Introduction
Schistosomiasis and soil-transmitted helminthiasis (STH) are among the top ten neglected tropical diseases (NTD) targeted for control/elimination by 2020 (WHO, 2012a ). Schistosomiasis is endemic in 78 countries, affecting almost 240 million people worldwide, and more than 700 million people are at risk (Bruun and Aagaard-Hansen, Reference Bruun and Aagaard-Hansen2008). On the other hand, more than a billion people or 24% of the world's population is infected with STH infections worldwide (Pullan et al. Reference Pullan, Smith, Jasrasaria and Brooker2014). Both diseases cause severe and subtle morbidity, including significant educational and nutritional effects in children (Mwinzi et al. Reference Mwinzi, Montgomery, Owaga, Mwanje, Muok, Ayisi, Laserson, Muchiri, Secor and Karanja2012). These parasitic diseases are prevalent in areas with favourable climatic and environmental conditions, unhygienic eating habits, poor water supply, poor sanitation and personal hygiene conditions which facilitate their transmission (Midzi et al. Reference Midzi, Mduluza, Chimbar, Tshuma, Charimar, Mhlanga, Manangazira, Munyat, Phiri, Mutambu, Midzi, Anastancia, Muranzi, Rusakaniko and Mutapi2014).
The World Health Organization's 2020 Roadmap on Neglected Tropical Diseases (WHO, 2012a ) and the London 2012 declaration to combat the NTD disease (WHO, 2012b ) are major driving forces that have led to the recent international commitment to escalate the control of NTDs. School-based de-worming programmes using anti-helminths therapy is the adopted strategy for prevention and control of schistosomiasis and STH infections in school children (WHO, 2006). However, identifying areas where both diseases occur singly or co-distributed will increase the efficiency of integrated control programming required in the implementation of the preventive chemotherapy treatment (Hanson et al. Reference Hanson, Weaver, Zoerhoff, Kabore, Linehan, Doherty, Engels, Savioli and Ottesen2012). Therefore, maps of the co-geospatial distribution of at-risk population and disease burden at national and district levels will ensure a cost-effective planning and delivery of control activities (Schur et al. Reference Schur, Hürlimann, Garba, Traore, Ndir, Ratard, Tchuem Tchuente, Kristensen, Utzinger and Vounatsou2011).
Although, high resolution spatial risk maps for mono distribution of schistosomiasis and STH have been provided at country level respectively (Ekpo et al. Reference Ekpo, Hürlimann, Schur, Oluwole, Abe, Mafe, Obiageli, Nebe, Isiyaku, Olamiju, Kadiri, Poopola, Braide, Saka, Mafiana, Kristensen, Utzinger and Vounatsou2013; Oluwole et al. Reference Oluwole, Ekpo, Karagiannis-Voules, Abe, Olamiju, Isiyaku, Okoronkwo, Saka, Nebe, Braide, Mafiana, Utzinger and Vounatsou2015), operationally, co-distribution maps of schistosomiasis and STH are required at district levels for integrated control of both diseases in the context of preventive chemotherapy. This study contributes to the design of such maps for Ogun State, Nigeria.
Materials and methods
Study area
Ogun State is one of the 36 states of the Federal Republic of Nigeria, located in the south-western part of the country within longitude 2°45′E and 3°55¹E and latitude 7°01′N and 7°18′N. The state is divided into 20 administrative areas known as the local government areas (LGAs) with an estimated population of 5 million inhabitants. There are 1314 public primary schools in Ogun State with an average of 100 pupils enrolled in each school. The health system is decentralized, comprising PHC (Primary Health Care) facilities in the community and General hospitals at District headquarters. Tertiary health institutions are in the State capital, Abeokuta and in some major cities in the State.
Selection of schools and survey for schistosomiasis and STH infection
The study was carried out between February and December 2013. Systematic point sampling method was used to select the schools. This was done by placing a 15 km by 15 km grid on the GIS map of Ogun State and geo-referencing the centres of the grids. The ArcGIS version 10.1. software was used to extract the geographical coordinates and with the aid of Google Map (https://www.google.com.ng/maps/), the closest primary school to the centroid of each grid were selected for the study. A total of 46 schools were selected, but only 42 schools participated in the study due to logistic and accessibility constraints.
Using the WHO guideline on the survey for helminthiasis in schools, a minimum of 50 and a maximum of 60 children submitted stool and urine samples per school (WHO, 2002). Selection of children in each school was carried out after stratification by class grade from Primary 1 to 6. A quota was then allocated to each class grade with proportional allocation according to the number of students in each grade. Finally, the participating children were randomly selected in each grade. In schools were pupils were not up to 50 students, the entire pupils were recruited into the study.
Each pupil submitted a single stool and urine sample which were taken to the laboratory and processed for microscopic examination. Ten ml of each urine sample were centrifuged for 5min at 5000 revolutions per minute (RPM), and the sediment examined for ova of Schistosoma haematobium under the microscope. One gram of each stool sample was processed using ethyl ether concentration method prepared in sodium–acetate–acetic acid–formalin solution (SAF) (Endriss et al. Reference Endriss, Elizabeth, Rohr, Rohr and Weiss2005). The processed stool samples were then examined for ova of STH and Schistosoma mansoni. The ova of Schistosomes and each of the STH parasite observed in samples were counted. The intensity of S. haematobium was expressed as the number of ova per 10 ml of urine while the intensity of each STH parasite and S. mansoni were expressed as number of ova per gram of stool sample. We used SAF solution method to process the stool sample because it helps in sample fixation, thereby preserving the morphology of STH species eggs in the stool sample for up to 40 days; an advantage that is not possible with the Kato Katz method (Glinz et al. Reference Glinz, Silué, Knopp, Lohourignon, Yao, Steinmann, Rinaldi, Cringoli, N'Goran and Utzinger2010). This was important as most of the study sites were quite far from the Laboratory and processing of stool samples on the day of collection was not feasible if the quality is to be assured. More importantly, this approach (formol-ether/acetyl-acetate concentration method prepared in SAF) has been proven to be more sensitive than the Kato Katz method (Glinz et al. Reference Glinz, Silué, Knopp, Lohourignon, Yao, Steinmann, Rinaldi, Cringoli, N'Goran and Utzinger2010) especially in areas where transmission is low (Knopp et al. Reference Knopp, Mgeni, Khamis, Steinmann, Stothard, Rollinson, Hanspeter Marti and Utzinger2008; Nikolay et al. Reference Nikolay, Brooker and Pullan2014). Besides, urine filtration kit and Kato-Katz kit recommended by WHO are not readily available in Nigeria market and can only be obtained by special request from WHO.
Geo-coordinates of study locations
The geo-coordinate location (latitude and longitude) of each school was verified using a handheld Global Positioning System (GPS) Garmin 12XL (Garmin Corp, USA). Coordinates were obtained and recorded in decimal degree unit.
Ethical approval and inform consent form
Ethical clearance for the study was given by the Federal Ministry of Health, Abuja and the Ogun State Ministry of Health, Abeokuta. Approval for the study was also obtained from the Ogun State Ministry of Education, Science and Technology with an approval reference number ESS.1/382/99. Permission and approval were also obtained from the Zonal Educational Officers, Educational Secretaries of each LGA and Head teachers of each school visited. Verbal and written informed consent was obtained from parents of the students of the selected schools after explaining the aim and objective of the study to them. Only students that agreed to participate in the study and whose parent consented by signing the informed consent forms or gave verbal consent were allowed to participate in the study. All children in the schools selected for the survey were treated by the Schistosomiasis and STH control unit of the Ogun State Ministry of Health.
Data analysis
Data were entered using Microsoft excel, cleaned for error and analysis were executed using STATA version 12 (Stata Corp LP, USA). Descriptive statistics were used to compute prevalence and mean intensity of Schistosoma and STH infections by sex, age group and LGA. The Chi-square test using the complex samples crosstabs procedure was used to explore the relationship between prevalence of infections and sex, age groups and LGAs. The Kruskal–Wallis test was used to compare the differences in geometrical mean intensity of infection.
Spatial analysis
Co-ordinate of schools sampled and their prevalence data for Schistosoma and STH infections collected were entered using Microsoft excel 2007 and then imported into ArcGIS 9.3. This was then used to display the prevalence and distribution of each Schistosoma and STH parasite infections in Ogun State, Nigeria.
Results
Demographics
A total of 2148 pupils submitted both urine and stool samples for examination, 1114 (51.9%) of them were males while 1034 (48.1%), were females. The age distribution of the pupils that participated in the study were 495 (23.0%), 867 (40.4%), 663 (30.9%) and 123 (5.7%) for age groups 5–7 years, 8–10 years, 11–13years and ≥14 years respectively. The standard deviation of the mean age of participants was 9.7 ± 2.0 with no significant difference between boys and girls
Prevalence of Schistosoma and STH infections
Of the 2148 pupils examined, the overall prevalence of Schistosoma infections (at least one of S. haematobium or S. mansoni) was 4.0% (95% CI = 3.2–4.9) with prevalence ranging from 1.7 to 32.3% in LGAs and 1.6 to 32.6% in schools. Prevalence of Schistosoma infection by sex and age group is shown in Table 1. Schistosoma infections were higher in females than in males, but it was not statistically significant (χ 2 = 0.8179, P > 0.05). We observed an increase in the number of infected students with Schistosoma infections as the age increases with the highest prevalence observed in age group ≥14 years, but these differences were not statistically significant (χ 2 = 3.2486, P > 0.05). Aggregated prevalence of Schistosoma parasites per LGAs in Ogun State (Table 1) shows that prevalence of Schistosoma infections was highest in Ewekoro LGA (32.3%, 95% CI = 22.9–42.8).
Table 1. Prevalence of schistosomiasis and soil-transmitted helminth species in Ogun State in 2013
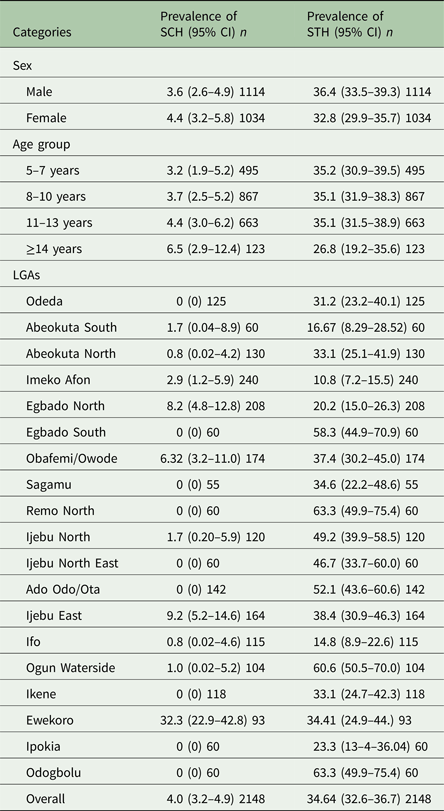
*n, Number examined.
On the other hand, the overall prevalence of STH infections (at least one infection of Ascaris lumbricoides or hookworm or Trichuris trichiura) was 34.6% (95% CI = 32.6–36.7) with prevalence ranging from 10.8 to 63.3% in LGAs and 3.3 to 72.01% in schools. Prevalence of STH infections by sex and age group is shown in Table 2. STH infections were higher in male school children than in females, but the difference was not statistically significant (χ 2 = 3.0191, P > 0.05). STH infections among the age group seem to decrease with increasing age, although this was not statistically significant (χ 2 = 3.5145, P > 0.05). Aggregated prevalence of STH parasites per LGAs in Ogun State shows that the highest prevalence of STH infections was observed in Odogbolu and Remo North LGAs both having (63.3%, 95% CI = 49.9–75.4) while the least was in Imeko Afon LGA with (10.8%, 95% CI = 7.2–15.5) (Table 1)
Table 2. Prevalence of Schistosoma and STH parasite in Ogun State in 2013
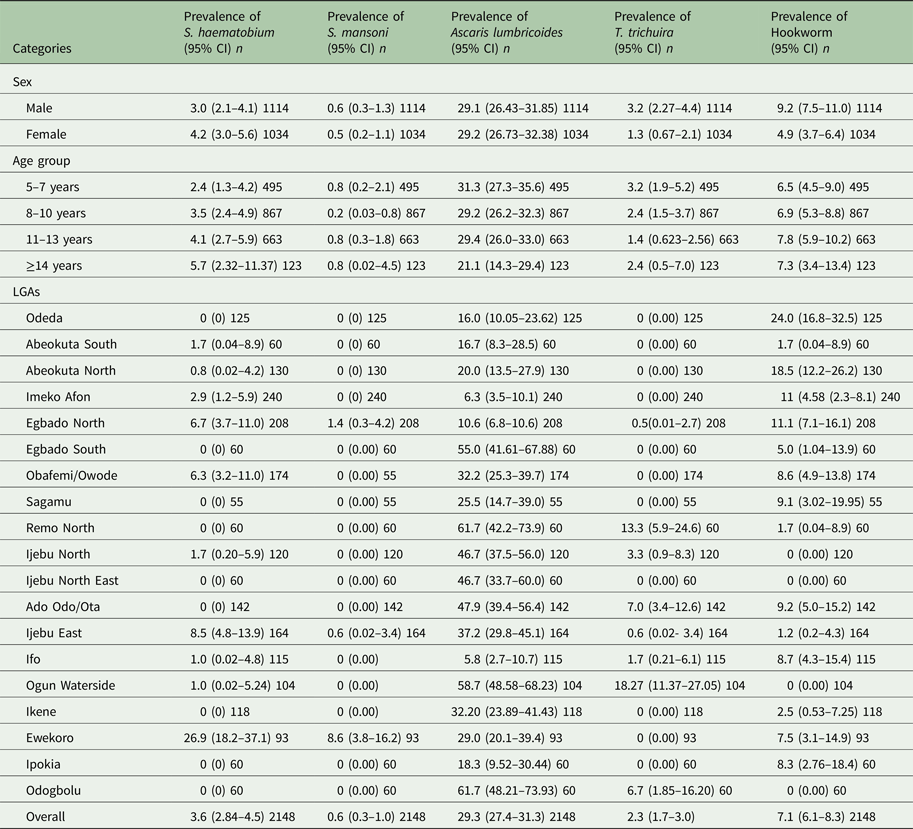
*n, Number examined.
Prevalence and intensity of Schistosoma and STH parasites in Ogun State
Schistosoma haematobium infection was the most common (3.6%, 95% CI = 2.8–4.5) Schistosoma spp among school children in Ogun state. Prevalence of S. mansoni was low (0.6%) (Table 2). There was no significant difference (P > 0.05) in infection with S. haematobium and S. mansoni in both sex and age group. The prevalence of each species of Schistosoma parasite per LGA is shown in Table 2. Among the STH parasites, A. lumbricoides was the most common (29.3%, 95% CI = 27.4–31.3), while T. trichiura has the least prevalence of (2.3%, 95% CI = 1.7–3.0). Infection was significantly higher in males than in females for both hookworm (χ 2 = 14.4621, P < 0.05) and T. trichiura infection (χ 2 = 9.3773, P < 0.05) but there was no significant difference (χ 2 = 0.0441) in the prevalence of A. lumbricoides infection between sexes. Prevalence of STH infection was not statistically different (P > 0.05) between age groups. The prevalence of each parasite species per LGA is also shown in Table 2.
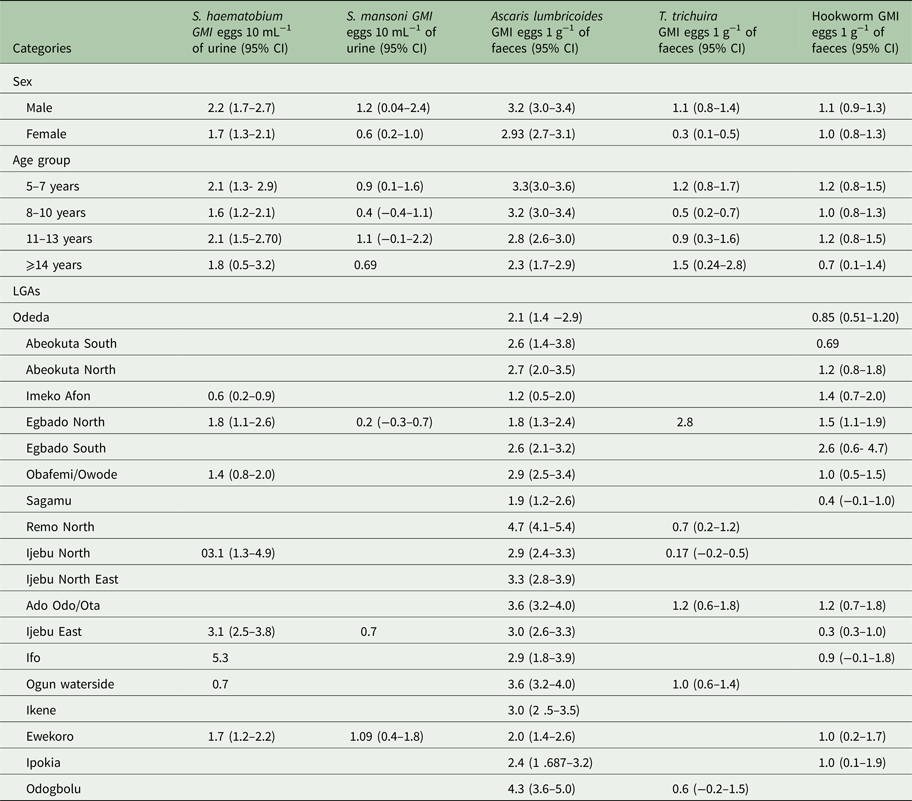
The Arithmetic and Geometric mean intensity of infections for the schistosomes and STH parasites by sex, age and LGA is provided in Tables 3 and 4, respectively. The intensity of infection was observed to be higher in males than in females for all parasites (Tables 3 and 4), but only in T. trichiura was there a significant difference in infection intensity (χ 2 = 6.490, P < 0.05). Between the age groups, the intensity of infection was highest in 5–7 years than other age groups. The intensity of infection significantly reduces (χ 2 = 12.953, P < 0.05) with an increase in age.
Table 3. Arithmetic mean intensity (AMI) of Schistosoma and STH parasites in Ogun State in 2013
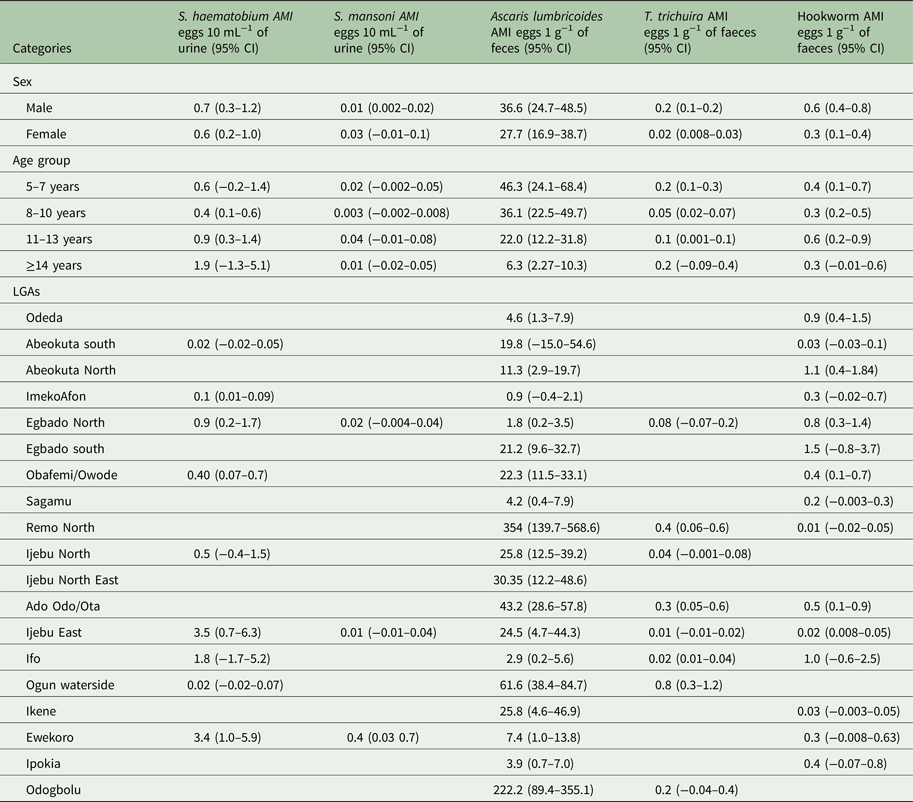
Table 4. Geometric mean intensity (GMI) of Schistosoma and STH parasites in Ogun State in 2013
Spatial co-distribution of schistosomiasis and STH infections in Ogun State
Schstosoma haematobium was the most geographically spread infection, observed in 15 (35.7%) locations in 10 LGAs, whereas S. mansoni was seen in four (9.5%) locations in three LGAs. Co-occurrence of the two Schistosoma species was observed only in four locations in three LGAs (Fig. 1). Prevalence map for Schistosoma parasites in Ogun State based on WHO prevalence threshold for Preventive Chemotherapy is shown in Figs 2 and 3.

Fig. 1. Spatial distribution of the two Schistosoma parasites in Ogun State.

Fig. 2. Observed prevalence of Schistosoma haematobium in Ogun State.

Fig. 3. Observed prevalence of Schistosoma mansoni in Ogun State.
Ascaris lumbricoides infection was the most geographically spread of the STH parasites, observed in 40 (95.2%) locations in 19 LGAs. Hookworm was seen in 28 (66.7%) locations in 15 LGAs and T. trichiura was found in 10 (23.8%) locations in eight LGAs (Fig. 4) Co-distribution of hookworm and A. lumbricoides infections were observed in 21 (50%) locations in 13 LGAs, A. lumbricoides and T. trichiura co-existed in four locations in three LGAs. Co-occurrence of all three STH species was observed in six (14.3%) locations in five LGAs. Figures 5–7 shows the prevalence of each species of STH parasite in Ogun State using WHO prevalence threshold.

Fig. 4. Spatial distribution of STH parasite in Ogun State.

Fig. 5. Observed prevalence of Ascaris lumbricoides in Ogun State.

Fig. 6. Observed prevalence of hookworm in Ogun State.

Fig. 7. Observed prevalence of Trichuris trichiura in Ogun State.
Spatial co-distribution of Schistosoma and STH infections in Ogun State is shown in Fig. 8. The two infections were observed in 15 (35.7%) of the 42 sampled schools. Aggregating by LGAs, 10 LGAs were identified to be endemic for both Schistosomiasis and STH (Table 2). These include Abeokuta South, Abeokuta North, Imeko Afon, Yewa North, Obafemi Owode, Ijebu North, Ijebu East, Ifo, Ogun waterside and Ewekoro.

Fig. 8. Map of co-distribution of STH and Schistosoma infections in Ogun State.
Discussion
Map of co-distribution of schistosomiasis and STH infections and the number of people infected or at risk are essential for the implementation of cost-effectively integrated control programme (Magalhães et al. Reference Magalhães, Archie, Clements, Patil, Gething and Brooker2011; Schur et al. Reference Schur, Hürlimann, Garba, Traore, Ndir, Ratard, Tchuem Tchuente, Kristensen, Utzinger and Vounatsou2011; Chammartin et al. Reference Chammartin, Guimarães, Scholte, Bavia, Utzinger and Vounatsou2014). Here, we present for the first time the co-spatial distribution maps of schistosomiasis and STHs at LGA (implementation unit) for Ogun State to support integrated control like other studies in Kenya, Uganda, Cameroon and Zimbabwe (Handzel et al. Reference Handzel, Karanja, Addiss, Hightower, Rosen, Colley, Andove, Slutsker and Secor2003; Kabatereine et al. Reference Kabatereine, Standley, Sousa-Figueiredo, Fiona, Fleming, Stothard, Talisuna and Alan Fenwick2012; Tchuem Tchuente et al. Reference Tchuem Tchuente, Noumedem, Ngassam, Kenfack, Gipwe, Dankoni, Tarini and Zhang2013).
The prevalence and intensities of both diseases point out the fact that Schistosomiasis/STH infection remains a major public health problem in Ogun State (Ekpo et al. Reference Ekpo, Olabinke, Oluwole, Adeluola and Gbemisola2012, Reference Ekpo, Hürlimann, Schur, Oluwole, Abe, Mafe, Obiageli, Nebe, Isiyaku, Olamiju, Kadiri, Poopola, Braide, Saka, Mafiana, Kristensen, Utzinger and Vounatsou2013; Oluwole et al. Reference Oluwole, Ekpo, Karagiannis-Voules, Abe, Olamiju, Isiyaku, Okoronkwo, Saka, Nebe, Braide, Mafiana, Utzinger and Vounatsou2015). Schistosomiasis is a focal disease, prevalent in areas where there are high human–water contact practices with water bodies such as dams, rivers or irrigation canals, poor sanitation and poor access to potable water (Ekpo et al. Reference Ekpo, Hürlimann, Schur, Oluwole, Abe, Mafe, Obiageli, Nebe, Isiyaku, Olamiju, Kadiri, Poopola, Braide, Saka, Mafiana, Kristensen, Utzinger and Vounatsou2013)
Schistosoma haematobium was the most widely distributed and most prevalent among the two species of Schistosoma found in this study. The finding also corroborates the recent mapping of schistosomiasis in Nigeria using compiled survey data from 1950 to 2010 which revealed that S. haematobium is the most spatially distributed of three Schistosoma species in Nigeria (Ekpo et al. Reference Ekpo, Hürlimann, Schur, Oluwole, Abe, Mafe, Obiageli, Nebe, Isiyaku, Olamiju, Kadiri, Poopola, Braide, Saka, Mafiana, Kristensen, Utzinger and Vounatsou2013). The reason for wider distribution of S. haematobium than S. mansoni may be due to the ecology and distribution of their snail intermediate host (Madsen et al. Reference Madsen, Coulibaly and Furu1987; Madsen, Reference Madsen1992) which indirectly influenced the distribution of the parasites (Opisa et al. Reference Opisa, Odiere, Jura, Karanja and Mwinzi2011).
Schistosoma mansoni was prevalent in four locations. These locations are not far from perennial water bodies which permit the sustained transmission of S. mansoni in these places and are similar to other observations of S. mansoni infection among people living close to a permanent water body (Lwambo et al. Reference Lwambo, Siza, Brooker, Bundy and Guyatt1999; Handzel et al. Reference Handzel, Karanja, Addiss, Hightower, Rosen, Colley, Andove, Slutsker and Secor2003; Woodhall et al. Reference Woodhall, Wiegand and Wellman2013; Nagi et al. Reference Nagi, Chadeka, Sunahara, Mutungi, Dan Justin, Kaneko, Ichinose, Matsumoto, Njenga, Hashizume, Shimada and Hamano2014). This may be due to inability of S. mansoni to develop in the snail intermediate host at high water temperature (>15 °C) (Pflüger, Reference Pflüger1980) that may occur in seasonal water bodies.
Our findings show that prevalence of schistosomiasis increase as the age increases from age group 5–7 peaking at age group >14. This observation corroborates earlier findings which show that prevalence of shistosomiasis is highest among age group 10–15 years (Agnew-Blais et al. Reference Agnew-Blais, Carnevale, Gropper, Shilika, Bail and Ngoma2010; Senghor et al. Reference Senghor, Diallo, Sylla, Doucouré, Ndiath, Gaaye, Djuikwo-Teukeng, Cheikh and Sokhna2014). This age group is known to be very active and are involved in recreational activities like swimming in schisto-infested water which made them at higher risk than the lower age group.
The prevalence of schistosomiasis in Ewekoro LGA is higher than what is observed in other LGAs. This finding may be a reflection of their high level of exposure to infested water than other study sites in other LGAs. As shown in the map the selected study sites in Ewekoro LGA are very close to the river system. In addition, personal interaction with the school teachers and parents of the pupils during data collection shows that pupils from the selected schools come from a community where they do not have access to pipe borne water but depend solely on the river for their domestic activities. They also confirm that most of the children do engage in recreational activities especially swimming in the river
Ascaris lumbricoides was the most spatially distributed and prevalent among the STH, followed by hookworm and T. trichiura. This pattern of infection has been observed in earlier studies on STH infections in the south-western part of the country (Ibidapo and Okwa, Reference Ibidapo and Okwa2008; Ekpo et al. Reference Ekpo, Olabinke, Oluwole, Adeluola and Gbemisola2012). Most studies on STH infections in endemic countries, both in sub-Sahara Africa and other continents have identified A. lumbricoides as the most common of the STH infections in the world (Pullan et al. Reference Pullan, Gething, Smith, Mwandawiro, Sturrock, Gitong, Hay and Brooker2011; Chammartin et al. Reference Chammartin, Scholte, Malone, Bavia, Nieto, Utzinger and Vounatsou2013; Lai et al. Reference Lai, Zhou, Utzinger and Vounatsou2013; Schule et al. Reference Schule, Clowes, Kroidl, Kowuor, Nsojo, Mangu, Riess, Geldmacher, Laubender, Mhina, Maboko, Löscher, Hoelscher and Saathoff2014). Hence, this observation corroborates findings from other researches that incriminate A. lumbricoides as the main parasite in most STH infections. This is due to the ability of the ova of A. lumbricoides to withstand harsh environmental condition in the soil until a favourable condition for its embryonation is available
Ascaris lumbricoides was found in 19 LGAs, with increasing prevalence, from the western to the eastern part of the state. Hence, higher prevalence of A. lumbricoides is observed in Ogun waterside, Ijebu East, Ijebu North, Remo North, Odogbolu and Ijebu North East LGAs. The current study shows that hookworm is widely distributed in Ogun State, as we have earlier reported (Oluwole et al. Reference Oluwole, Ekpo, Karagiannis-Voules, Abe, Olamiju, Isiyaku, Okoronkwo, Saka, Nebe, Braide, Mafiana, Utzinger and Vounatsou2015). The distribution of hookworm infection in Ogun State is due to its geographical location in the equatorial zone. This zone is known to be suitable for the development of hookworm larvae (Lai et al. Reference Lai, Zhou, Utzinger and Vounatsou2013). However, the low prevalence of hookworm observed in Ogun Waterside, Ijebu East, Ijebu North, Remo North, Odogbolu and Ijebu North East LGAs of the state, may reflect the unfavourable environmental conditions for the survival of eggs and larvae of hookworm (Brooker et al. Reference Brooker, Clements and Bundy2006) rather than improvement in the socioeconomic status of the people living in this area, when compared with Odeda, Abeokuta North, Ewekoro, Egbado North, Egbado south, Ifo and Ipokia LGAs with higher prevalence of hookworm. The high prevalence of STH (>20% WHO prevalence threshold) observed in the study, established the prerequisite for mass drug administration (MDA) for STH in Ogun State. However, studies have shown that there is a high level of re-infection among school-age children within 6 months after MDA (Ekpo et al. Reference Ekpo, Olabinke, Oluwole, Adeluola and Gbemisola2012; Jia et al. Reference Jia, Melville, Utzinger, King and Zhou2012; Yap et al. Reference Yap, Du, Wu, Jiang, Chen, Zhou, Hattendorf, Utzinger and Steinmann2013). Hence, there is need to compliment deworming programme with other interventions such as health education on good hygienic practice and environmental sanitation. This will help reduce transmission of the disease (Campbell et al. Reference Campbell, Savage, Gray, Atkinson, Magalhães, Nery, McCarthy, Velleman, Wicken, Traub, Gail, Williams, Andrews and Clements2014; Echazú et al. Reference Echazú, Bonanno, Juarez, Cajal, Heredia, Caropres, Camino, Caro, Vargas, Paredes and Krolewieck2015)
Limitation of the study
In the selection of study sites, potentially high-risk areas for schistosomiasis disease (areas close to water bodies) was not given special consideration since the focus of the study was to model the co-distribution of schistosomiasis and STH across Ogun State using geostatistical methods. Hence, the prevalence of schistosomiasis in each LGA may have been underestimated.
Concluding remarks
This study provides information on the distribution and prevalence of Schistosoma and STH infections in Ogun State. Based on our results, integrated control of both diseases will be required in 10 LGAs of the state while STH control only is required in 10 LGAs. These evidence-based data may be useful for donors to allocate resources for supporting deworming activities in the State. The prevalence and spatial risk map of the two diseases are useful for state program managers as decision-support tools to help facilitate integrated control of the two diseases.
Acknowledgments
We are grateful to the children, parents, and teachers of the schools selected for the study for their co-operation and participation. Our appreciation also goes to the primary health care centres that gave us space for laboratory analysis of urine and fecal samples during the field data collection.
Financial support
Funding for the study was provided by the European Foundation Initiative for African Research into Neglected Tropical Diseases (EFINTD), Senior Postdoctoral fellowship grant (AZ:86 527) to UFE.
Conflict of interest
None.













