Introduction
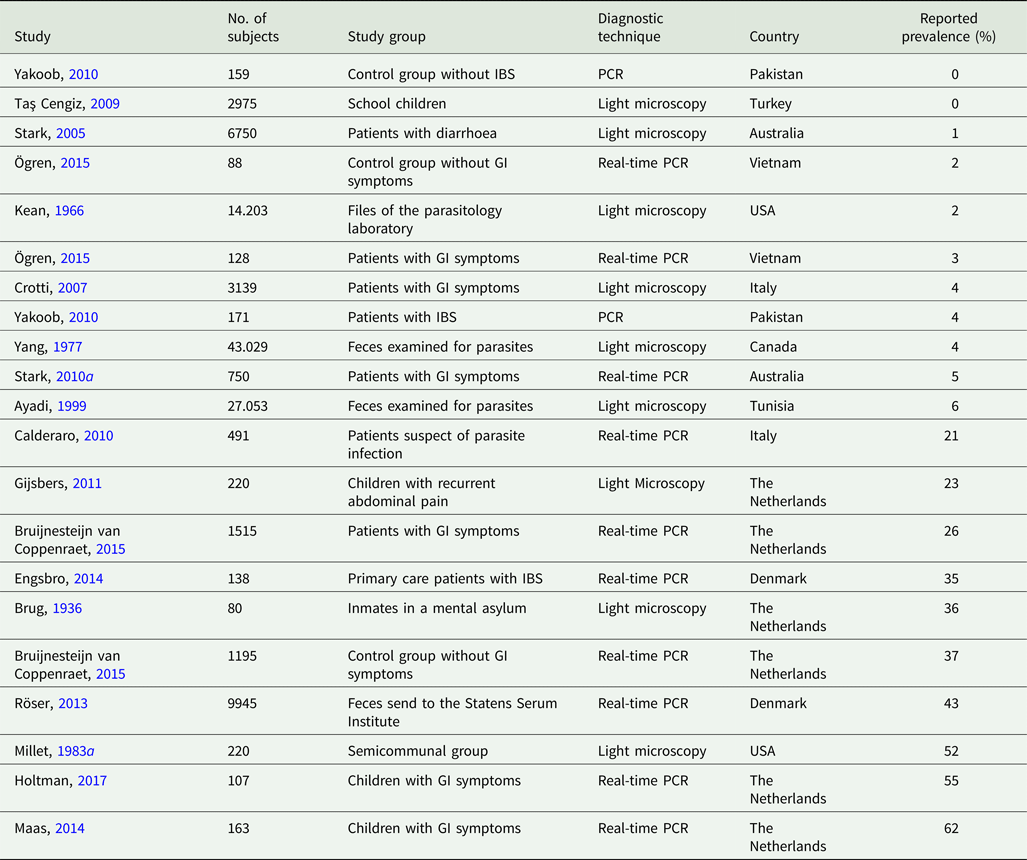
Dientamoeba fragilis (D. fragilis) is a protozoan parasite of the human intestine. While its pathogenic status is often disputed, most clinicians believe it is the cause of abdominal pain and diarrhoea as it is frequently found in patient suffering from these disorders (Lagace-Wiens et al., Reference Lagace-Wiens, VanCaeseele and Koschik2006; Vandenberg et al., Reference Vandenberg, Peek, Souayah, Dediste, Buset, Scheen, Retore, Zissis and Van Gool2006; Banik et al., Reference Banik, Barratt, Marriott and Harkness2011). Dientamoeba fragilis is reported with a prevalence ranging from 0 to 62%, depending on region, population and detection methods used (Table 1). Most prevalence studies were performed using light microscopy. Nowadays polymerase chain reaction (PCR) is used and this results in higher prevalences (Stark et al., Reference Stark, Al-Qassab, Barratt, Stanley, Roberts, Marriott, Harkness and Ellis2011). Infections are more prevalent in females than in males (Barratt et al., Reference Barratt, Harkness, Marriott, Ellis and Stark2011). There is no consensus on differences in infection rates between adults and children (Barratt et al., Reference Barratt, Harkness, Marriott, Ellis and Stark2011).
Table 1. Regional prevalence of Dientamoeba fragilis. Studies were ordered by increasing prevalence
In this review, the pathogenicity, diagnostic approach, treatment and follow-up of patients infected with D. fragilis will be discussed.
Dientamoeba fragilis: biology and pathogenesis
In 1918, M.W. Jepps and C. Dobell were the first to describe D. fragilis as a non-pathogenic amoeba (Jepps and Dobell, Reference Jepps and Dobell1918). Later C. Dobell postulated that D. fragilis was a flagellate, closely related to Histomonas (Dobell, Reference Dobell1940). Subsequent research by various groups dismissed the original statement from Jepps and Dobell that D. fragilis is a non-pathogenic amoeba and showed it is a flagellate lacking flagella (Desser and Yang, Reference Desser and Yang1976; Preiss et al., Reference Preiss, Ockert, Bromme and Otto1990; Grendon et al., Reference Grendon, DiGiacomo and Frost1995; Windsor et al., Reference Windsor, Rafay, Shenoy and Johnson1998; Dickinson et al., Reference Dickinson, Cohen and Schlenker2002; Norberg et al., Reference Norberg, Nord and Evenga2003; Stark et al., Reference Stark, Beebe, Marriott, Ellis and Harkness2005, Reference Stark, Barratt, Roberts, Marriott, Harkness and Ellis2010a; Banik et al., Reference Banik, Barratt, Marriott and Harkness2011; Ögren et al., Reference Ögren, Dienus, Löfgren, Einemo, Iveroth and Matussek2015). Faecal–oral transmission is considered the most likely route of infection in humans (Stark et al., Reference Stark, Roberts, Marriott, Harkness and Ellis2012). Many people harbouring D. fragilis also carry other gastrointestinal protozoa, known for transmission through the faecal–oral route (Windsor et al., Reference Windsor, Rafay, Shenoy and Johnson1998; Ayadi and Bahri, Reference Ayadi and Bahri1999; Girginkardeşler et al., Reference Girginkardeşler, Kurt, Kilimcioǧlu and Ok2008; Stark et al., Reference Stark, Barratt, Chan and Ellis2016). This could explain the high prevalence of D. fragilis found in groups with poor hygiene (Millet et al., Reference Millet, Spencer, Chapin, Yatabe, Brewer and Garcia1983a), but it does not match with the generally higher prevalence in developed countries. Humans are considered as the preferred host of D. fragilis, but animals have also been reported to serve as natural hosts (Stark et al., Reference Stark, Barratt, Chan and Ellis2016). While most domestic animals do not normally carry D. fragilis (Stark et al., Reference Stark, Roberts, Marriott, Harkness and Ellis2012), pigs are a natural host of D. fragilis (Cacciò et al., Reference Cacciò, Sannella, Manuali, Tosini, Sensi, Crotti and Pozio2012) and thus may form a substantial source of human infections. After ingestion, D. fragilis multiply in the large intestine of a permissive host, where the trophozoites, precysts and cysts develop and are shedded in the stool ensuring subsequent spread to a new host. It was initially assumed that D. fragilis does not have a cyst stage (Jepps and Dobell, Reference Jepps and Dobell1918). Although a putative precyst form has been described several times, it is only recently that the existence of a (pre-)cyst stage is more generally recognized (Stark et al., Reference Stark, Garcia, Barratt, Phillips, Roberts, Marriott, Harkness and Ellis2014; Stark et al., Reference Stark, Barratt, Chan and Ellis2016).
A suitable animal model was unavailable for a long period, but in 2013, a mouse model was published (Munasinghe et al., Reference Munasinghe, Vella, Ellis, Windsor and Stark2013). All D. fragilis-infected mice displayed colonic inflammation and weight loss, but uninfected mice had lower levels of intestinal inflammation. Furthermore, cysts orally administered to mice resulted in an infection with D. fragilis (Munasinghe et al., Reference Munasinghe, Vella, Ellis, Windsor and Stark2013). This animal model supports the perception that D. fragilis should be considered as a pathogen.
While D. fragilis was originally described as a non-pathogenic protozoan organism, over the years many reports appeared supporting the pathogenic potential of D. fragilis (Spencer et al., Reference Spencer, Garcia and Chapin1979, Reference Spencer, Chapin and Garcia1982; Lagace-Wiens et al., Reference Lagace-Wiens, VanCaeseele and Koschik2006; Banik et al., Reference Banik, Barratt, Marriott and Harkness2011). Nevertheless, the debate on this subject is not yet closed. Case reports state that patients harbouring D. fragilis have symptoms correlated to infection and have clinical improvement after eradication (Hakansson, Reference Hakansson1936; Desser and Yang, Reference Desser and Yang1976; Shein and Gelb, Reference Shein and Gelb1983; Butler, Reference Butler1996; Dickinson et al., Reference Dickinson, Cohen and Schlenker2002). Larger studies with more patients provide evidence for a correlation between infection and symptoms, concluding that D. fragilis could be pathogenic (Kean and Malloch, Reference Kean and Malloch1966; Preiss et al., Reference Preiss, Ockert, Bromme and Otto1990; Grendon et al., Reference Grendon, DiGiacomo and Frost1995; Windsor et al., Reference Windsor, Rafay, Shenoy and Johnson1998; Ayadi and Bahri, Reference Ayadi and Bahri1999; Norberg et al., Reference Norberg, Nord and Evenga2003; Stark et al., Reference Stark, Beebe, Marriott, Ellis and Harkness2005; Rayan et al., Reference Rayan, Ismail and el Gayar2007; Stark et al., Reference Stark, Barratt, Roberts, Marriott, Harkness and Ellis2010a; Banik et al., Reference Banik, Barratt, Marriott and Harkness2011; Ögren et al., Reference Ögren, Dienus, Löfgren, Einemo, Iveroth and Matussek2015). Conclusions on the pathogenic nature of D. fragilis are based mainly on eradication studies reporting relief of symptoms in patients after treatment (Spencer et al., Reference Spencer, Garcia and Chapin1979; Spencer et al., Reference Spencer, Chapin and Garcia1982; Millet et al., Reference Millet, Spencer, Chapin, Yatabe, Brewer and Garcia1983a; Cuffari et al., Reference Cuffari, Oligny and Seidman1998; Borody et al., Reference Borody, Warren, Wettstein, Robertson, Recabarren and Fontela2002; Girginkardeler et al., Reference Girginkardeler, Balcıog, Ertan and Ok2003; Norberg et al., Reference Norberg, Nord and Evenga2003; Bosman et al., Reference Bosman, Benninga, van de Berg, Kooijman and van Gool2004; Vandenberg et al., Reference Vandenberg, Peek, Souayah, Dediste, Buset, Scheen, Retore, Zissis and Van Gool2006; Kurt et al., Reference Kurt, Girginkardeşler, Balcioğlu, Ozbilgin and Ok2008). In 2011, Barratt et al. published a review about D. fragilis in which they studied the literature until 2011 and stated the following about the parasite: ‘when found in patients with gastrointestinal symptoms without any other pathogen, D. fragilis should be considered as cause of the symptoms and thus patients should receive appropriate treatment’ (Barratt et al., Reference Barratt, Harkness, Marriott, Ellis and Stark2011).
However, the correlation between the presence of the parasite and clinical symptoms is not always obvious or sometimes even absent (Keystone et al., Reference Keystone, Yang, Grisdale, Pillon, Giardia, Unit and Hospital1984; De Wit et al., Reference De Wit, Koopmans, Kortbeek, Van Leeuwen, Bartelds and Van Duynhoven2001; De Jong et al., Reference De, Jong, Korterink, Benninga, Hilbink, Widdershoven and Deckers-kocken2014; Bruijnesteijn van Coppenraet et al., Reference Bruijnesteijn van Coppenraet, Dullaert-de Boer, Ruijs, van der Reijden, van der Zanden, Weel and Schuurs2015; Krogsgaard et al., Reference Krogsgaard, Engsbro, Stensvold, Nielsen and Bytzer2015). In a study performed between 1996 and 1999 in the Netherlands, there was a higher D. fragilis prevalence in 574 control patients (14.6%) compared with 857 patients presented at a general practitioner with symptoms of a gastroenteritis (10.3%) (De Wit et al., Reference De Wit, Koopmans, Kortbeek, Van Leeuwen, Bartelds and Van Duynhoven2001). The same was observed by Bruijnesteijn van Coppenraet et al., who saw a higher D. fragilis prevalence in 1195 control-group patients (37.3%) than in 1515 patients with gastrointestinal symptoms (25.7%) (Bruijnesteijn van Coppenraet et al., Reference Bruijnesteijn van Coppenraet, Dullaert-de Boer, Ruijs, van der Reijden, van der Zanden, Weel and Schuurs2015). A case–control study in the Netherlands, comparing 132 children with chronic abdominal pain to a control group of 77 patients without symptoms, did not report a significant difference in the prevalence of D. fragilis between the two groups, nor a correlation between clinical or microbiological response and treatment (De Jong et al., Reference De, Jong, Korterink, Benninga, Hilbink, Widdershoven and Deckers-kocken2014). This suggests there is no association between chronic abdominal pain and a D. fragilis infection. The control group consisted of children admitted to a mental health institution which could bias the outcome, and humans in semicommunal groups have a higher prevalence of intestinal protozoan infections compared with the overall population (Millet et al., Reference Millet, Spencer, Chapin, Garcia, Yatabe and Stewart1983b).
Several case studies/series suggest significant symptom relieve upon successful treatment of a D. fragilis infection (Spencer et al., Reference Spencer, Garcia and Chapin1979; Spencer et al., Reference Spencer, Chapin and Garcia1982; Millet et al., Reference Millet, Spencer, Chapin, Yatabe, Brewer and Garcia1983a; Cuffari et al., Reference Cuffari, Oligny and Seidman1998; Borody et al., Reference Borody, Warren, Wettstein, Robertson, Recabarren and Fontela2002; Girginkardeler et al., Reference Girginkardeler, Balcıog, Ertan and Ok2003; Norberg et al., Reference Norberg, Nord and Evenga2003; Bosman et al., Reference Bosman, Benninga, van de Berg, Kooijman and van Gool2004; Vandenberg et al., Reference Vandenberg, Peek, Souayah, Dediste, Buset, Scheen, Retore, Zissis and Van Gool2006; Kurt et al., Reference Kurt, Girginkardeşler, Balcioğlu, Ozbilgin and Ok2008) but others fail to statistically proof this (Röser et al., Reference Röser, Simonsen, Stensvold, Olsen, Bytzer, Nielsen and Mølbak2014). Röser and et al. performed a placebo-controlled double-blind trial in Denmark with 96 infected children in 2014, treating them with either metronidazole or placebo but did not observe significant differences in clinical outcome between these two groups. Parasitological eradication 2 weeks after treatment was significantly more frequent in the metronidazole group, suggesting a initial positive effect of antibiotic treatment. However, this difference in parasitoligical eradication rapidly changed 8 weeks after completion of treatment. The amount of infections in the placebo group decreased, whereas the infections in the treatment group increased (Röser et al., Reference Röser, Simonsen, Stensvold, Olsen, Bytzer, Nielsen and Mølbak2014). This suggest a self-limiting disease in the controls (Wenrich, Reference Wenrich1944), and/or re-infection in the treatment group through contact with infectious family members or environment.
The reasons for the different outcomes in studies on pathogenicity and symptom relieve of D. fragilis are unclear. Firstly, there are reports on different subtypes have different virulence factors, comprising both pathogenic and non-pathogenic variants, or even that the subtypes consist of two different species (Johnson and Clark, Reference Johnson and Clark2000; Hussein et al., Reference Hussein, Al-mohammed and Hussein2009; Dunwell, Reference Dunwell2013). Most laboratory diagnostic tests do not distinguish between subtypes. This situation also exists with, e.g. the non-pathogenic Escherichia coli (E. coli) and its pathogenic enterotoxigenic (ETEC), enteroinvasive (EIEC), enterohaemorrhagic (EHEC), enteroadherent (EAEC) and enteropathogenic (EHEC) subvariants. Due to the presence of specific virulence factors, ETEC, EIEC, EAEC and EHEC are all being recognized as pathogenic diarrhoea-causing variants of the generally harmless human commensal E. coli (Hart et al., Reference Hart, Batt, Fletcher, Embaye and Saunders1989; Robins-Browne and Hartland, Reference Robins-Browne and Hartland2002). Based on 18S rRNA sequence differences, two major D. fragilis genotypes have been described, but the overall significance with regard to pathogenicity (if any) is unclear (Johnson and Clark, Reference Johnson and Clark2000; Peek et al., Reference Peek, Reedeker and Van Gool2004; Windsor et al., Reference Windsor, Macfarlane and Clark2006; Hussein et al., Reference Hussein, Al-mohammed and Hussein2009; Dunwell, Reference Dunwell2013; Cacciò et al., Reference Cacciò, Sannella, Bruno, Stensvold, David, Guimarães, Manuali, Magistrali, Mahdad, Beaman, Maserati, Tosini and Pozio2016). Barratt et al. detected the presence of RNA and coding genes in the transcriptome of D. fragilis known to be possible cytotoxic virulence factors such as cysteine peptidases, saposin-like proteins and a leukotriene A4 hydrolase-like peptidase, pointing to a pathogenic character of D. fragilis (Barratt et al., Reference Barratt, Cao, Stark and Ellis2015). Future molecular studies can hopefully distinguish a commensal, non-pathogenic subtype/species from a pathogenic one (Barratt et al., Reference Barratt, Cao, Stark and Ellis2015).
Secondly, host factors could influence virulence and clinical symptoms of infection with D. fragilis, such as the use of immune compromising medication and comorbidity. Furthermore, it is generally accepted that D. fragilis infections can be self-limiting, as shown in a study where spontaneous clearance was reported in 41% of 93 untreated patients within a 180-day period in a retrospective follow-up study (Van Hellemond et al., Reference Van Hellemond, Molhoek, Koelewijn, Wismans and van Genderen2012). Immune responses against D. fragilis have been described (Chan et al., Reference Chan, Stewart, Guan, Robb, Fuite, Chan, Diaz-Mitoma, King, MacDonald and Mackenzie1996). Hence it could be that only the first infection with D. fragilis results in obvious clinical symptoms.
Interestingly, the reported PCR-based D. fragilis prevalence in the Netherlands (Maas et al., Reference Maas, Dorigo-Zetsma, de Groot, Bouter, Plötz and van Ewijk2014; Bruijnesteijn van Coppenraet et al., Reference Bruijnesteijn van Coppenraet, Dullaert-de Boer, Ruijs, van der Reijden, van der Zanden, Weel and Schuurs2015; Holtman et al., Reference Holtman, Kranenberg, Blanker, Ott, Lisman-van Leeuwen and Berger2017) and Denmark (Röser et al., Reference Röser, Simonsen, Nielsen, Stensvold and Mølbak2013; Engsbro et al., Reference Engsbro, Stensvold, Nielsen and Bytzer2014) is relatively high compared with other developed countries like Australia (Stark et al., Reference Stark, Barratt, Roberts, Marriott, Harkness and Ellis2010a) or Italy (Calderaro et al., Reference Calderaro, Gorrini, Montecchini, Peruzzi, Piccolo, Rossi, Gargiulo, Manca, Dettori and Chezzi2010). A first explanation for this relatively high prevalence could be the low specificity of the diagnostic PCR used. All these Dutch and Danish studies use the PCR technique described by Verweij et al. (Reference Verweij, Mulder, Poell, van Middelkoop, Brienen and van Lieshout2007). Stark et al. commented in their recent review that the observed high prevalence of these studies may reflect artefacts of this PCR test rather than a true high incidence (Stark et al., Reference Stark, Barratt, Chan and Ellis2016). A study published in 2016 tested 420 animal samples and demonstrated that the PCR test by Verweij et al. displays cross-reactivity with other trichomonads commonly found in animals (Chan et al., Reference Chan, Barratt, Roberts, Phillips, Šlapeta, Ryan, Marriott, Harkness, Ellis and Stark2016). Contradictory another recent European study from Italy found a lower incidence while also using the PCR technique described by Verweij et al. (Calderaro et al., Reference Calderaro, Gorrini, Montecchini, Peruzzi, Piccolo, Rossi, Gargiulo, Manca, Dettori and Chezzi2010). A second explanation for the high D. fragilis prevalence in the Netherlands and Denmark might be in the high density of pig farms in these two countries. Pigs are a natural host of D. fragilis (Cacciò et al., Reference Cacciò, Sannella, Manuali, Tosini, Sensi, Crotti and Pozio2012); contamination is plausible since pig sheds are built close to residential areas and pig manure, used as fertilizer, is injected into agricultural land. Denmark and the Netherlands produces a lot of pig meat (Danish Agriculture and Food Council, 2016). The surface areas of the Netherlands and Denmark are relatively small while their population sizes are high, resulting in highest numbers of people and pigs per km2 in Europe. This results in high chance of humans to be infected with D. fragilis from pigs. Given that several stable genetic lineages of D. fragilis (variants) have been reported (Johnson and Clark, Reference Johnson and Clark2000; Hussein et al., Reference Hussein, Al-mohammed and Hussein2009; Dunwell, Reference Dunwell2013; Cacciò et al., Reference Cacciò, Sannella, Bruno, Stensvold, David, Guimarães, Manuali, Magistrali, Mahdad, Beaman, Maserati, Tosini and Pozio2016), it may well be that one of these variants represents a ‘zoonotic’ pig strain, and that these pig-derived strains are not causing much symptomology in humans. Therefore, the high prevalence of non-symptomatic patients with a D. fragilis infection could be due to a high infection rate with a non-pathogenic variant or subtype from pigs or may even be false positive as the used PCR might have detected related but distinct species (Chan et al., Reference Chan, Barratt, Roberts, Phillips, Šlapeta, Ryan, Marriott, Harkness, Ellis and Stark2016).
Summarizing the mode of pathogenesis and the putative virulence factors of this parasite is still largely unclear and every D. fragilis infection is considered similar. If however differences in the pathogenic subtypes exist, and also virulence and immune responses that influence the outcome of the infection are being unravelled, it will not only resolve the dispute on pathogenicity but also allow better management of our patients.
For now we recommend health care professionals to approach D. fragilis the way Barratt et al. did in 2011; if D. fragilis is found in patients with gastro-intestinal symptoms and no other aetiological factor is found, adequate treatment is required (Barratt et al., Reference Barratt, Harkness, Marriott, Ellis and Stark2011).
Dientamoeba fragilis: clinical features
The most frequently documented symptoms in patients infected with D. fragilis are abdominal pain and diarrhoea (Vandenberg et al., Reference Vandenberg, Peek, Souayah, Dediste, Buset, Scheen, Retore, Zissis and Van Gool2006). Other associated manifestations are weight loss, anorexia, flatus, fatigue, looseness of stools, nausea, vomiting and anal pruritis (Norberg et al., Reference Norberg, Nord and Evenga2003). But asymptomatic presence has also been reported (Bruijnesteijn van Coppenraet et al., Reference Bruijnesteijn van Coppenraet, Dullaert-de Boer, Ruijs, van der Reijden, van der Zanden, Weel and Schuurs2015). The described duration of illness differs between patients with a widespread variation between long-standing symptoms and self-limiting disease (Wenrich, Reference Wenrich1944). Patients can present general abdominal tenderness during physical examination. Clinical presentation makes it difficult to differentiate a D. fragilis infection from other diseases as symptoms are rather general and none represent specific diagnostic criteria for an ongoing D. fragilis infection.
Dientamoeba fragilis: laboratory diagnostic methods
Many approaches have been used to identify D. fragilis in stool samples of persons with gastro-intestinal complaints. Since the first description of D. fragilis in 1918, diagnostic laboratories found more sensitive and specific detection methods. Most Western microbiological laboratories provide diagnostic tests for D. fragilis, such as microscopy and PCR (Table 2). The available diagnostic tests, their advantages and putative pitfalls are briefly described below.
Table 2. Commonly used laboratory diagnostic methods for the detection of Dientamoeba fragilis in human stool samples

a Assuming required infrastructure is already present and thus of no consequence on price per test.
b There is some discussion as some PCRs may detect a potentially non-pathogenic Dientamoeba fragilis subspecies from pigs (Stark et al., Reference Stark, Barratt, Chan and Ellis2016).
Microscopy
Although microscopy can be easily performed in most settings, D. fragilis is hard to identify microscopically due to its morphological similarity to some related protozoa. In addition, the vegetative form is relatively fragile and when damaged it is difficult to be recognized (hence, the name). Therefore, routine microscopic examination of feces for the presence of D. fragilis requires a highly trained and skilled technologist. Higher sensitivity is achieved when examination of the stool is performed immediately after defecation, but in most clinical settings, this is difficult to realize. Usually in the daily routine, stool samples arrive in the laboratory hours after defecating (if not days), and it is a considerable burden for the physician to elaborately explain the complex sampling procedure, and for the patient to correctly collect and timely deliver the fresh stool sample to the laboratory. An additional problem is the phasic secretion from intestinal parasites (Van Gool et al., Reference Van Gool, Weijts, Lommerse and Mank2003). A high load of D. fragilis can be found on one day, with almost no detection load a few days later. The sensitivity of diagnosing D. fragilis increases with more than 30% when the stool samples are examined 3 consecutive days compared with only once (Hiatt et al., Reference Hiatt, Markell and Ng1995). In conclusion, microscopy, when performed accurately, is a logistically challenging and time-consuming diagnostic method with a relatively low sensitivity.
Triple Feces Test
In order to overcome the problems associated with phasic secretion, laboratories implemented the Triple Feces Test (TFT). The TFT is a microscopic diagnostic test which combines sampling on 3 consecutive days with a fixative, a concentration method and a permanent stain. In 2003, van Gool et al. compared single microscopic examination with TFT using 544 stool samples, finding a significant difference in detection rate, in favour of TFT (Van Gool et al., Reference Van Gool, Weijts, Lommerse and Mank2003). The authors suggest that TFT can be an effective method for the detection of intestinal parasites (Van Gool et al., Reference Van Gool, Weijts, Lommerse and Mank2003). A disadvantage of TFT is the diagnostic delay of 3 days (as sampling is on 3 successive days). Another problem is the difficulty some patients have with collecting the right amount of stool. If too much stool sample is mixed with the fixative, the preservation of the parasite is compromised and the test can be inconclusive. Insufficient amounts of stool sample also can result in an inconclusive test result and both result in even longer delay. Finally a skilled microscopist is required to reliably discriminate D. fragilis from the morphologically similar protozoa and other particles in stool.
Polymerase chain reaction
PCR assay amplifies and detects specific D. fragilis DNA. Distinction is made between conventional and real-time PCR. Conventional PCR consists of two steps: the first is amplification of the DNA in a block-thermocycler, and the second step is electrophoresis to visualize the DNA. The two steps need to be performed subsequently in two different apparatuses, with the risk of contamination when the amplified DNA is transferred from the PCR machine to the visualization set-up. In real-time PCR, both steps, amplification and visualization of the generation of the PCR product, are performed simultaneously in a single machine. This reduces not only the risk of contamination but also eliminates manual labour and hence putative sample mix-up. Compared with conventional PCR and/or microscopic examination, real-time PCR has a persistent superior sensitivity and specificity in the detection of D. fragilis (Calderaro et al., Reference Calderaro, Gorrini, Montecchini, Peruzzi, Piccolo, Rossi, Gargiulo, Manca, Dettori and Chezzi2010; Stark et al., Reference Stark, Beebe, Marriott, Ellis and Harkness2006, Reference Stark, Barratt, Roberts, Marriott, Harkness and Ellis2010b; Stensvold and Nielsen, Reference Stensvold and Nielsen2012). Furthermore, due to the higher sensitivity, one stool sample is enough, so there is no diagnostic delay of 3 days as seen with the TFT. Importantly, PCR requires a carefully designed setup that is laboratory- and reagent-specific to prevent lowered sensitivity and/or false positives (Rychlik, Reference Rychlik1995; Chan et al., Reference Chan, Barratt, Roberts, Phillips, Šlapeta, Ryan, Marriott, Harkness, Ellis and Stark2016). Many different specific D. fragilis PCR have been described, see for instance Stark et al. (Reference Stark, Barratt, Chan and Ellis2016) for a summary. However, one has to realize that each PCR has its own specific specificity and sensitivity (Rijsman et al., Reference Rijsman, Monkelbaan and Kusters2016).
Alternative diagnostic methods
Serology testing with an indirect immunofluorescence assay is currently not available in routine diagnostics as it has been previously only used as a research tool. Furthermore, the diagnostic value is unclear since a high seroprevalence in asymptomatic individuals was found generating possible false-positive test results (Chan et al., Reference Chan, Stewart, Guan, Robb, Fuite, Chan, Diaz-Mitoma, King, MacDonald and Mackenzie1996). No rapid immunochromatographic test for the detection of D. fragilis is available. Reliable commercial immunoassays are not yet available for the detection of D. fragilis, although preliminary studies proved these tests have potential (Chan et al., Reference Chan, Ming Xu and Mackenzie1993). Culture techniques are available, but have an inferior sensitivity and specificity compared with PCR and are both labour-, resource- and time-consuming (Stark et al., Reference Stark, Barratt, Roberts, Marriott, Harkness and Ellis2010b).
Dientamoeba fragilis: treatment
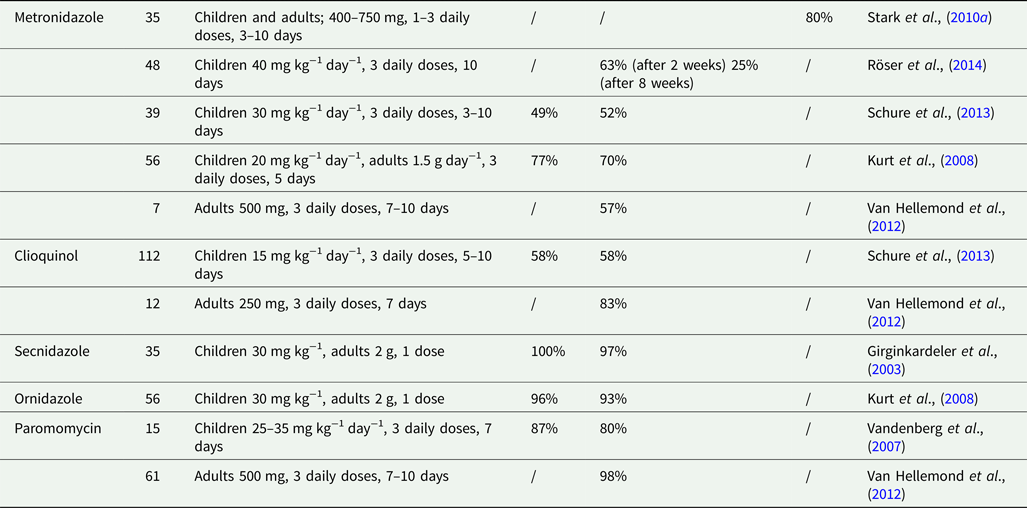
Regardless of its putative and much debated role as a pathogen in clinical practice, D. fragilis is often rationally treated with a single-drug regimen based on a restricted set of antibiotics. Most treatment regimens are based on studies with small numbers, making them relatively difficult to interpret knowing that D. fragilis infections are potentially self-limiting (Wenrich, Reference Wenrich1944; Van Hellemond et al., Reference Van Hellemond, Molhoek, Koelewijn, Wismans and van Genderen2012). Most doctors prescribe antibiotics based on their clinical experience and habits. Although often prescribed, metronidazole (Stark et al., Reference Stark, Barratt, Roberts, Marriott, Harkness and Ellis2010a; Schure et al., Reference Schure, de Vries, Weel, van Roon and Faber2013; Röser et al., Reference Röser, Simonsen, Stensvold, Olsen, Bytzer, Nielsen and Mølbak2014) is less effective when compared with other agents, such as clioquinol (Schure et al., Reference Schure, de Vries, Weel, van Roon and Faber2013), paromomycin (Vandenberg et al., Reference Vandenberg, Souayah, Mouchet, Dediste and van Gool2007; Van Hellemond et al., Reference Van Hellemond, Molhoek, Koelewijn, Wismans and van Genderen2012), secnidazole (Girginkardeler et al., Reference Girginkardeler, Balcıog, Ertan and Ok2003) and ornidazole (Kurt et al., Reference Kurt, Girginkardeşler, Balcioğlu, Ozbilgin and Ok2008). Clinical guidelines differ throughout the world as they are based on small cohort studies since large-scale double-blind randomized placebo-controlled trials have not been described in the literature. Below we will briefly discuss the most relevant published data, which are summarized in Table 3.
Table 3. Overview antibiotic regimes for Dientamoeba fragilis infection
Metronidazole
Metronidazole is one of the most commonly prescribed antibiotics for the treatment of D. fragilis infections. An in vitro study published in 2012 tested 11 agents, including metronidazole, paromomycin, iodoquinol and tetracycline. They found that 5-nitroimidazole derivatives, such as ornidazole, ronidazole and metronidazole have the lowest minimal lethal concentrations to eradicate D. fragilis, suggesting that these agents could be good therapeutic options (Nagata et al., Reference Nagata, Marriott, Harkness, Ellis and Stark2012). Stark et al. analysed 39 patients in retrospect who were treated for a D. fragilis infection. All patients were diagnosed using real-time PCR. From the patients who received metronidazole, 80% was released from complaints and had parasitological eradication. Six out of 28 patients receiving metronidazole still harboured D. fragilis 2–4 weeks after treatment, which indicated either failure of treatment, relapse or reinfection. The dose and duration of treatment did not correlate with clinical outcome. All patients receiving paramomycin or iodoquinol had a parasitological and clinical effect, and thus a higher eradication rate when compared with metronidazole (Stark et al., Reference Stark, Barratt, Roberts, Marriott, Harkness and Ellis2010a). A more recent placebo-controlled double-blind study from 2014 in Denmark found no significant difference in clinical improvement between a placebo group and children who were treated with metronidazole. Eradication from D. fragilis was significantly higher in the metronidazole group 2 weeks after ending treatment, but this difference reduced after 8 weeks. The data do not provide evidence for the effectiveness of metronidazole as routine treatment for D. fragilis-positive children with chronical gastro-intestinal complaints (Röser et al., Reference Röser, Simonsen, Stensvold, Olsen, Bytzer, Nielsen and Mølbak2014).
Clioquinol
Clioquinol, an 8-hydroxyquinoline derivative, is an antiprotozoal drug used for D. fragilis treatment. A retrospective analysis from the Netherlands in 2013 studied 238 children infected with D. fragilis. The infections were diagnosed using real-time PCR. One hundred and fifty-one patients underwent treatment; 112 received clioquinol (15 mg kg−1 day−1 in three daily doses, during 5–10 days) and 39 metronidazole (30 mg kg−1 day−1 in three daily doses, during 3–10 days). Clioquinol had a significant better clinical effect compared with metronidazole. There was a comparable parasitological eradication rate between clioquinol and metronidazole after treatment (Schure et al., Reference Schure, de Vries, Weel, van Roon and Faber2013). The pre- and post-treatment time to PCR testing varied from 4 to 22 weeks. Post-treatment retesting time was chosen arbitrarily as no data exist on ideal post-treatment testing times. Early post-treatment PCR testing might have resulted in false-positive results due to the presence of residual DNA. On the other hand, late post-treatment testing could have resulted either in false-positive results from re-infection or false-negative results on account of D. fragilis being a self-limiting disease (Wenrich, Reference Wenrich1944; Van Hellemond et al., Reference Van Hellemond, Molhoek, Koelewijn, Wismans and van Genderen2012). Since clioquinol is a general well-known antibiotic with relative few side-effects and with better treatment outcomes than metronidazole, we advise the use of clioquinol 15 mg kg−1 in children and 250 mg in adults, in three daily doses during 7 days. Alternative treatment options should be considered if clioquinol is inadequate (see flow-chart).
Alternative treatment options
Other antibiotic treatment options for a D. fragilis infection are secnidazole, ornidazole and paromomycin. In 2003, Girginkardeşler et al. studied 35 patients infected with D. fragilis in Turkey. They observed parasitological eradication after treatment with secnidazole in 34 patients and all gastro-intestinal symptoms either disappeared (77.1%) or diminished (22.9%) (Girginkardesler et al., Reference Girginkardeler, Balcıog, Ertan and Ok2003). A single-dose ornidazole, when compared with metronidazole, resulted in a significantly better parasitological and clinical outcome. Patients treated with metronidazole suffered more from side-effects, such as nausea, a dry mouth and a metallic taste (Kurt et al., Reference Kurt, Girginkardeşler, Balcioğlu, Ozbilgin and Ok2008). Children treated with paromomycin had a parasitological eradication and clinical improvement in a Dutch study (Vandenberg et al., Reference Vandenberg, Souayah, Mouchet, Dediste and van Gool2007). When compared with clioquinol or metronidazole, paromomycin appeared to be more effective in adults in a retrospective cohort study by Van Hellemond et al. (Reference Van Hellemond, Molhoek, Koelewijn, Wismans and van Genderen2012). In this study, D. fragilis infections spontaneously cleared in 41% of untreated cases, an indication for the frequent self-limiting character of D. fragilis infections, or alternatively of the poor performance of the microscopic method to establish infection (Van Hellemond et al., Reference Van Hellemond, Molhoek, Koelewijn, Wismans and van Genderen2012). Combination therapy (doxycyclin with iodoquinol or secnidazole, nitazoxinid and doxycylin) seems to be adequate, but considerably causes side-effects (Stark et al., Reference Stark, Barratt, Roberts, Marriott, Harkness and Ellis2010a). Moreover, unconventional treatment with various natural dry plant extracts, such as pomegranate, garlic, wormseed and ginger root, show no potential of eradicating D. fragilis (Barratt et al., Reference Barratt, Ellis, Harkness, Marriott and Stark2013).
Follow-up
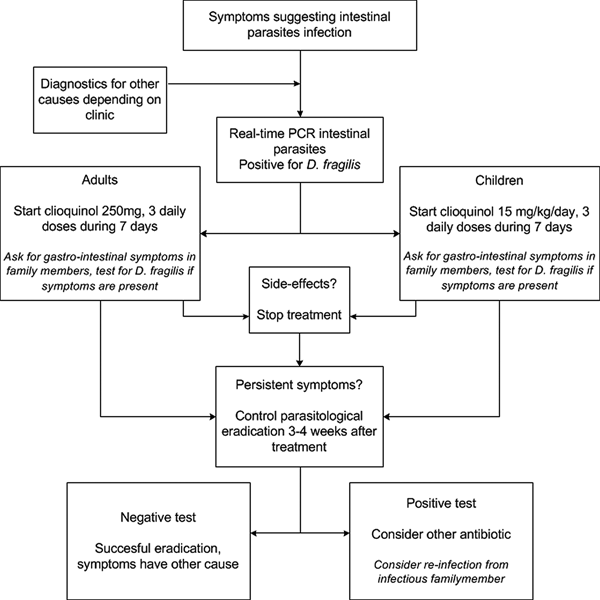
There is no solid data available on the clinical follow-up after positive D. fragilis testing, and studies are needed to analyse a post-treatment work-up. Based on our experience, we advise to repeat testing, especially if symptoms persist 3–4 weeks after completion of therapy. Post-treatment samples for microscopy-based testing can be obtained almost immediately following eradication, but with PCR-based testing, one should observe a ‘DNA-wash out’ period of a week in order to avoid false positive due to the presence of dead organisms in the feces. For patients remaining positive, a second treatment with an alternative antibiotic is advisable (see Fig. 1). One should be aware of possible re-infection from (asymptomatically) infected family members, and additional testing of family members might be indicated in those cases where initial therapy fails.
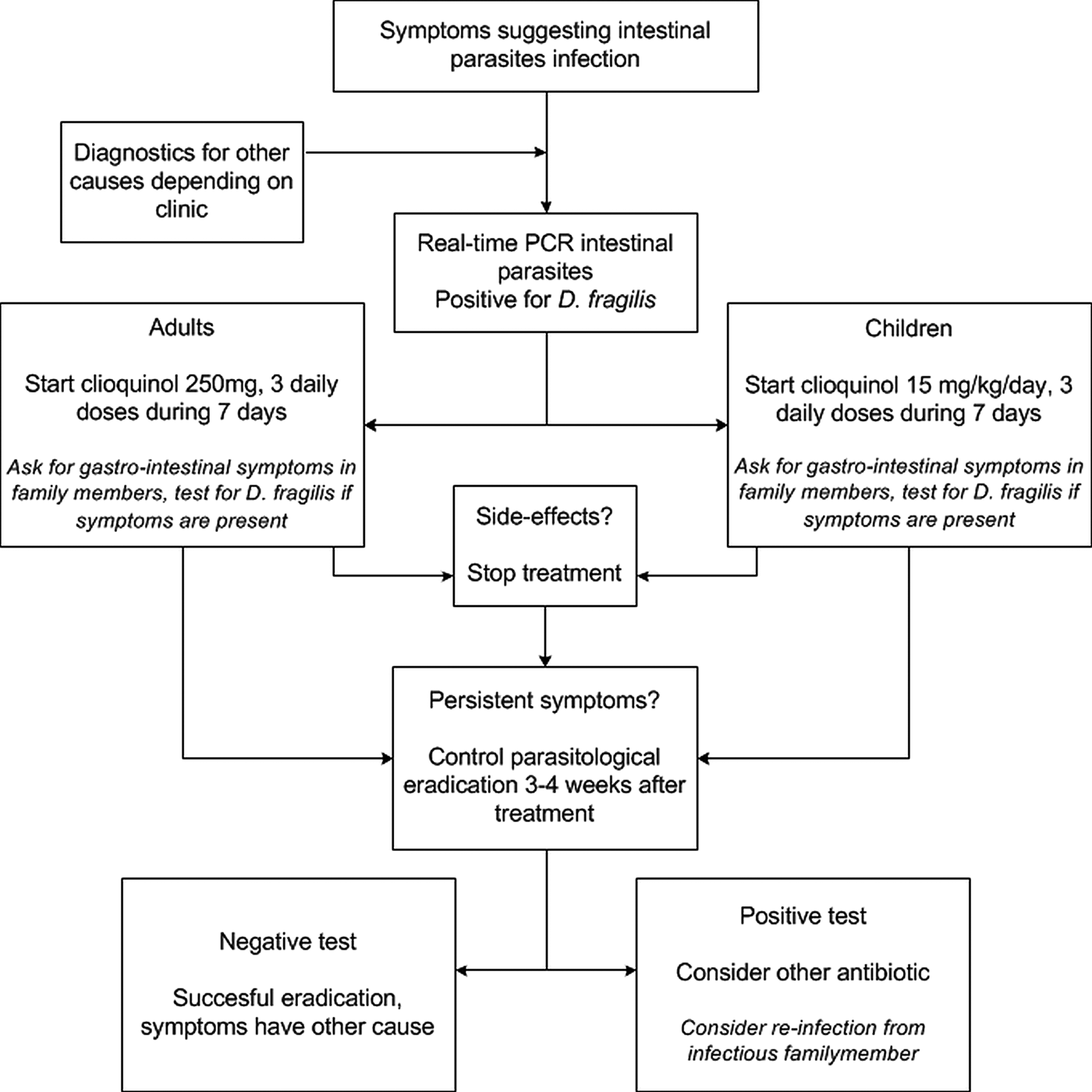
Fig. 1. Flow-chart for diagnostic and therapeutic approach for a Dientamoeba fragilis infection.
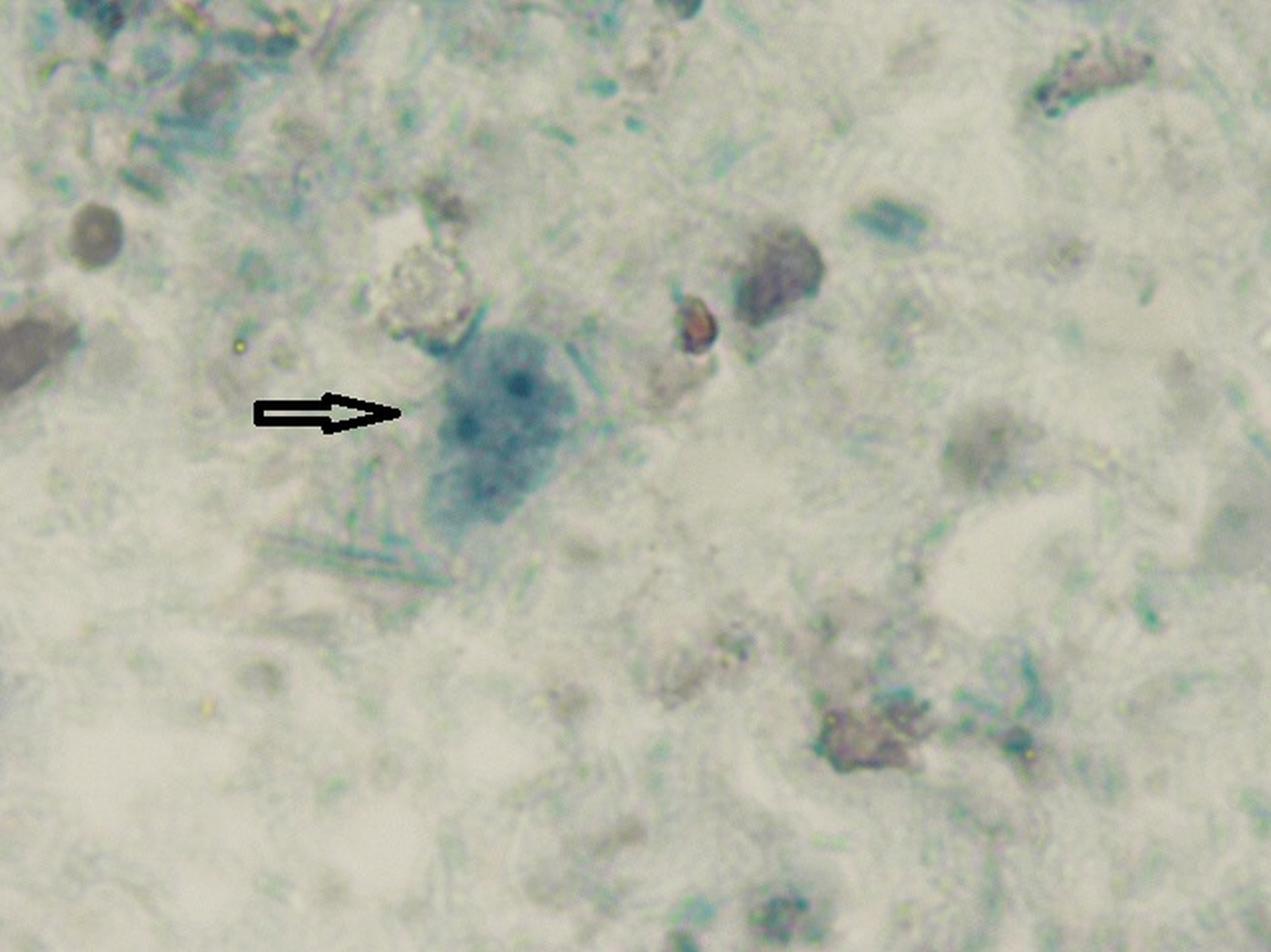
Fig. 2. Microscopic image Dientamoeba fragilis in a stool sample. Oil-immersion using bright-field microscopy at ×1000 magnification of a permanent staining was with chlorazol black. The characteristic fragmented nuclei are clearly visible within the trophozoid.
Conclusion and guideline for the physician
There is a discussion on the pathogenic status of D. fragilis in patients with gastro-intestinal complaints. Consensus on the best diagnostic and therapeutic approach is lacking. For D. fragilis, a well-designed, in-laboratory-validated real-time PCR is the best diagnostic test with regard to sensitivity and specificity (Cacciò et al., Reference Cacciò, Sannella, Bruno, Stensvold, David, Guimarães, Manuali, Magistrali, Mahdad, Beaman, Maserati, Tosini and Pozio2016). Also with regard to patient comfort and time to result, real-time PCR outperforms ‘second best’ test, i.e. the Triple Feces Test (Bruijnesteijn van Coppenraet et al., Reference Bruijnesteijn van Coppenraet, Wallinga, Ruijs, Bruins and Verweij2009; Stark et al., Reference Stark, Barratt, Roberts, Marriott, Harkness and Ellis2010b). In our opinion, health care professionals should see D. fragilis as an aetiological factor in patients with gastro-intestinal symptoms especially if other probable causes for these symptoms are absent (Barratt et al., Reference Barratt, Harkness, Marriott, Ellis and Stark2011). The best treatment remains scientific unclear. We advise medical professionals to prescribe antibiotics for D. fragilis infections in patients with gastrointestinal complaints. If doing so we prefer clioquinol 250 mg in three daily doses during 7 days in adults (Van Hellemond et al., Reference Van Hellemond, Molhoek, Koelewijn, Wismans and van Genderen2012) and in children 15 mg kg−1 day−1 in three daily doses for 7 days (Schure et al., Reference Schure, de Vries, Weel, van Roon and Faber2013). Repeated testing is advised 3–4 weeks after treatment if symptoms persist and if positive, treatment with an alternative antibiotic regime and screening of family members should be considered.
Acknowledgements
We would like to thank E.P. van Zuuk for providing us with the light microscopic image of D. fragilis (Fig. 2).
Author contributions
All authors have substantially contributed to the conception and design, acquisition and analysis of data, and the drafting of the manuscript.
Financial support
No grant support was obtained for the writing of this manuscript.
Conflict of interest
None.
Ethical standards
Not applicable.