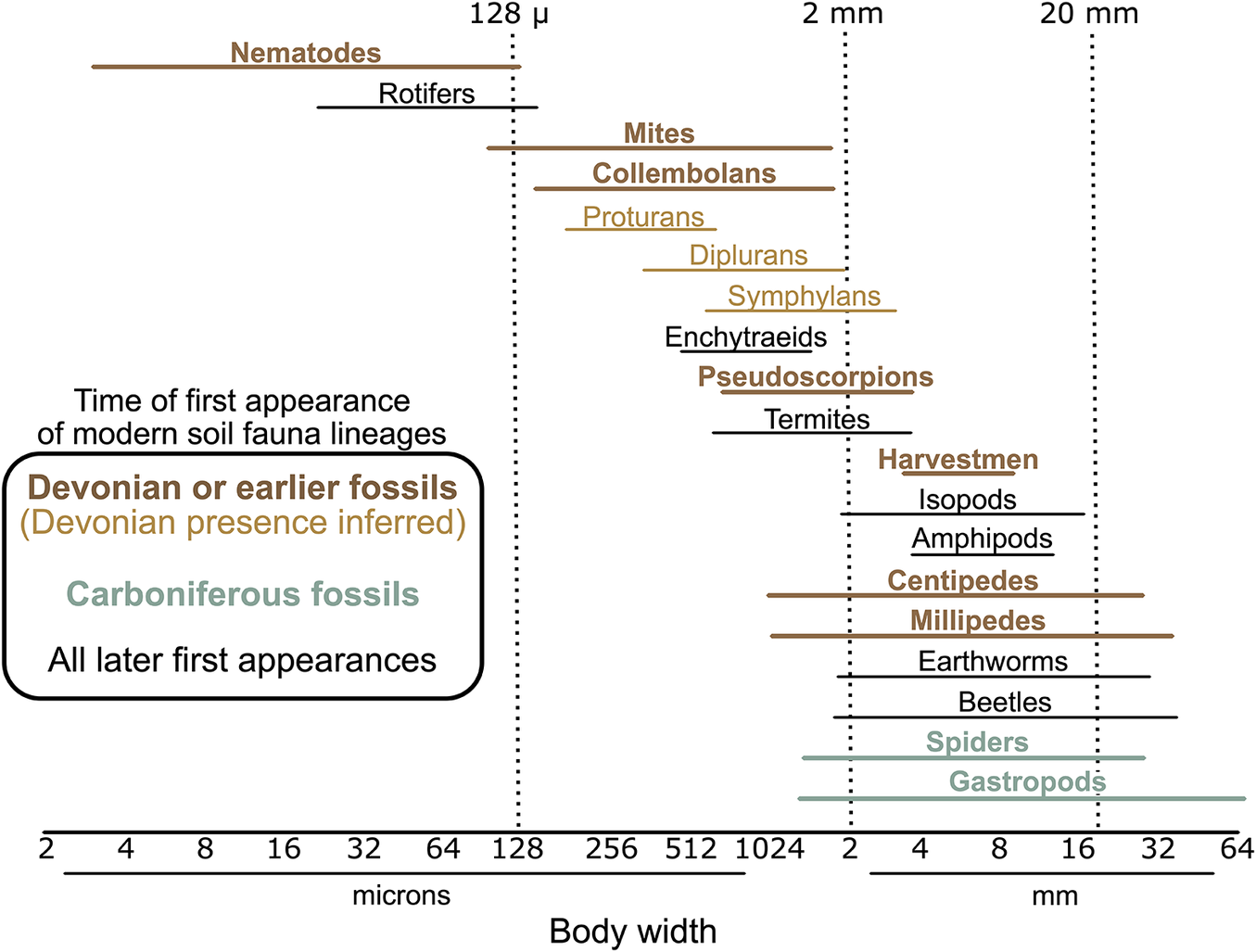
Figure 6. Classic representation of the modern soil fauna (frequently reproduced, originally from Swift et al. [Reference Swift, Heal, Anderson and Anderson1979]) redrawn to highlight 400 Myr of continuity. Half of the lineages depicted can be traced back to the Devonian, whether directly via fossils (bold) or inferred from the fossil preservation of a sibling lineage. Other lineages not depicted also would have been present in the Devonian (e.g., tardigrades, pauropods). Modern land snail lineages are Cretaceous or younger, but the ecology was represented among Carboniferous fossils with lineages now extinct. Overall, the only ecologies depicted here likely to have been newly added as late as the Cenozoic may have been tallitrid amphipod “lawn shrimp.”
By the late Carboniferous, the fundamental phylogenetic structure within the three primary lineages of terrestrial arthropods had been established (Figs. 1C, 4). Each extant arachnid order is likely to have been a distinct lineage. Not all modern insect orders existed, but paleopteran and polyneopteran insects were diverse, and the basic subdivisions within the Paraneoptera and the Holometabola also were present—all within about 20 Myr of the first winged insects (Nel et al. Reference Nel, Roques, Nel, Prokin, Bourgoin, Prokop, Szwedo, Azar, Desutter-Grandcolas and Wappler2013; Schachat et al. Reference Schachat, Labandeira, Saltzman, Cramer, Payne and Boyce2018, Reference Schachat, Goldstein, DeSalle, Bobo, Boyce, Payne and Labandeira2023a). Among myriapods, a Carboniferous centipede fossil of the derived scolopendromorphs indicates their geophilomorph sibling lineage also would be distinct, even if their crown-group is not expected until later in the Permian (Shear and Edgecombe Reference Shear and Edgecombe2010; Benavides et al. Reference Benavides, Edgecombe and Giribet2023). Millipede fossils, including some with fused tergites and cylindrical body outline, suggest the derived eugnathan helminthomorphs were present, including divergence of their superorders (Shear and Edgecombe Reference Shear and Edgecombe2010; Rodriguez et al. Reference Rodriguez, Jones, Sierwald, Marek, Shear, Brewer, Kocot and Bond2018).
For many of these taxa, no fossils are yet known, and assumptions must be made regarding continuity of form and ecology with modern relatives, but at least the stem-group ancestry of all those lineages would have been present and distinct. Where molecular phylogenetic information is available within lineages, crown-groups can be surprisingly young, for example, a Cenozoic crown for the basal mesothelid spiders (Xin et al. Reference Xin, Liu, Chen, Ono, Li and Kuntner2015), thereby hinting at the potential of important turnover within lineages undersampled by the fossil record. And important modern innovations should not be assumed to be present in ancient stem-groups, such as the burrowing of geophilomorph centipedes. However, where Paleozoic fossils are available, continuity of form and ecology between stem and crown tends to exist. In some cases, long-term consistency of form and ecology can lead to eventual diversification as the Earth system and its biota continues to change, as with the mygalomorph spiders present since the Triassic but diversifying in the Cenozoic alongside ants and other non-volant insects as potential prey (Dunlop and Penney Reference Dunlop and Penney2012; Garrison et al. Reference Garrison, Rodriguez, Agnarsson, Coddington, Griswold, Hamilton, Hedin, Kocot, Ledford and Bond2016).
Although several highly diverse lineages are understudied, such as mites and millipedes, many groups exhibit a stable ecological presence extending from the Paleozoic to modern systems. Our perception of faunal change and turnover since the Carboniferous, such as we have it, is dictated by innovation and novelty through time in a few key lineages, such as tetrapods, winged insects, and spiders—alongside later terrestrializations such as Mesozoic earthworms and styllomatophoran snails.
Expanding Away from the Interface
In the soil crusts that would have existed throughout the Proterozoic, terrestrial life would have been concentrated at the thin interface between soil and atmosphere where cyanobacteria-based productivity and nitrogen fixation occurred. Bryophyte-grade land plants joined these systems in the Ordovician but would not have changed the basic geometry and fundamental thinness of soil crusts. Although surface roughness may have increased in some environments as bryophytes and the other simple thalloid forms that followed (Tomescu and Rothwell Reference Tomescu, Rothwell, Greb and DiMichele2006; Edwards et al. Reference Edwards, Honegger, Axe and Morris2018) accumulated dead material or baffled dust from the air (Williams et al. Reference Williams, Buck and Beyene2012), that building up of the substrate would have amounted to elevating the interface without thickening the living vegetation or increasing its stature. The living green edge may have changed albedo or altered surface hydrology by increasing water retention and impeding water penetrance but would still have had little reach above or into the substrate, except via assistance from mycorrhizal associations. That changed with the late Silurian and Devonian radiation of rooted vascular plants, but unevenly so: through the Devonian, much of the landscape appears to have remained occupied by soil crusts and bryophytes rather than trees and shrubs (Boyce et al. Reference Boyce, Hotton, Fogel, Cody, Hazen, Knoll and Hueber2007; Hobbie and Boyce Reference Hobbie and Boyce2010).
As terrestrial animals evolved, they appear to have tracked the opportunities afforded by the vegetation. Most of the higher-level lineages present by the Devonian are now diminutive members of the soil fauna and presumably were so then as well. Some of these lineages can be found at considerable depth in modern soils (Edwards Reference Edwards1961) by virtue of being small enough for passive occupation of interstitial pores rather than due to active burrowing. Evolution of a more expansive range of ecologies can be traced over later time, particularly in arthropods. Pauropods, symphylans, and the basal bristly millipedes are all tiny soil fauna among the myriapods; active deep-burrowing capacity is an innovation of more derived millipedes. Similarly, among centipede myriapods, the basal scutigeromorphs are fast, long-legged runners across open surfaces, but speed can be compromised in more derived centipedes due to trade-offs related to burrowing capacity, ultimately culminating with the worm-like geophilomorphs—but only well after the evolution of deep-rooting trees. Proturan, dipluran, and collembolan hexapods along with the basal apterygote insects are mostly small to tiny components of the soil and litter; it is of course the more derived insects that took the greatest of leaps with flight in Carboniferous forests. Sadly, the arachnids have not yet achieved flight but answered insects in their own way by adding to their early diversity of soil and surface predators with the Mesozoic evolution of web-based prey capture in Araneomorph spiders (Blackledge et al. Reference Blackledge, Scharff, Coddington, Szüts, Wenzel, Hayashi and Agnarsson2009; Kallal et al. Reference Kallal, Kulkarni, Dimitrov, Benavides, Arnedo, Giribet and Hormiga2020).
This expansion within terrestrial ecosystems mirrors the expansion of ecological tiering through time in the marine realm (Bottjer and Ausich Reference Bottjer and Ausich1986; Fig. 7). This correspondence between the evolution of marine and terrestrial systems ultimately reflects an energy flux from above in both cases, although the intervening mechanics are distinct. In the marine realm, photic zone productivity rains down to accumulate passively on the substrate surface where available for exploitation by shallow deposit feeders. Beginning in the Ordovician, this flux is increasingly intercepted by filter feeders before reaching the substrate surface, initially those filter feeders rooted on the substrate and taking advantage of water currents to access large volumes of water, and later dominated by burrowing forms that pump water past the substrate surface into the sediment. Whereas the marine benthos has been structured by the fauna and its innovations in capturing a photosynthetic flux uniformly sourced from the photic zone above, photosynthetic life itself has structured terrestrial systems via the expansion in size of the vegetation, from thalloid forms limited to the thin substrate/atmosphere interface, to large trees that can grow many meters above the substrate and extend deep in the subsurface with roots, effectively pumping photosynthetic activity down into the soil.
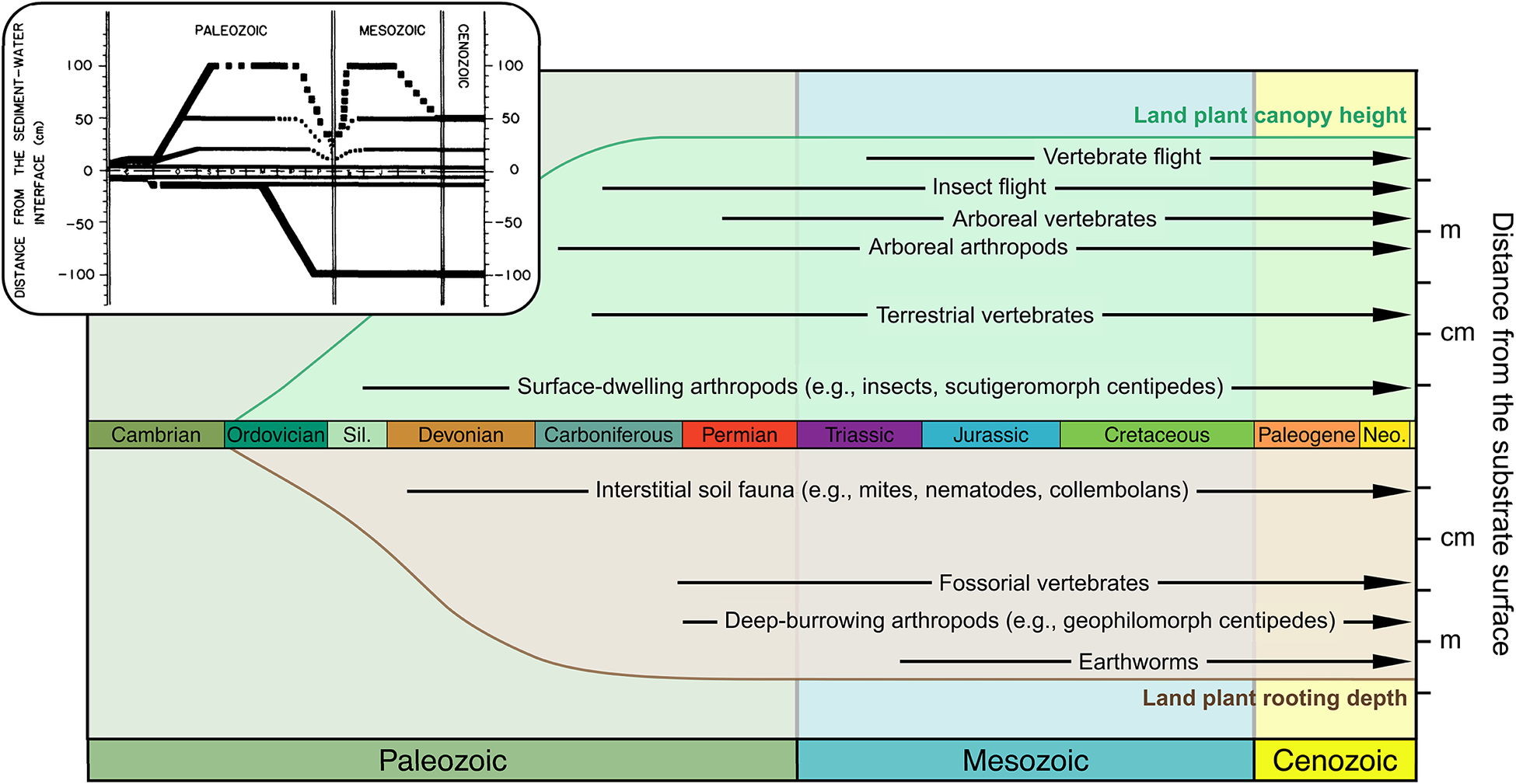
Figure 7. Correspondence of the evolution of ecological tiering in marine and terrestrial realms. Inset depiction of the benthic marine fauna is a classic from the pages of Paleobiology (Bottjer and Ausich Reference Bottjer and Ausich1986). In both cases, activity is initially limited to the substrate surface before expanding above and below the interface. Both patterns are structured by the availability of photosynthetic productivity. In the marine benthos, productivity rains down from the photic zone above, initially concentrating animal activity with deposit feeding in the local accumulation of detritus at the sediment–water interface. Thereafter, filter feeders took passive advantage of water currents for suspension feeding to intercept descending organic matter before it reached the sediments, followed by more reliance on active pumping of water into the sediments for suspension feeding at depth. In terrestrial systems, the physical extent of land plants (upper bounds shown in green/brown shading) directly structures animal communities. The earliest land plants were themselves limited to the soil surface before the vascular plant lineage achieving greater stature, including shrubs and trees, and pumping primary productivity deep into the soil via roots. Evolution of active burrowing and flight among land animals followed the evolution of structural innovations in land plants.
Evolution of Herbivory
Paleontological interest in herbivory typically focuses on the relative digestibility of different plant organs (Shear and Selden Reference Shear, Selden, Gensel and Edwards2001; Labandeira Reference Labandeira2007), as well as the physical act of feeding: tetrapod teeth and jaw mechanics (Christensen Reference Christensen2014; Mihlbachler et al. Reference Mihlbachler, Campbell, Ayoub, Chen and Ghani2016), as well as insect mouthparts and the damage left behind in plant tissues (Labandeira Reference Labandeira1997; Wilf et al. Reference Wilf, Labandeira, Kress, Staines, Windsor, Allen and Johnson2000; Labandeira et al. Reference Labandeira, Wilf, Johnson and Marsh2007; Schachat et al. Reference Schachat, Payne and Boyce2023b). However, the prevalence of soil fauna among early terrestrial life suggests that a key consideration may, instead, be nutrient stoichiometry and the role of herbivore body size in avoiding the stoichiometric challenges unique to terrestrial herbivory (Boyce Reference Boyce, Ibarra, Nelsen and D’Antonio2023). Land or sea, the biochemical composition of most life roughly follows the Redfield ratio of 106:16:1 for C:N:P abundance, but land plants dramatically skew those ratios due to a superabundance of carbon in their cell wall materials. Rather than a C:N ratio of 7:1, plant leaves average 36:1, and wood can have ratios greater than 1000:1 (Elser et al. Reference Elser, Fagan, Denno, Dobberfuhl, Folarin, Huberty, Interlandi, Kilham, McCauley and Schulz2000; Smyth et al. Reference Smyth, Titus, Trofymow, Moore, Preston and Prescott2016). A recurring concern in evolutionary studies has been the acquisition of gut symbionts with the enzymatic capacity to depolymerize cellulose to its glucose monomers (Reisz and Sues Reference Reisz, Sues and Sues2000; Shear and Selden Reference Shear, Selden, Gensel and Edwards2001; Reisz and Fröbisch Reference Reisz and Fröbisch2014; Dunlop and Garwood Reference Dunlop and Garwood2017), but sugar consumption alone can only fuel movement and behavior. Growth and reproduction require the nitrogen and phosphorous that are integral to proteins, nucleic acids, and membrane phospholipids but are diluted in land plants by an overabundance of carbon, predominantly in the form of cell wall polymers.
How herbivores confront these stoichiometric challenges varies with body size (Boyce Reference Boyce2023). Herbivores among the soil micro- and mesofauna, including some nematodes, tardigrades, collembolans, and mites, can focus on consumption of individual cell contents on a cell-by-cell basis, bypassing the stoichiometric extremes of cell wall material. Larger insect herbivores can at least still selectively avoid the most nutrient-poor materials within a plant tissue, like cuticle and veins. Tetrapod herbivores must cope with the full stoichiometric imbalance inherent in consuming entire plants or plant organs; to do so requires capacious guts and consumption of large volumes of food to ensure access to adequate nutrients despite the low nutritional content (Hirakawa Reference Hirakawa2001; Karasov and del Rio Reference Karasov and del Rio2020). The opportunities and challenges of herbivory at different body sizes then play out over the Paleozoic with the sequential appearances of cell-scale microfauna herbivory, tissue-scale insect herbivory, and organ-scale tetrapod herbivory spanning 150 Myr between documentation of microfauna herbivory in the Early Devonian and tetrapod herbivory attaining its modern prominence in the late Permian (Labandeira Reference Labandeira1997, Reference Labandeira1998; Reisz and Sues Reference Reisz, Sues and Sues2000; Iannuzzi and Labandeira Reference Iannuzzi and Labandeira2008; Poinar et al. Reference Poinar, Kerp and Hass2008; Gorman Reference Gorman2013; Labandeira et al. Reference Labandeira, Tremblay, Bartowski and Hernick2014).
Maximum herbivore body size will then have impacted secondary consumers (Knoll and Follows Reference Knoll and Follows2016). Animal food webs before the Carboniferous consisted of soil detritivores—including consumers of fungi and bacteria—and cell-by-cell herbivores along with their predators (Fig. 8). Such a system avoids the stoichiometric challenges of herbivory but is necessarily small. No chain of predatory consumption could get far into larger sizes if rooted in a trophic foundation of animals measured in microns. That system changed during the late Paleozoic, and insect herbivory may have constituted an inflection point. As herbivory evolved among winged insects in the Carboniferous, herbivore sizes increased. Given the sizes attained by some paleodictyopteroids (Kukalová-Peck and Richardson Reference Kukalová-Peck and Richardson1983), the body mass of Carboniferous insect herbivores likely exceeded by nine orders of magnitude that of an herbivorous mite in the Devonian. With primary productivity shunted directly to much larger herbivores, insect predators could be larger still.

Figure 8. Transitions in Paleozoic food webs. Before the Carboniferous, cell-by-cell herbivory from the microfauna provided the only direct trophic interaction between animals and plants. Otherwise, animal communities were founded on decomposition, with detritivores feeding on the microbial life engaged in the decay of plant biomass. With detritivores and cell-by-cell herbivores necessarily small, the maximum sizes achieved by higher-order consumers also were small. With the appearance of insect herbivory in the Carboniferous, terrestrial vertebrate predators became prominent—although vertebrate communities were also subsidized by aquatic feeding, not depicted. Only with vertebrate herbivory reaching its modern prevalence later in the Permian did vertebrate communities begin to approach the large body sizes that would be achieved in the Mesozoic. For alternative representations of Paleozoic trophic ecology, see Habgood et al. (Reference Habgood, Hass and Kerp2003) and Labandeira (Reference Labandeira2005).
Overall maximum body sizes, of course, were ultimately tied to tetrapods and their gradual terrestrialization. In the Carboniferous, tetrapod food webs were heavily subsidized by aquatic feeding, as even tetrapods on land as adults would have had aquatic juveniles (Schoch Reference Schoch2009). It was after insect herbivory became systemic in the late Carboniferous that fully terrestrial tetrapods appeared with total-group amniotes. And, just as large insect herbivores led to large insect predators, the eventual establishment of abundant tetrapod herbivory over the Permian was a prerequisite for the enormous increases in size among tetrapods in the Mesozoic (Fig. 8).
A Second Wave?
Over the 150 Myr of the Silurian through Carboniferous, at least nine separate animal lineages found their way onto land: arachnids, myriapods, hexapods, nematodes, tardigrades, tetrapods, onychophorans, and two gastropod lineages (Fig. 9). This early cohort was associated with the Paleozoic rise of the land plant vegetation and establishment of the modern range of terrestrial environments, and many of its lineages have been fundamental to defining our modern world. That early cohort, however, represents a minority of all the modern independently terrestrial animal lineages. In the subsequent 150 Myr including the Permian through Jurassic, enchytraeid, leech, and earthworm annelids are the only known additions to the system, perhaps along with oniscidean isopods. After this comparative lull, however, the most recent 150 Myr of the Cretaceous and Cenozoic have seen the terrestrialization of dozens of gastropod lineages and between 2 and 10 lineages of brachyuran decapods depending on where one draws the line of “terrestrial.” In addition to the brachyurans, other crustacean terrestrializations include astacid decapods with burrowing crayfish, anomuran decapods like the mighty coconut crab, and diminutive talitrid amphipods. Rotifers also may have a terrestrial history no older than the Cretaceous (Tang et al. Reference Tang, Obertegger, Fontaneto and Barraclough2014).
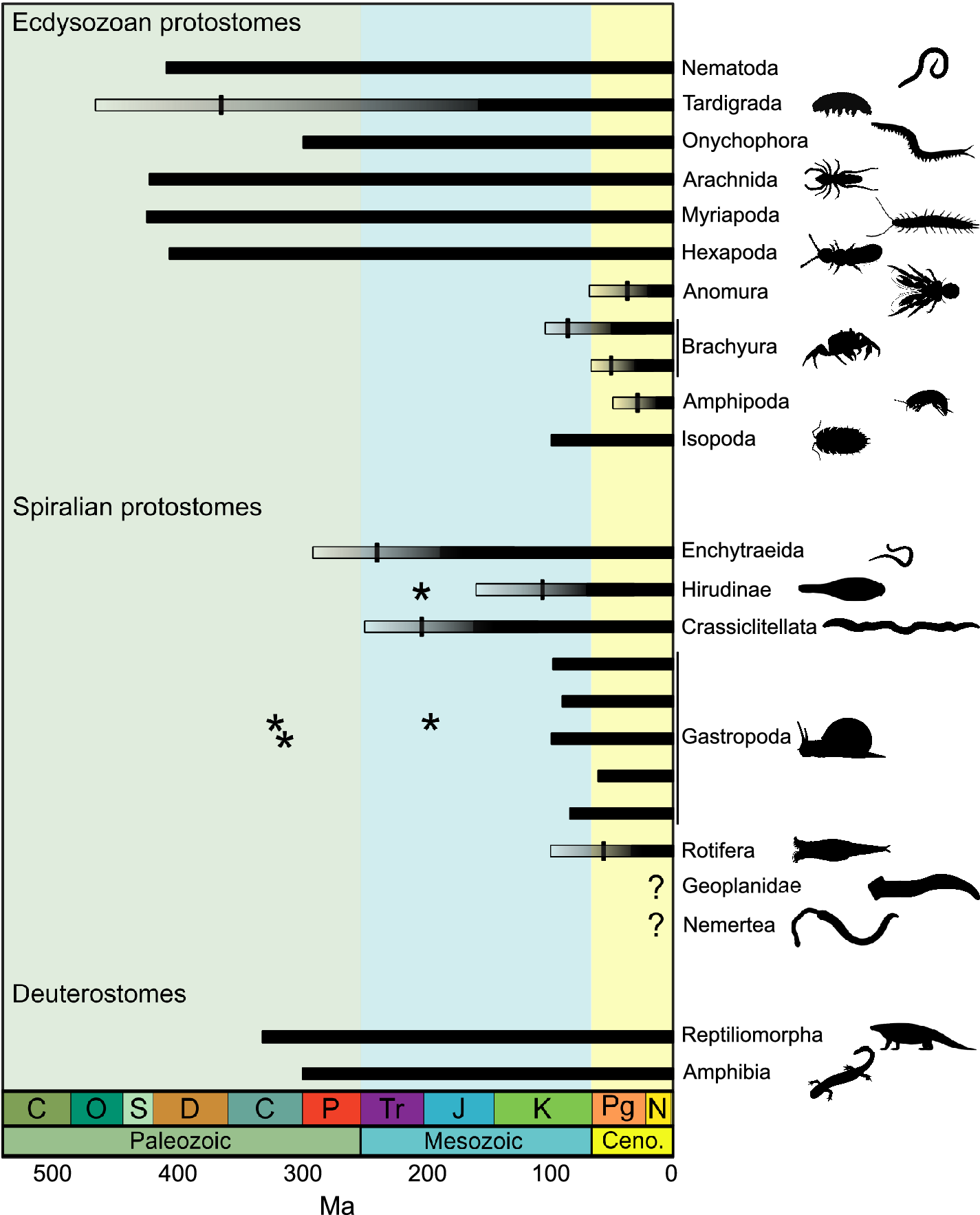
Figure 9. Timing of independent derivations of terrestrial animal lineages. Ranges based on the fossil record are depicted with solid bars. Lineage ages based on time-calibrated phylogenies are depicted with a gradient reflecting 95% confidence intervals; median age is indicated with a tick mark. With neither fossils nor adequate phylogenetic attention, the origins of land planarians and nemerteans are difficult to constrain but may well be old (Sola et al. Reference Sola, Sluys, Gritzalis and Riutort2013; Benítez-Álvarez et al. Reference Benítez-Álvarez, Leal-Zanchet, Oceguera-Figueroa, Ferreira, de Medeiros Bento, Braccini, Sluys and Riutort2020). Asterisks indicate fossils documenting land animal lineages now extinct among annelids and gastropods (Solem and Yochelson Reference Solem and Yochelson1979; Bomfleur et al. Reference Bomfleur, Kerp, Taylor, Moestrup and Taylor2012; Jochum et al. Reference Jochum, Yu and Neubauer2020). Six distinct animal phyla had achieved terrestriality by the Carboniferous, most prominently the arthropods with arachnids, myriapods, and hexapods (Poinar et al. Reference Poinar, Kerp and Hass2008; Bishop et al. Reference Bishop, Walmsley, Phillips, Quayle, Boisvert and McHenry2015; Garwood et al. Reference Garwood, Edgecombe, Charbonnier, Chabard, Sotty and Giribet2016; Wolfe et al. Reference Wolfe, Daley, Legg and Edgecombe2016; Brookfield et al. Reference Brookfield, Catlos and Suarez2021; Howard et al. Reference Howard, Giacomelli, Lozano-Fernandez, Edgecombe, Fleming, Kristensen, Ma, Olesen, Sørensen and Thomsen2022). Later additions included clusters of independent lineages among annelids, gastropod mollusks, and pancrustacean arthropods (Bandel and Riedel Reference Bandel and Riedel1994; Kano et al. Reference Kano, Chiba and Kase2002; Dayrat et al. Reference Dayrat, Conrad, Balayan, White, Albrecht, Golding, Gomes, Harasewych and de Frias Martins2011; Bracken-Grissom et al. Reference Bracken-Grissom, Cannon, Cabezas, Feldmann, Schweitzer, Ahyong, Felder, Lemaitre and Crandall2013; Broly et al. Reference Broly, Maillet and Ross2015; Romero et al. Reference Romero, Pfenninger, Kano and Klussmann-Kolb2016; Bullis et al. Reference Bullis, Herhold, Czekanski-Moir, Grimaldi and Rundell2020; Copilaş-Ciocianu et al. Reference Copilaş-Ciocianu, Borko and Fišer2020; Erséus et al. Reference Erséus, Williams, Horn, Halanych, Santos, James, des Châtelliers and Anderson2020; Balashov Reference Balashov2021; Harzhauser and Neubauer Reference Harzhauser and Neubauer2021; Tingting and Neubauer Reference Tingting and Neubauer2021; Wolfe et al. Reference Wolfe, Ballou, Luque, Watson-Zink, Ahyong, Barido-Sottani and Chan2023). From the top, included brachyuran decapod lineages are gecarcinids and sesarmids; included gastropod lineages are cyclophoroids, helicinids, hydrocenids, ellobiids, and styllomatophorans. This figure is far from complete. Groups like gastropods and decapods present a spectrum of semi-terrestrial forms difficult to judge, e.g., are crayfish terrestrial if burrowing on dryland down to the water table (Butler Reference Butler2002; Welch and Eversole Reference Welch and Eversole2006; Marin and Tiunov Reference Marin and Tiunov2023)? With greater phylogenetic resolution, some groups would resolve into multiple separately terrestrial sublineages (e.g., bdelloid versus monogonont rotifers, abundant nematode lineages, even distinct clades of Nemertea: Kiontke and Fitch [Reference Kiontke and Fitch2013]; Kvist et al. [Reference Kvist, Laumer, Junoy and Giribet2014]; Tang et al. [Reference Tang, Obertegger, Fontaneto and Barraclough2014]) and scattered terrestrial species can be found in lineages otherwise aquatic, e.g., arboreal polychaete annelids (Glasby et al. Reference Glasby, Kitching and Ryan1990). Silhouettes from PhyloPic.org.
Terrestrialization means different things. If terrestrialization means the establishment of a complex multicellular biota on land, that process happened in the Paleozoic. If terrestrialization just means the act of leaving the water, however, that evolutionary activity continues to this day. The distinction matters but is muddled. The latter definition appears to be in effect for many studies focusing on the terrestrialization of individual groups. For such work, Cenozoic lineages can be useful and evocative as they are still in process; we can watch crabs leave the surf. However, applying this knowledge from recent transitions to the original evolution of a terrestrial fauna is complicated by the earlier Paleozoic constituting an entirely different context. Any animal lineage newly on land in the Devonian or a later time would be entering a far more complex system with greater trophic opportunities, more plant biomass, and more opportunity to shelter from environmental extremes than in the Silurian.
If there has been a second pulse of terrestrialization since the Cretaceous, another change in environmental context worth noting would be that there may also have been more terrestrial productivity with the diversification of flowering plants (Boyce et al. Reference Boyce, Fan and Zwieniecki2017); how much should be read into this correspondence, however, is unclear. First, considering the vegetation, the evolution of nitrogen-based symbioses with fungi and prokaryotes has dramatically increased, hinting at fundamental changes in nutrient availability through time (Boyce et al. Reference Boyce, Ibarra, Nelsen and D’Antonio2023). However, that transition was initiated in the Jurassic, before angiosperm evolution, thereby casting flowering plants as more of a result of global change rather than proximal cause and complicating potential links to newly terrestrial animal lineages. Second, considering the fauna, many of the terrestrial latecomers are concentrated in a few key lineages, like brachyuran crustaceans and pulmonate gastropods, that were themselves new to the mid-Mesozoic. So, if the pattern happened to hinge on key exaptations within these lineages, they simply would not have been available earlier in time. Furthermore, a Pull of the Recent may be in effect in the sense that we cannot be confident of a complete census of terrestrial lineages for any time before the present. As hinted at by Carboniferous snail shells and Triassic annelid cocoons (Fig. 9), lineages can also be lost to extinction (Dayrat et al. Reference Dayrat, Conrad, Balayan, White, Albrecht, Golding, Gomes, Harasewych and de Frias Martins2011). Thus, terrestrialization may be a question of turnover, rather than just accumulation, but that loss would be invisible to molecular phylogenetics and incompletely captured by paleontology. A fully resolved view of Carboniferous tetrapods would involve a much larger cohort of independently terrestrialized lineages beyond the modern persistence of amniotes and lissamphibians (Schoch Reference Schoch2014), a perspective more closely resembling the complexity of our modern terrestrial cohorts of decapod crustaceans and pulmonate gastropods. We are likely never to know if similar complexities existed with the Mesozoic terrestrializations of clitellate annelids. These concerns are compounded by the preservation potential of a variety of lineages being limited to amber, which was essentially unavailable as a mode of fossil preservation before the Cretaceous.
A final consideration is that we do not even have amber preservation for land planarians, nemerteans, and others; all we know is that they are on land now. Perhaps they are recent additions to terrestrial systems, perhaps not. Extensive molecular phylogenetic sampling of understudied lineages like planarians, nemerteans, isopods, and others could be helpful. For fossils and phylogenies alike, an understanding of terrestrialization will require a greater emphasis on the activity underfoot in the soil and litter.
Acknowledgments
We thank S. Schachat, G. Vermeij, and an anonymous reviewer for helpful feedback on the manuscript and S. Schachat for figure revisions—in particular, for holding us to a higher aesthetic standard on Figure 6. Inset for Figure 7 reprinted from Paleobiology with permission. M.P.N. kindly acknowledges support from the Negaunee Integrative Research Center (Field Museum).
Competing Interests
The authors declare no competing interests.











