INTRODUCTION
Neospora caninum is an obligate intracellular cyst-forming coccidian parasite with a worldwide distribution. Neospora caninum is a major cause of abortion in cattle and causes severe neuromuscular disease in dogs (Dubey, Reference Dubey2003; McInnes et al. Reference McInnes, Irwin, Palmer and Ryan2006). The disease is responsible for considerable economic losses to the beef and dairy industries, which are mainly linked to the lower reproductive performance of affected cattle (Thurmond and Hietala, Reference Thurmond and Hietala1997; Dubey, Reference Dubey2003), but also to reduced milk production, premature culling and decreased weight gain (Thurmond and Hietala, Reference Thurmond and Hietala1996; Barling et al. Reference Barling, McNeill, Thompson, Paschal, McCollum, Craig and Adams2000; Hernandez et al. Reference Hernandez, Risco and Donovan2001). Prevalence rates determined in seroepidemiological studies carried out at farm level may reach 100% (Dubey, Reference Dubey1999).
Neospora caninum has a heteroxenous prey–predator life cycle. The definitive carnivorous host (DH) sheds unsporulated oocysts into the environment following predation of intermediate hosts (IH) containing N. caninum tissue cysts and IH become infected after ingestion of sporulated oocysts in water or food, or in the case of carnivores and omnivores, also through the consumption of infected tissues. In addition, N. caninum can be transmitted transplacentally in several host species (Dubey, Reference Dubey2003). Up to now, the only confirmed DH are canids from the genus Canis, namely dogs (Canis familiaris) (McAllister et al. Reference McAllister, Dubey, Lindsay, Jolley, Wills and McGuire1998), coyotes (C. latrans) (Gondim et al. Reference Gondim, McAllister, Pitt and Zemlicka2004b ), dingoes (C. lupus dingo) (King et al. Reference King, Slapeta, Jenkins, Al-Qassab, Ellis and Windsor2010) and grey wolves (C. lupus lupus) (Dubey et al. Reference Dubey, Jenkins, Ferreira, Choudhary, Verma and Kwok2014).
A wide range of domestic and wildlife species were shown to be susceptible to N. caninum infection, but their role in domestic and sylvatic transmission cycles still needs to be elucidated (Almería, Reference Almería2013). Little is known about clinical neosporosis in wildlife. Disease in non-domestic carnivores was described only in a European pine marten and in blue and red foxes, in young animals, with similar neurological and dermatological presentations as in domestic dogs (Donahoe et al. Reference Donahoe, Lindsay, Krockenberger, Phalen and Šlapeta2015). N eospora caninum infection in artiodactyls is associated with stillbirths and systemic disease in very young animals (Donahoe et al. Reference Donahoe, Lindsay, Krockenberger, Phalen and Šlapeta2015).
Dogs become infected when fed with unfrozen, raw or undercooked beef. Other possible infection sources include feeding on abortion tissues from bovines, predation of small wildlife mammals and feeding on animal carcasses or offal from game animals left in the field (Gondim et al. Reference Gondim, McAllister, Mateus-Pinilla, Pitt, Mech and Nelson2004a ). Though transplacental infection is considered the main mechanism for parasite transmission in cattle, the presence of dogs as a source of environmental contamination with oocysts, was found to be crucial to maintain the life cycle (Dubey, Reference Dubey2003; Dubey et al. Reference Dubey, Schares and Ortega-Mora2007). Dogs should therefore be regarded as a potential risk factor, not only for cattle but also for natural populations sharing the same habitat. In Portugal, this is especially important in regards to nature conservation areas located in the region Alentejo, which has the largest inventory of grass-fed, extensively raised cattle in the country. Neospora caninum was identified for the first time as a cause of abortion in Portuguese cattle farms in 2001 (Thompson et al. Reference Thompson, Canada, do Carmo Topa, Silva, Vaz and Rocha2001) and isolated from an aborted fetus in 2002 (Canada et al. Reference Canada, Meireles, Rocha, Sousa, Thompson, Dubey, Romand, Thulliez and Correia da Costa2002). Subsequent studies in intensive dairy farms, showed a prevalence of 28% in randomly sampled farms and 46% in farms with abortion problems (Canada et al. Reference Canada, Carvalheira, Meireles, Correia da Costa and Rocha2004).
The serological screening of N. caninum in dogs is an indirect means to evaluate environmental contamination, since seropositive animals probably already shed oocysts. The prevalence of antibodies to N. caninum in wildlife will mirror the impact of environmental contamination and reflect the susceptibility of native IH species to infection, therefore providing further evidence on the circulation of N. caninum between domestic and wild ecosystems (Almería, Reference Almería2013).
This study aimed to evaluate the exposure to N. caninum in dogs and wild animals in a wildlife conservation area in the southeast of Portugal.
MATERIALS AND METHODS
Sample characterization
Serological tests were performed on serum samples from 286 dogs and serum (n = 126) and lung extracts (n = 51) from 177 wild animals. Wildlife species included 32 European rabbits (Oryctolagus cuniculus), 34 Egyptian mongoose (Herpestes ichneumon), 26 wild boars (Sus scrofa), 25 foxes (Vulpes vulpes), 17 common genets (Genetta genetta), 14 red deer (Cervus elaphus), six wildcats (Felis silvestris), six stone martens (Martes foina), six wildcats (F. silvestris), six European badgers (Meles meles), two European otters (Lutra lutra), six European polecats (Mustela putorius), one garden dormouse (Eliomys quercinus), one common rat (Rattus norvegicus), three western Mediterranean mice (Mus spretus) and one wood mouse (Apodemus sylvaticus). Sampling was performed between 2010 and 2013 in the frame of the project LIFE Habitat Lince Abutre (LIFE08 NAT/P/000227). Samples were obtained in a wildlife conservation area in the southeast of Portugal involving a total of 24 freguesias (the smallest administrative unit in Portugal) belonging to 13 municipalities in the NUTS2 regions Alentejo and Algarve (Fig. 1). The sampling area is largely characterized by a peneplain landscape, generally not exceeding 200 m above sea level. The climate is typically Mediterranean Csa (Köppen climate classification), with warm to hot, dry summers and mild to cool, wet winters. Average monthly temperatures are above 22·0 °C during the warmest month and range between 18 and −3 °C in the coldest month (Kottek et al. Reference Kottek, Grieser, Beck, Rudolf and Rubel2006), while the average precipitation is <800 mm per year (www.ipma.pt). Data on sex, age and sampling conditions of wildlife species (captured/hunted/road kill animals) were recorded whenever possible. Samples were sent to the National Institute for Veterinary and Agrarian Research (INIAV) for the diagnosis of several diseases that may affect the Iberian lynx and black vulture populations in this particular area. Samples and animal data were kindly provided for the purposes of this study by the national veterinary authority Direcção-Geral de Alimentação e Veterinária (DGAV).
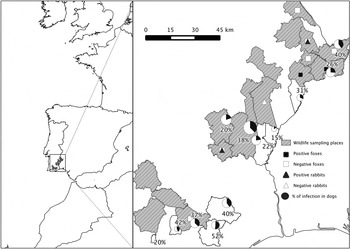
Fig. 1. Survey area: wildlife sampling places, sample prevalence of N eospora caninum in dogs and location of positive and negative foxes and rabbits. © EuroGeographics for the administrative boundaries.
Neospora caninum production and purification
Neospora caninum tachyzoites used to prepare the antigen suspension for the N. caninum modified agglutination test (N-MAT) and to coat Indirect Fluorescent Antibody Test (IFAT) slides were propagated by continuous passage in Vero cell (African Green Monkey kidney epithelial cells) cultures using Dulbecco's Minimal Essential Medium (DMEM) supplemented with 100 UI mL−1 penicillin, 100 µg mL−1 streptomycin, 2 mm l-glutamine, 20 mm HEPES and fetal calf serum (10% to initiate cultures and 2% for maintenance) at 37 °C in closed 75 cm2 cell culture flasks. Tachyzoites were harvested after 3–4 days of infection. At this stage, most of the cell layer was confluent and N. caninum tachyzoites intracellular. The cell monolayer was scraped from the cell culture flask into the supernatant and the resulting suspension was passed through a 21 G syringe to release tachyzoites from cells, centrifuged at 770 g for 15 min, at 4 °C, and the pellet was resuspended in phosphate-buffered saline (PBS). Tachyzoites were separated from host cell debris, using columns made of Whatman CF 11 cellulose powder, as described by Dempster (Reference Dempster1984). The purified parasites were used immediately for N-MAT antigen or IFAT slide preparation.
N-MAT antigen and IFAT slide preparation
The N-MAT antigen was prepared as described by Packham et al. (Reference Packham, Sverlow, Conrad, Loomis, Rowe, Anderson, Marsh, Cray and Barr1998) with minor changes (Waap et al. Reference Waap, Cardoso, Marcelino, Malta, Cortes and Leitão2011). Briefly, cell-free parasites were washed once in 20 mL PBS and the pellet was resuspended in 2–3 mL of 37% formaldehyde diluted in PBS to 6% formaldehyde. Parasites were fixed overnight at 4 °C and then washed three times in 20 mL PBS to remove formaldehyde. The fixed tachyzoites were counted on a Neubauer chamber and resuspended to a final concentration of 35–45 × 106 tachyzoites mL−1 in filtered (0·2 µ m pore size) alkaline buffer (pH 8·7), containing 7·02 g NaCl, 3·09 g H3BO3, 24 mL 1 N NaOH, 50 mg eosin Y, 1·0 g sodium azide and 4 mg mL−1 bovine albumin (fraction V) per litre.
IFAT slide preparation was based on the procedure proposed by Shkap et al. (Reference Shkap, Reske, Pipano, Fish and Baszler2002). Briefly, purified tachyzoites were washed and suspended in a 4% formaldehyde solution in PBS, on ice, during 30 min. The fixed tachyzoites were washed three times to remove formaldehyde and the pellet was suspended in 1 mL PBS. Parasites were counted and diluted in PBS to a final concentration of 2 × 106 tachyzoites mL−1, distributed in 6 µL drops on slides, dried at 37 °C and fixed in cold acetone (−20 °C) for 10 min.
Screening of anti-N. caninum-specific IgG antibodies in dogs
Serum samples from dogs were screened for specific anti-N. caninum antibodies by the IFAT at three dilutions (1:50, 1:100 and 1:200) using rabbit antidog IgG-FITC secondary antibody (Sigma Aldrich) diluted at 1:750 in 0·01% Evans blue solution. Positive and negative IFAT controls (canine origin, VMRD, Inc) were included in each testing round. Bovine serum albumin was added to the serum and conjugate diluting buffers in order to reduce non-specific binding of antibodies. Slides were mounted with 50% glycerol in PBS (pH 8·5) and viewed under a Zeiss fluorescence microscope with a 40× objective. Complete peripheral fluorescence was regarded as a positive reaction, while partial, apical or absent fluorescence was considered a negative reaction.
Analysis of anti-N. caninum-specific IgG antibodies in wildlife species
Lung tissue samples were collected during necropsy and processed as described by Ferroglio et al. (Reference Ferroglio, Rossi and Gennero2000) to prepare lung extracts for serological analysis. Lung extracts were shown to be suitable for the screening of specific antibodies against several pathogens in wildlife, including Encephalitozoon (Waller et al. Reference Waller, Lyngset, Elvander and Morein1980), Francisella tularensis (Mörner et al. Reference Mörner, Sandström and Mattsson1988), canine parvovirus (Duarte et al. Reference Duarte, Fernandes, Santos and Tavares2012) and Toxoplasma gondii (Jakubek et al. Reference Jakubek, Mattsson, Mörner, Mattsson and Gavier-Widén2012; Waap et al. Reference Waap, Nunes, Vaz and Leitão2016).
Serum samples and lung extracts were screened for IgG antibodies to N. caninum at 1:50, 1:150 and 1:450 dilutions using N-MAT according to previously established procedures (Packham et al. Reference Packham, Sverlow, Conrad, Loomis, Rowe, Anderson, Marsh, Cray and Barr1998). Each testing round included positive and negative control sera from cattle and dogs tested by IFAT and ELISA, as well as an antigen control (PBS instead of serum). Agglutination of tachyzoites as a diffuse opacity covering at least half of the well was considered a positive reaction and sedimentation as a button or ring a negative reaction. Positive and doubtful results, characterized by an indistinct agglutination pattern, were tested by the IFAT at 1:50, 1:100 and 1:200 dilutions, in order to confirm the serological status of animals. Sera from foxes were assayed with rabbit antidog IgG-FITC secondary antibody (Sigma Aldrich) and rabbits were tested with donkey antirabbit IgG secondary antibody, Alexa Fluor 488 (Thermo Fisher Scientific). Proteins A and G can bind with strong affinity to immunoglobulin of several species and are commonly applied in ELISA tests for multi-species diagnosis (Zhang et al. Reference Zhang, Wang, Fang, Nie, Feng, Zhou and Zhao2010; Schaefer et al. Reference Schaefer, White, Schaaf, Mohammed and Wade2011) Therefore, confirmation of positive and doubtful results in the other wildlife species was attempted with Alexa Fluor 488 proteins A and G conjugates (Thermo Fisher Scientific).
Data analysis
The 95% confidence intervals (CI) for prevalence data were calculated by Wilson's score method assuming a perfect test using EpiTools Epidemiological Calculator (Sergeant, Reference Sergeant2016). Geographical distribution maps were constructed using Quantum Geographic Information System (QGIS) software 2.0.1.
RESULTS
The overall seroprevalence in dogs at the established cut-off of 1:50 was 32·5% (CI 27·3–38·1). Considering the three dilutions used for titration of sera, 22 dogs were positive at the cut-off dilution 1:50, 17 dogs had an endpoint titre at 1:100 dilution and 54 had endpoint titres ≥1:200 (Table 1). Overall, reading of results at 1:50 dilution was difficult, due to considerable non-specific binding of antibodies, visible as a strong apical fluorescence, which frequently extended to more than half of the tachyzoite membranes.
Table 1. Number (n) of lung extracts (LE) and sera from dogs and wildlife animals tested, percentage of infection in the total sample (%) and NAT and IFAT titres in confirmed results

In wildlife animals tested by the N-MAT, a distinct agglutination pattern indicating the presence of antibodies to N. caninum was observed in two foxes and 15 rabbits, while six rabbits, two foxes, two wild boars and two Egyptian mongoose were considered doubtful. Positive reactivity was only detected in sera; all lung extracts tested negative. Two foxes and one rabbit had an N-MAT endpoint titre at 1:50 dilution, and 14 rabbits had endpoint titres ≥1:450 (Table 1). Confirmation of N-MAT positive and doubtful results by IFAT showed the presence of antibodies to N. caninum in both positive foxes and eight seropositive rabbits, while one of the doubtful fox sera and one of the doubtful rabbit sera were also found to be positive. One fox and one rabbit had an IFAT titre of 1:50, two rabbits had a titre of 1:100 and two foxes and five rabbits had titres ≥200 (Table 1). Seropositive foxes came from two out of eight freguesias sampled, and seropositive rabbits came from two out of three freguesias (Fig. 1). At the positive sampling sites, antibodies to N. caninum were found in 1 of 12 and 2 of 6 foxes and 3 of 12 and 5 of 17 rabbits. Positive foxes included one adult female and one juvenile and one subadult male. No data on sex and age were available for rabbits. Only animals with a positive reaction in both assays were considered seropositive; therefore, the percentage of infected individuals in the sample was considered to be 12% (CI 4·2–30%) in foxes and 25% (CI 13·3–42·1%) in rabbits. Evaluation of the protein A/G system for confirmation of doubtful results in the two Egyptian mongoose and two wild boar sera included titration of Alexa Fluor 488 proteins A and G conjugates (Thermo Fisher Scientific) and titration of control sera. The binding of the conjugate was assessed with N. caninum slides and positive and negative dog sera, and with T. gondii slides and positive and negative fox, pig, wild boar and Egyptian mongoose sera. However, except for dogs and foxes, the fluorescent signal was too weak to establish the seropositivity of sera (data not shown). Based on these results, the protein A/G system was found to be unsuitable to confirm infection in Egyptian mongoose and wild boars and this species was therefore considered to be negative.
DISCUSSION
The aim of this study was to determine the presence of specific antibodies anti-N. caninum in samples from dogs and wild animals in a nature conservation area in the southeast of Portugal.
Several serological techniques have been employed to screen for anti-N. caninum antibodies in different animal species, including the IFAT, usually using the cut-off of 1:50 in dogs (Björkman and Uggla, Reference Björkman and Uggla1999; Silva et al. Reference Silva, Lobato, Mineo and Mineo2007; Uzeda et al. Reference Uzeda, Costa, Santos, Pinheiro, De Almeida, McAllister and Gondim2007; Yakhchali et al. Reference Yakhchali, Javadi and Morshedi2010; Robbe et al. Reference Robbe, Passarelli, Gloria, Di Cesare, Capelli, Iorio and Traversa2016; Wang et al. Reference Wang, Yao, Zhang, Wang, Ma, Liu, Zheng, Zhang, Liu and Zhang2016), the N-MAT and various ELISAs. The competitive cELISA and N-MAT are the most commonly used tests in wildlife, because these techniques do not require the use of species-specific secondary antibodies (Almería, Reference Almería2013). Though the assay principle of the cELISA makes this test attractive to be used in all species, validation data are absent and several authors have stated a lack of test agreement with IFAT results (Capelli et al. Reference Capelli, Natale, Nardelli, Frangipane di Regalbono and Pietrobelli2006; Sobrino et al. Reference Sobrino, Dubey, Pabón, Linarez, Kwok, Millán, Arnal, Luco, López-Gatius, Thulliez, Gortázar and Almería2008).
In this study, we opted to use the IFAT for the serological survey in dogs. The IFAT has been widely employed for N. caninum serology in dogs and is generally accepted as the reference test (Björkman and Uggla, Reference Björkman and Uggla1999; Capelli et al. Reference Capelli, Natale, Nardelli, Frangipane di Regalbono and Pietrobelli2006; Silva et al. Reference Silva, Lobato, Mineo and Mineo2007). Several authors have also claimed that the IFAT is more specific than the ELISA, because the use of crude tachyzoite antigen as coating antigen in soluble extract-based ELISAs may be a cause of cross-reactivity with T. gondii and other related apicomplexan parasites (Björkman and Hemphill, Reference Björkman and Hemphill1998; Silva et al. Reference Silva, Lobato, Mineo and Mineo2007). Though many authors consider the dilution 1:50 the most appropriate cut-off for seropositivity in dogs (Björkman and Uggla, Reference Björkman and Uggla1999; Silva et al. Reference Silva, Lobato, Mineo and Mineo2007; Uzeda et al. Reference Uzeda, Costa, Santos, Pinheiro, De Almeida, McAllister and Gondim2007; Yakhchali et al. Reference Yakhchali, Javadi and Morshedi2010; Robbe et al. Reference Robbe, Passarelli, Gloria, Di Cesare, Capelli, Iorio and Traversa2016; Wang et al. Reference Wang, Yao, Zhang, Wang, Ma, Liu, Zheng, Zhang, Liu and Zhang2016), in this study, interpretation of results was difficult, with a high percentage of sera producing strong apical reactions, in many instances with incomplete peripheral fluorescence of fixed tachyzoites. Others, when using the IFAT for the serological screening of N. caninum in different species made similar observations (Paré et al. Reference Paré, Hietala and Thurmond1995; Mineo et al. Reference Mineo, Silva, Costa, von Ancken, Kasper, Souza, Cabral, Costa and Mineo2001; Galvão et al. Reference Galvão, Rezende-Gondim, Chaves, Schares, Ribas and Gondim2015). Apical, unspecific fluorescence may arise from serological cross-reactivity with other apicomplexan species (Paré et al. Reference Paré, Hietala and Thurmond1995), due to the presence of conserved apical complex antigens (Conrad et al. Reference Conrad, Sverlow, Anderson, Rowe, BonDurant, Tuter, Breitmeyer, Palmer, Thurmond, Ardans, Dubey, Duhamel and Barr1993). Nevertheless, according to other authors, the IFAT for N. caninum shows little cross-reactivity with frequent apicomplexan parasites of dogs, such as T. gondii, Sarcocystis spp., Babesia canis and Hammondia heydorni (Dubey and Lindsay, Reference Dubey and Lindsay1993; Trees et al. Reference Trees, Guy, Tennant, Balfour and Dubey1993; Dubey et al. Reference Dubey, Lindsay, Adams, Gay, Baszler, Blagburn and Thulliez1996; Gondim et al. Reference Gondim, Meyer, Peters, Rezende-Gondim, Vrhovec, Pantchev, Bauer, Conraths and Schares2015), but the extent to which antibodies to other protozoa (e.g. Cystoisospora spp., Cryptosporidium spp.) may cross-react is unknown. Since reading of results depends on the subjective assessment of the observer, this emphasizes even more the need for reassessment of cut-offs and harmonization of IFAT reagents and slide preparation procedures.
Wildlife species were assayed by a serial testing strategy, using the N-MAT as the first screening assay and the IFAT to confirm results. The use of two tests to determine the infection status of wild animals is generally recommended, due to the lack of validated tests for wildlife species. The choice of the N-MAT was based on the higher sensitivity, compared with the IFAT, possibly resulting from a better exposure of epitopes of suspended whole formalin-fixed tachyzoites, while the IFAT has proven to be more specific (Packham et al. Reference Packham, Sverlow, Conrad, Loomis, Rowe, Anderson, Marsh, Cray and Barr1998).
Information on N. caninum prevalence in dogs in Portugal is available from only one study (Maia et al. Reference Maia, Cortes, Brancal, Lopes, Pimenta, Campino and Cardoso2014), which recorded an overall prevalence of 7·9% in dogs by cELISA. The seroprevalence found in the regions Alentejo (5·5%) and Algarve (8·8%) was substantially lower than in the present survey. This may be explained by differences in sample size, time and place of sampling, as well as the serological screening assay used. Prevalence data from other European countries vary likewise substantially. Similar infection rates between 32 and 32·7% were recorded in Italy (Robbe et al. Reference Robbe, Passarelli, Gloria, Di Cesare, Capelli, Iorio and Traversa2016) and Romania (Gavrea et al. Reference Gavrea, Mircean, Pastiu and Cozma2012), and rates up to 43·6% were reported from Spain (Collantes-Fernández et al. Reference Collantes-Fernández, Gómez-Bautista, Miró, Alvarez-García, Pereira-Bueno, Frisuelos and Ortega-Mora2008; Regidor-Cerrillo et al. Reference Regidor-Cerrillo, Pedraza-Diaz, Rojo-Montejo, Vazquez-Moreno, Arnaiz, Gomez-Bautista, Jimenez-Palacios, Ortega-Mora and Collantes-Fernandez2010), while studies carried out in Austria (Wanha et al. Reference Wanha, Edelhofer, Gabler-Eduardo and Prosl2005), Czech Republic (Václavek et al. Reference Václavek, Sedlák, Hůrková, Vodrázka, Sebesta and Koudela2007), Serbia (Kuruca et al. Reference Kuruca, Spasojevic-Kosic, Simin, Savovic, Laus and Lalosevic2013) and Poland (Ploneczka and Mazurkiewicz, Reference Płoneczka and Mazurkiewicz2008; Goździk et al. Reference Goździk, Wrzesień, Wielgosz-Ostolska, Bień, Kozak-Ljunggren and Cabaj2011) found a lower prevalence, ranging between 4·9 and 21·7%.
Infection of dogs in both urban and rural environments is likely to occur through feeding on raw or poorly cooked beef, and vertical transmission from bitches to successive litters has also been described (Dubey et al. Reference Dubey, Schares and Ortega-Mora2007). However, dogs in rural/nature conservation areas are more probable to predate potentially infected small mammals and birds and to have access to aborted fetal tissues from cattle and wild ruminants, carcasses from wild animals and hunting offal, which may explain the high infection rate found in the present survey.
Concerning the wildlife species tested in this study, anti-N. caninum-specific antibodies were detected only in foxes and rabbits. This is the first evidence of exposure to N. caninum in wild animals in Portugal. Unfortunately, it was not possible to establish a correlation between the seropositivity found in dogs, foxes and rabbits, mainly because most sampling areas of these species were not coincident. This may be explained by the difficulty to match the sampling from hunted, captured or road kill wild animals with the sampling from dogs in the same areas. Spatial analysis is also hampered by the low seroprevalence of N. caninum in wildlife and because some species of wild animals have a wide home range. Spatial analysis to correlate infection in dogs and wild animals will therefore require a greater geographical coverage and a more representative sample size. The percentage of infection found in foxes in the present study (12%) is higher than that reported in most studies carried out in Europe. Thus, studies using the IFAT recorded infection rates between 0·9 and 4·4% in foxes in Britain (Hamilton et al. Reference Hamilton, Gray, Wright, Gangadharan, Laurenson and Innes2005), Czech Republic (Bártová et al. Reference Bártová, Slezáková, Nágl and Sedlák2016), Ireland (Murphy et al. Reference Murphy, Walochnik, Hassl, Moriarty, Mooney, Toolan, Sanchez-Miguel, O'Loughlin and McAuliffe2007) and Spain (Sobrino et al. Reference Sobrino, Dubey, Pabón, Linarez, Kwok, Millán, Arnal, Luco, López-Gatius, Thulliez, Gortázar and Almería2008), while studies carried out by ELISA showed a prevalence of 1·5% in Hungary (Jakubek et al. Reference Jakubek, Farkas, Pálfi and Mattsson2007) and absence of infection among 221 foxes sampled in different parts of Sweden (Jakubek et al. Reference Jakubek, Bröjer, Regnersen, Uggla, Schares and Björkman2001). However, in some areas, exposure of foxes to N. caninum may be high, as shown by the prevalence of 69·8% determined by the N-MAT in the Spanish Pyrenees (Marco et al. Reference Marco, Ferroglio, López-Olvera, Montané and Lavín2008). Despite the seroprevalence and the occasional finding of N. caninum-like oocysts in feces, red foxes were not shown to be definitive hosts (Dubey et al. Reference Dubey, Hemphill, Calero-Bernal, Schares, Dubey, Hemphill, Calero-Bernal and Schares2017). Nonetheless, a study on the feeding habits of foxes in Britain showed that foxes feed mainly on medium-sized mammals, primarily rabbits, corresponding to 74% of mass ingested (Baker et al. Reference Baker, Furlong, Southern and Harris2006), and there is evidence that foxes select rabbits when they are abundant, feeding on small mammals and fruits/seeds when lagomorphs are scarce (Díaz-Ruiz et al. Reference Díaz-Ruiz, Delibes-Mateos, García-Moreno, López-Martín, Ferreira and Ferreras2013). The infection rate of 25% found in the present survey suggests therefore that rabbits could be the main source of infection with N. caninum to foxes and that fluctuation of the rabbit population may account for the variable seroprevalence found in different areas. In fact, though Almería et al. (Reference Almería, Vidal, Ferrer, Pabón, Fernández-de-Mera, Ruiz-Fons, Alzaga, Marco, Calvete, Lavin, Gortazar, López-Gatius and Dubey2007) did not detect antibodies in any of 251 wild rabbits tested in Spain, rabbits were shown to be natural IH of N. caninum by molecular methods, with an infection prevalence of 10·5% (Hughes et al. Reference Hughes, Thomasson, Craig, Georgin, Pickles and Hide2008). In addition, antibodies to N. caninum were found in 1·2 and 1·85% of animals tested by the IFAT in rabbit farms in Northern Italy and Northern Egypt, respectively (Ibrahim et al. Reference Ibrahim, Huang, Salem, Talaat, Nasr, Xuan and Nishikawa2009; Machacova et al. Reference Machacova, Bártová, Sedlak, Budikova and Piccirillo2015). The lower prevalence in farmed rabbits can be explained by the fact that caged animals in industrial units have fewer opportunities to become infected with N. caninum oocysts.
Though seropositivity to N. caninum was described in several Mustelidae, Herpestidae, wild boars, wild cats and wild ruminants (Almería, Reference Almería2013; Donahoe et al. Reference Donahoe, Lindsay, Krockenberger, Phalen and Šlapeta2015), none of these species in the present survey were positive. Since the prevalence of infection can be low, the number of animals analysed may not be representative enough and further investigations on these species are needed, in order to assess their role in the sylvatic cycle of N. caninum in the area studied.
Conclusion
Our results show a high prevalence N. caninum in dogs in a wildlife conservation area in Portugal and suggest that rabbits could be a reservoir of infection to dogs, foxes and other wildlife carnivores. The high seroprevalence in dogs and foxes at 1:50 dilution may be caused by a too low cut-off and should be interpreted carefully. Prevalence of N. caninum in dogs and wildlife will need to be taken into account to define adequate prevention measures to reduce transmission to cattle. Asides cattle management practices aimed at reducing vertical transmission in affected farms, prevention strategies should address more incisively horizontal transmission, i.e. discourage feeding dogs with fresh raw meat and restricting as much as possible the access of dogs to ruminants, calving areas and livestock feed and water. Additionally, hunters should take special care in regards to the safe disposal of tissues and organs from hunted animals, in order to prevent infection of dogs and wild carnivores.
ACKNOWLEDGEMENTS
The authors are grateful to project LIFE Habitat Lince Abutre (LIFE08 NAT/P/000227) for the samples used in this study and associated data, with special thanks to DGAV and Patrícia Tavares Santos. The authors also acknowledge Lucinda Marques and Maria do Carmo Ramos (INIAV/Parasitology Laboratory) for invaluable technical assistance.
FINANCIAL SUPPORT
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
CONFLICT OF INTEREST
None.




