Article contents
Development of a low-cost copro-LAMP assay for simultaneous copro-detection of Toxocara canis and Toxocara cati
Published online by Cambridge University Press: 17 February 2021
Abstract
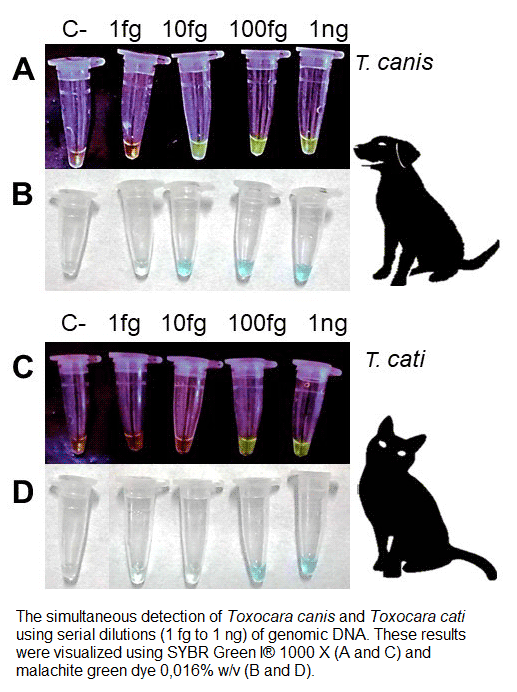
Toxocariasis is a zoonotic disease caused mainly by Toxocara canis and Toxocara cati and diagnosis in dogs and cats is an important tool for its control. For this reason, a new coprological loop-mediated isothermal amplification (LAMP) assay was developed for the simultaneous detection of these species. The primer set was designed on a region of the mitochondrial cox-1 gene. Amplification conditions were evaluated using a temperature gradient (52°C to 68°C), different incubation times (15–120 min), and different concentrations of malachite green dye (0.004–0.4% w/v). The analytical sensitivity was evaluated with serial dilutions of genomic DNA from T. canis and T. cati adult worms, and with serial dilutions of DNA extracted from feces using a low-cost in-house method. The specificity was evaluated using genomic DNA from Canis lupus familiaris, Felis catus, Escherichia coli, Toxascaris leonina, Ancylostoma caninum, Echinococcus granulosus sensu stricto and Taenia hydatigena. The LAMP assay applied to environmental fecal samples from an endemic area showed an analytical sensitivity of 10–100 fg of genomic DNA and 10−5 serial dilutions of DNA extracted from feces using the low-cost in-house method; with a specificity of 100%. Additionally, the total development of the assay was carried out in a basic laboratory and per-reaction reagent cost decreased by ~80%. This new, low-cost tool can help identify the most common agents of toxocariasis in endemic areas in order to manage prevention strategies without having to rely on a laboratory with sophisticated equipment.
Keywords
Information
- Type
- Research Article
- Information
- Copyright
- Copyright © The Author(s) 2021. Published by Cambridge University Press
References
- 9
- Cited by


