Article contents
Allee effect in a manipulative parasite within poikilothermic host under temperature change
Published online by Cambridge University Press: 09 September 2021
Abstract
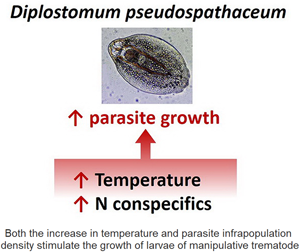
Temperature and intraspecific competition are important factors influencing the growth of all organisms, including parasites. The temperature increase is suggested to stimulate the development of parasites within poikilothermic hosts. However, at high parasite densities, this effect could be diminished, due to stronger intraspecific competition. Our study, for the first time, addressed the joint effects of warming and parasite abundances on parasite growth in poikilothermic hosts. The growth of the common fish parasite larvae (trematode Diplostomum pseudospathaceum) within the rainbow trout at different infection intensities and temperatures (15°C and 18°C) was experimentally investigated. The results showed that temperature was positively correlated with both parasite infection success and growth rates. The growth rates increased much more compared to those in many free-living poikilothermic animals. Atypically for a majority of parasites, D. pseudospathaceum larvae grow faster when abundant (Allee effect). The possible causes for this phenomenon (manipulation cost sharing, etc.) are discussed in this study. Importantly, limited evidence of the interaction between temperature and population density was found. It is likely that temperature did not change the magnitude of the Allee effect but affected its timing. The impact of these effects is supposed to become more pronounced in freshwater ecosystems under current climate changes.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press
Footnotes
These authors contributed equally to this work
References
- 5
- Cited by



