Introduction
NADH:ubiquinone oxidoreductase [EC 7.1.1.2], eukaryotic complex I, is the largest and the most complicated enzyme of the respiratory chain. Its subunits are encoded by both the nuclear and mitochondrial genomes (Chomyn et al., Reference Chomyn, Mariottini, Cleeter, Ragan, Matsuno-Yagi, Hatefi, Doolittle and Attardi1985; Walker et al., Reference Walker, Arizmendi, Dupuis, Fearnley, Finel, Medd, Pilkington, Runswick and Skehel1992). It couples the transfer of two electrons from NADH to ubiquinone to the translocation of four protons across the mitochondrial inner membrane. The proposed mechanism includes conformation changes, electrostatic interactions and water molecules that constitute proton-translocation pathways (Grba and Hirst, Reference Grba and Hirst2020; Kampjut and Sazanov, Reference Kampjut and Sazanov2020). Complex I has an L-shaped structure with a hydrophilic peripheral and a hydrophobic membrane domain. The hydrophilic arm contains two enzymatically distinct regions: the N-module involved in the oxidation of NADH and subsequent electron transport, forming a tip of the arm, and the Q-module, which contains Fe–S clusters, through which electrons are transferred to ubiquinone, forming the interface between two domains. The hydrophobic P-module (composed of the ND1, ND2, ND4 and ND5 multi-protein modules taking part in the proton pumping) is embedded in the inner mitochondrial membrane (Yagi and Matsuno-Yagi, Reference Yagi and Matsuno-Yagi2003; Brandt, Reference Brandt2006, Reference Brandt2013; Berrisford and Sazanov, Reference Berrisford and Sazanov2009). The core of this enzyme consists of 14 essential subunits that are fairly conserved across different domains of life (Gabaldón et al., Reference Gabaldón, Rainey and Huynen2005). Mammalian complex I additionally contains up to 32 accessory subunits that are not directly associated with energy conservation (Carroll et al., Reference Carroll, Fearnley, Skehel, Shannon, Hirst and Walker2006; Kmita and Zickermann, Reference Kmita and Zickermann2013). These proteins may be involved in the regulation of enzymatic activity, stability of the complex or auxiliary functions, for example, the fatty acid synthesis (Janssen et al., Reference Janssen, Nijtmans, van den Heuvel and Smeitink2006; Pereira et al., Reference Pereira, Videira and Duarte2013). Two of the most commonly used inhibitors of the mitochondrial complex I are rotenone (stabilizing the semiquinone intermediate within the complex) and capsaicin (antagonizing either formation or release of the quinol product) (Degli Esposti, Reference Degli Esposti1998; Okun et al., Reference Okun, Lummen and Brandt1999).
In addition to complex I, another NADH dehydrogenase, NDH2, has been discovered in the mitochondria of several organisms. It catalyses the transfer of electrons from NADH to ubiquinone without pumping protons out of the matrix (Matus-Ortega et al., Reference Matus-Ortega, Salmeron-Santiago, Flores-Herrera, Guerra-Sanchez, Martinez, Rendon and Pardo2011). In extreme cases (for example, in Saccharomyces cerevisiae), the complex I is completely missing and its function is taken by the alternative dehydrogenases (Overkamp et al., Reference Overkamp, Bakker, Kotter, van Tuijl, de Vries, van Dijken and Pronk2000).
Trypanosomatids (class Kinetoplastea) is a group of obligate parasitic flagellates confined exclusively to insects (monoxenous species) or transmitted by insects or annelids to vertebrates or plants (Lukeš et al., Reference Lukeš, Butenko, Hashimi, Maslov, Votýpka and Yurchenko2018; Maslov et al., Reference Maslov, Opperdoes, Kostygov, Hashimi, Lukeš and Yurchenko2019). Functionality of the trypanosomatid complex I has long been debated. Bioinformatics analysis identified 29 orthologue genes of the prototypical eukaryotic subunits and further 34 genes encoding unique accessory proteins in genomes of dixenous Trypanosoma brucei, T. cruzi and Leishmania major (Opperdoes and Michels, Reference Opperdoes and Michels2008; Perez et al., Reference Perez, Lapaille, Degand, Cilibrasi, Villavicencio-Queijeiro, Morsomme, Gonzalez-Halphen, Field, Remacle, Baurain and Cardol2014; Opperdoes et al., Reference Opperdoes, Butenko, Flegontov, Yurchenko and Lukeš2016). These genomic data suggest that trypanosomatid complex I is composed of over 60 subunits and its molecular mass is over 2 MDa, which is twice as large as its bovine or yeast counterpart (Abdrakhmanova et al., Reference Abdrakhmanova, Zickermann, Bostina, Radermacher, Schagger, Kerscher and Brandt2004; Carroll et al., Reference Carroll, Fearnley, Skehel, Shannon, Hirst and Walker2006). The mitochondrial DNA of Trypanosoma spp. encodes eight complex I subunits. ND1–ND5 are orthologues to the mitochondrial subunits (which participate in protons pumping and bind ubiquinone and rotenone), while ND7–ND9 are orthologues to the nuclear-encoded subunits NDUFS2 (Fe–S cluster and binding site for ubiquinone), NDUFS8 (two Fe–S clusters) and NDUFS3 in humans. The genes for ND4L and ND6 had been assigned to neither the mitochondrial nor to the nuclear DNA (Opperdoes and Michels, Reference Opperdoes and Michels2008). However, it has been proposed that these proteins are encoded in the mitochondria by CR3 and CR4 genes (Duarte and Tomás, Reference Duarte and Tomás2014). Recent data demonstrated that trypanosomatids possess all the proteins necessary for NAD+ regeneration by complex I: those involved in electron transfer, ubiquinone binding and reduction and proton pumping. Proteomic analysis confirmed the presence of both canonical and auxiliary subunits encoded in the nuclear genome of T. brucei. It was clearly showed that the complex I subunits are organized into the high molecular weight proteins in trypanosomal mitochondria (Panigrahi et al., Reference Panigrahi, Ziková, Dalley, Acestor, Ogata, Anupama, Myler and Stuart2008; Acestor et al., Reference Acestor, Zíková, Dalley, Anupama, Panigrahi and Stuart2011). However, none of the proteomic studies published to date has been able to detect complex I subunits encoded by the mitochondrial genome of trypanosomatids.
The importance of the complex I in trypanosomatids has been disputed. Indeed, both dyskinetoplastic Trypanosoma evansi and T. equiperdum thrive without it (Schnaufer et al., Reference Schnaufer, Domingo and Stuart2002). The natural T. cruzi mutants with deletions in ND4, ND5 and ND7 genes showed no alterations in mitochondrial bioenergetics compared to the wild type (Carranza et al., Reference Carranza, Kowaltowski, Mendonca, de Oliveira, Gadelha and Zingales2009). Long-term cultivated isolates of Leishmania tarentolae and Crithidia fasciculata have lost guide RNAs for editing of ND3, ND8 and ND9 genes and no complex I activity had been detected in them (Sloof et al., Reference Sloof, Arts, van den Burg, van der Spek and Benne1994; Thiemann et al., Reference Thiemann, Maslov and Simpson1994). Complex I is also not essential in the studied stages of T. brucei. Ablation of NDUFV1 and NDUFS7 in the procyclic and bloodstream forms did not produce any effect on the detected NADH dehydrogenase activity (Verner et al., Reference Verner, Čermáková, Škodová, Kriegová, Horváth and Lukeš2011; Surve et al., Reference Surve, Heestand, Panicucci, Schnaufer and Parsons2012), which was also not sensitive to the rotenone (Verner et al., Reference Verner, Čermáková, Škodová, Kováčová, Lukeš and Horváth2014). Of note, the presence of alternative NDH2 has been documented in T. brucei (Fang and Beattie, Reference Fang and Beattie2002; Verner et al., Reference Verner, Škodová, Poláková, Ďurišová-Benkovičová, Horváth and Lukeš2013) and Phytomonas serpens (Gonzalez-Halphen and Maslov, Reference Gonzalez-Halphen and Maslov2004; Čermáková et al., Reference Čermáková, Verner, Man, Lukeš and Horváth2007). Its elimination in both procyclic and bloodstream forms of T. brucei had only a modest effect on the viability of the tested cells (Verner et al., Reference Verner, Škodová, Poláková, Ďurišová-Benkovičová, Horváth and Lukeš2013; Surve et al., Reference Surve, Jensen, Heestand, Mazet, Smith, Bringaud, Parsons and Schnaufer2017).
The only trypanosomatid species with essential mitochondrial complex I known to date is P. serpens (Čermáková et al., Reference Čermáková, Verner, Man, Lukeš and Horváth2007). However, it lacks respiratory chain complexes III and IV (Nawathean and Maslov, Reference Nawathean and Maslov2000). The size of the complex I in that species is about 2.2 MDa and its NADH dehydrogenase activity, as well as mitochondrial membrane potential are sensitive to rotenone (Moyses and Barrabin, Reference Moyses and Barrabin2004; Verner et al., Reference Verner, Čermáková, Škodová, Kováčová, Lukeš and Horváth2014). It was demonstrated that the complex contains subunits NDUFA6 and NDUFA9 (Čermáková et al., Reference Čermáková, Verner, Man, Lukeš and Horváth2007).
Here, we investigated NADH dehydrogenase activity in P. serpens and seven monoxenous trypanosomatids: Blechomonas ayalai (Votýpka et al., Reference Votýpka, Suková, Kraeva, Ishemgulova, Duží, Lukeš and Yurchenko2013), Herpetomonas tarakana (Yurchenko et al., Reference Yurchenko, Kostygov, Havlová, Grybchuk-Ieremenko, Ševčíková, Lukeš, Ševčík and Votýpka2016), Kentomonas sorsogonicus (Votýpka et al., Reference Votýpka, Kostygov, Kraeva, Grybchuk-Ieremenko, Tesařová, Grybchuk, Lukeš and Yurchenko2014), Leptomonas seymouri (Wallace, Reference Wallace1977), Novymonas esmeraldas (Kostygov et al., Reference Kostygov, Dobáková, Grybchuk-Ieremenko, Váhala, Maslov, Votýpka, Lukeš and Yurchenko2016), Sergeia podlipaevi (Svobodová et al., Reference Svobodová, Zídková, Čepička, Oborník, Lukeš and Votýpka2007) and Wallacemonas raviniae (Kostygov et al., Reference Kostygov, Grybchuk-Ieremenko, Malysheva, Frolov and Yurchenko2014). We provide evidence that functional complex I is present in two more trypanosomatids. In these molecular complexes, we detected not only a majority of the nuclear DNA-encoded proteins, but (for the first time) also several subunits derived from the mitochondrial DNA. In all of them we also spotted MURF2, the protein of unknown function (Blum and Simpson, Reference Blum and Simpson1990).
Materials and methods
Cultivation of trypanosomatids
Phytomonas serpens (strain 9T) was grown at 27°C in brain heart infusion (BHI) medium (Becton, Dickinson and Co, Sparks, USA) supplemented with 10 μg mL−1 haemin (AppliChem, Darmstadt, Germany) (Lukeš et al., Reference Lukeš, Paris, Regmi, Breitling, Mureev, Kushnir, Pyatkov, Jirků and Alexandrov2006). Herpetomonas tarakana (strain OSR18) was cultivated at 27°C in the complete M199 medium (Sigma-Aldrich, St. Louis, USA) supplemented with 2 μg mL−1 haemin, 10% foetal bovine serum (FBS, Biosera, Kansas City, USA), 100 U mL−1 penicillin, 100 μg mL−1 streptomycin (Sigma-Aldrich), 2 μg mL−1 biopterin (Sigma-Aldrich) and 25 mm HEPES (AppliChem). Blechomonas ayalai (strain B08-376), K. sorsogonicus (strain MF08-01), L. seymouri (strain ATCC30220), N. esmeraldas (strain E262.01), S. podlipaevi (strain CER3) and W. raviniae (strain Mbr-04) were cultured at 23°C in BHI medium supplemented with 10 μg mL−1 haemin, 10% FBS, 100 U mL−1 penicillin, 100 μg mL−1 streptomycin.
Preparation of mitochondrial lysate
The mitochondria-enriched fractions from 5 × 108 cells were isolated by hypotonic lysis as described elsewhere (Horváth et al., Reference Horváth, Horáková, Dunajčíková, Verner, Pravdová, Šlapetová, Cuninková and Lukeš2005). Mitochondria were re-suspended in 0.5 m aminocaproic acid and 2% (w/v) dodecyl maltoside (both AppliChem). Lysis was performed for 1 h on ice and the lysates were centrifuged for 10 min at 20 000 × g at 4°C. The supernatants were recovered, and protein concentration was determined by the Bradford assay (Bradford, Reference Bradford1976).
In silico analyses
The genome of T. brucei [available from the TriTrypDB (Aslett et al., Reference Aslett, Aurrecoechea, Berriman, Brestelli, Brunk, Carrington, Depledge, Fischer, Gajria, Gao, Gardner, Gingle, Grant, Harb, Heiges, Hertz-Fowler, Houston, Innamorato, Iodice, Kissinger, Kraemer, Li, Logan, Miller, Mitra, Myler, Nayak, Pennington, Phan, Pinney, Ramasamy, Rogers, Roos, Ross, Sivam, Smith, Srinivasamoorthy, Stoeckert, Subramanian, Thibodeau, Tivey, Treatman, Velarde and Wang2010)] was used as a template to search for genes of nucleus-encoded complex I subunits and NDH2 in other trypanosomatid genomes – B. ayalai (Opperdoes et al., Reference Opperdoes, Butenko, Flegontov, Yurchenko and Lukeš2016), L. seymouri (Kraeva et al., Reference Kraeva, Butenko, Hlaváčová, Kostygov, Myškova, Grybchuk, Leštinová, Votýpka, Volf, Opperdoes, Flegontov, Lukeš and Yurchenko2015), N. esmeraldas (manuscript in preparation) and W. raviniae (manuscript in preparation) – using BLAST v.2.6.0+ (Camacho et al., Reference Camacho, Coulouris, Avagyan, Ma, Papadopoulos, Bealer and Madden2009).
NADH dehydrogenase activity assay
NADH dehydrogenase activity was measured in 1 mL NDH buffer (50 mm potassium phosphate buffer, pH 7.5, 1 mm EDTA, 0.2 mm KCN), containing 20–30 μg proteins from the mitochondrial lysates and 5 μL of 20 mm NADH (AppliChem). After the addition of 2 μL 10 mm coenzyme Q2 (Sigma-Aldrich), the change in absorbance at 340 nm was followed for 3 min (Čermáková et al., Reference Čermáková, Kovalinka, Ferenczyová and Horváth2019). A unit of activity was defined as the amount of enzyme that catalyses the oxidation of 1 nmol NADH per min, assuming an extinction coefficient of 6.2 L mmol−1 cm−1 (Gonzalez-Halphen and Maslov, Reference Gonzalez-Halphen and Maslov2004). Solutions of the inhibitors were freshly prepared. Capsaicin (Sigma-Aldrich) was dissolved in ethanol, rotenone (Serva, Heidelberg, Germany) and DPI (diphenyl iodonium, Sigma-Aldrich) – in dimethylsulphoxide and methanol, respectively. Rotenone and DPI were added to the assay mixture immediately before the start of the reaction, capsaicin was pre-incubated for 3 min. Native electrophoresis and in-gel activity staining methods were adapted from Zerbetto et al. (Reference Zerbetto, Vergani and Dabbeni-Sala1997) and Wittig et al. (Reference Wittig, Karas and Schagger2007) and performed as described previously (Verner et al., Reference Verner, Čermáková, Škodová, Kováčová, Lukeš and Horváth2014).
In-gel digestion and mass spectrometry analysis
Procedure was performed as previously described (Shevchenko et al., Reference Shevchenko, Tomas, Havlis, Olsen and Mann2006). Briefly, proteins were separated by native gradient gel, bands of interest were cut into small pieces and incubated in 100 mm ammonium bicarbonate buffer. The samples were reduced in 10 mm DTT (30 min, 56°C) and dehydrated in acetonitrile. Alkylation reaction was performed in the presence of 15 mm iodoacetamide (20 min, room temperature, dark) and samples were dehydrated as described above. For protein digestion, 500 ng of the sequencing grade trypsin (Promega, Madison, USA) and 1 mm CaCl2 were added and the samples were incubated on ice for 30 min (if digestion was incomplete, the reaction was incubated overnight at 37°C). Digested peptides were eluted with acetonitrile and dried in SpeedVac (Thermo Fisher Scientific, Waltham, USA).
For liquid chromatography-mass spectroscopy (LC-MS) analysis, the set of a Nano-trap column (Acclaim PepMap100 C18, 75 μm × 20 mm) and Nano-separation column (Acclaim PepMap C18, 75 μm × 500 mm, both Dionex, Sunnyvale, USA/Thermo Fisher Scientific) attached to the UltiMate 3000 RSLCnano system (Dionex) was used. The peptides were separated for 120 min in a 3–43% gradient of buffer B with two mobile phases used: 0.1% formic acid (v/v) (buffer A) and 80% acetonitrile (v/v) with 0.1% formic acid (buffer B). Spectral data were collected by using the Orbitrap Elite mass spectrometer (Thermo Fisher Scientific) operating in the data-dependent mode using the Top15 strategy for the selection of precursor ions for the HCD fragmentation (Michalski et al., Reference Michalski, Damoc, Lange, Denisov, Nolting, Muller, Viner, Schwartz, Remes, Belford, Dunyach, Cox, Horning, Mann and Makarov2012). Obtained datasets were processed by MaxQuant v.1.5.3.30 with built-in Andromeda search engine (Cox et al., Reference Cox, Neuhauser, Michalski, Scheltema, Olsen and Mann2011). The specific parameters for searching were: carbamidomethylation (C) as permanent modification and oxidation (M) and acetyl (protein N-terminus) as variable modifications. The search was performed against protein datasets of Phytomonas sp. (Hart1), T. brucei (TREU927), T. brucei (Lister 427), L. major (Friedlin) (TriTrypDB, downloaded 10.10.2020) and against a sequence database of U insertion/deletion editing in kinetoplastid mitochondria (Simpson et al., Reference Simpson, Wang, Thiemann, Alfonzo, Maslov and Avila1998).
Results
We have characterized NADH dehydrogenase activity in P. serpens and seven monoxenous trypanosomatids. We selected species from different clades of Trypanosomatidae (Lukeš et al., Reference Lukeš, Butenko, Hashimi, Maslov, Votýpka and Yurchenko2018). These are the members of the subfamilies Leishmaniinae (Kostygov and Yurchenko, Reference Kostygov and Yurchenko2017) (L. seymouri and N. esmeraldas), Strigomonadinae (Votýpka et al., Reference Votýpka, Kostygov, Kraeva, Grybchuk-Ieremenko, Tesařová, Grybchuk, Lukeš and Yurchenko2014) (K. sorsogonicus), Phytomonadinae (Yurchenko et al., Reference Yurchenko, Kostygov, Havlová, Grybchuk-Ieremenko, Ševčíková, Lukeš, Ševčík and Votýpka2016) (H. tarakana), Blechomonadinae (Votýpka et al., Reference Votýpka, Suková, Kraeva, Ishemgulova, Duží, Lukeš and Yurchenko2013) (B. ayalai), as well as two genera not formally classified into any subfamily – Sergeia (Svobodová et al., Reference Svobodová, Zídková, Čepička, Oborník, Lukeš and Votýpka2007) and Wallacemonas (Kostygov et al., Reference Kostygov, Grybchuk-Ieremenko, Malysheva, Frolov and Yurchenko2014). These species differ not only in host specificity (Dictyoptera, Diptera, Heteroptera or Siphonaptera), but also geographical distribution and particulars of their life cycle. Novymonas esmeraldas and K. sorsogonicus harbour endosymbiotic bacteria, which have been acquired by host species independently in evolution (Kostygov et al., Reference Kostygov, Butenko, Nenarokova, Tashyreva, Flegontov, Lukeš and Yurchenko2017; Silva et al., Reference Silva, Kostygov, Spodareva, Butenko, Tossou, Lukes, Yurchenko and Alves2018), while L. seymouri and B. ayalai are heavily infected with dsRNA viruses (Grybchuk et al., Reference Grybchuk, Akopyants, Kostygov, Konovalovas, Lye, Dobson, Zangger, Fasel, Butenko, Frolov, Votýpka, d'Avila-Levy, Kulich, Moravcová, Plevka, Rogozin, Serva, Lukeš, Beverley and Yurchenko2018a, Reference Grybchuk, Kostygov, Macedo, Votypka, Lukes and Yurchenko2018b).
In silico analyses
We examined the presence of 19 core subunits of both the membrane and peripheral domains of the complex I, whose human orthologues were identified in T. brucei (Duarte and Tomás, Reference Duarte and Tomás2014), and an alternative pathway enzyme, NDH2, in analysed species of trypanosomatids. The genomic data were available only for four species. The genomes of B. ayalai and L. seymouri are in TriTrypDB and two genomes were sequenced by us: N. esmeraldas (32 Mbp; N50 197 811 bp; 1422 scaffolds) and W. raviniae (27 Mbp; N50 58 925; 1386 scaffolds) (both unpublished data). The correspondent sequences of T. brucei TREU927 from the TriTrypDB (Aslett et al., Reference Aslett, Aurrecoechea, Berriman, Brestelli, Brunk, Carrington, Depledge, Fischer, Gajria, Gao, Gardner, Gingle, Grant, Harb, Heiges, Hertz-Fowler, Houston, Innamorato, Iodice, Kissinger, Kraemer, Li, Logan, Miller, Mitra, Myler, Nayak, Pennington, Phan, Pinney, Ramasamy, Rogers, Roos, Ross, Sivam, Smith, Srinivasamoorthy, Stoeckert, Subramanian, Thibodeau, Tivey, Treatman, Velarde and Wang2010) were used as queries to search the N. esmeraldas and W. raviniae assemblies with TBLASTN+ v.2.6.0 (Camacho et al., Reference Camacho, Coulouris, Avagyan, Ma, Papadopoulos, Bealer and Madden2009) using a threshold of 10−50. The obtained hits were reciprocally BLASTed against the NCBI database. In all the cases, the genes of interest were located in syntenic genomic positions. All tested genes were detected in the genomes of all analysed trypanosomatids (Table 1 and Supplementary Table 1). Of note, multiple copies for genes encoding subunits NDUFA8, NDUFB10 and NDUFA12 were documented in the genome of W. raviniae.
Table 1. In silico analysis of the selected complex I genes and alternative dehydrogenase NDH2 encoded by nuclear DNA
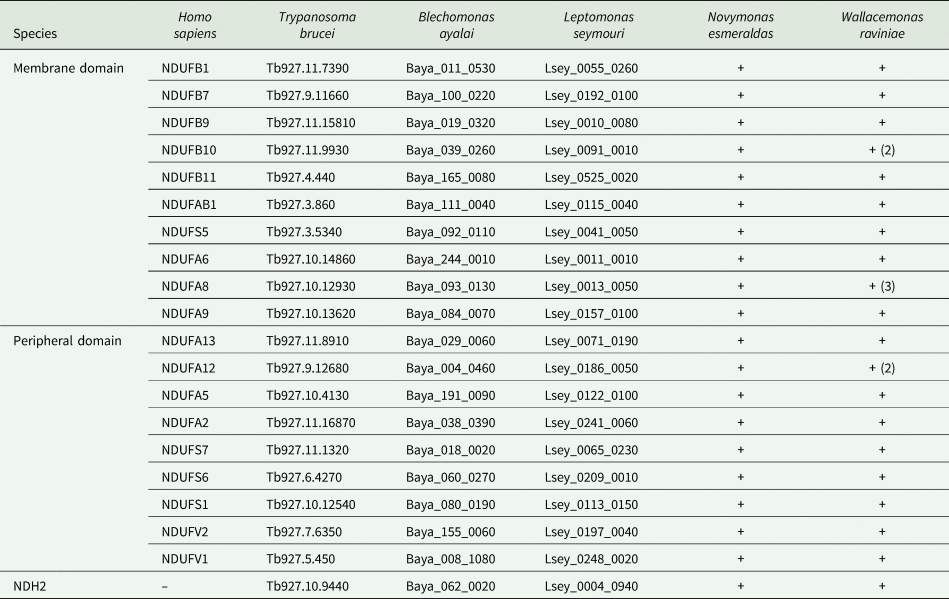
All selected genes were detected in all analysed trypanosomatid genomes. The table lists either the names of genes in the TriTrypDB that was used for T. brucei, B. ayalai and L. seymouri or the ‘+’ sign indicating the presence in unannotated databases for N. esmeraldas and W. raviniae. All genes were found in one copy, except for a few genes of W. raviniae, for which a higher copy number is given in parentheses. Names of H. sapiens orthologues are also provided.
NADH dehydrogenase activity
Mitochondrial proteins of the studied strains were separated in 2–12% clear native gradient gel and NADH dehydrogenase activity was detected by in-gel staining (Fig. 1A). In the high molecular weight range, we detected NADH dehydrogenase activity in all species tested. However, the intensity and number of active bands differed significantly. We also noticed significant differences when comparing two different types of native electrophoresis – clear native (Fig. 1A) and blue native (Fig. 1C). NADH dehydrogenase activity in the low molecular weight range was observed for K. sorsogonicus and N. esmeraldas. Its molecular weight around 130 kDa could correspond to the NDH2 dimer. For distinguishing different NADH dehydrogenase activities we performed in-gel staining in the presence of 100 μ m DPI (Fig. 1B), which inhibits NDH2 and incomplete complex I (Čermáková et al., Reference Čermáková, Verner, Man, Lukeš and Horváth2007). In the case of analysed trypanosomatids, DPI has inhibited most of the signals – strong bands remained visible only in the samples of P. serpens, N. esmeraldas and S. podlipaevi. This suggests that DPI-resistant activity in N. esmeraldas and S. podlipaevi corresponds to the complex I, as was previously shown in P. serpens (Čermáková et al., Reference Čermáková, Verner, Man, Lukeš and Horváth2007).
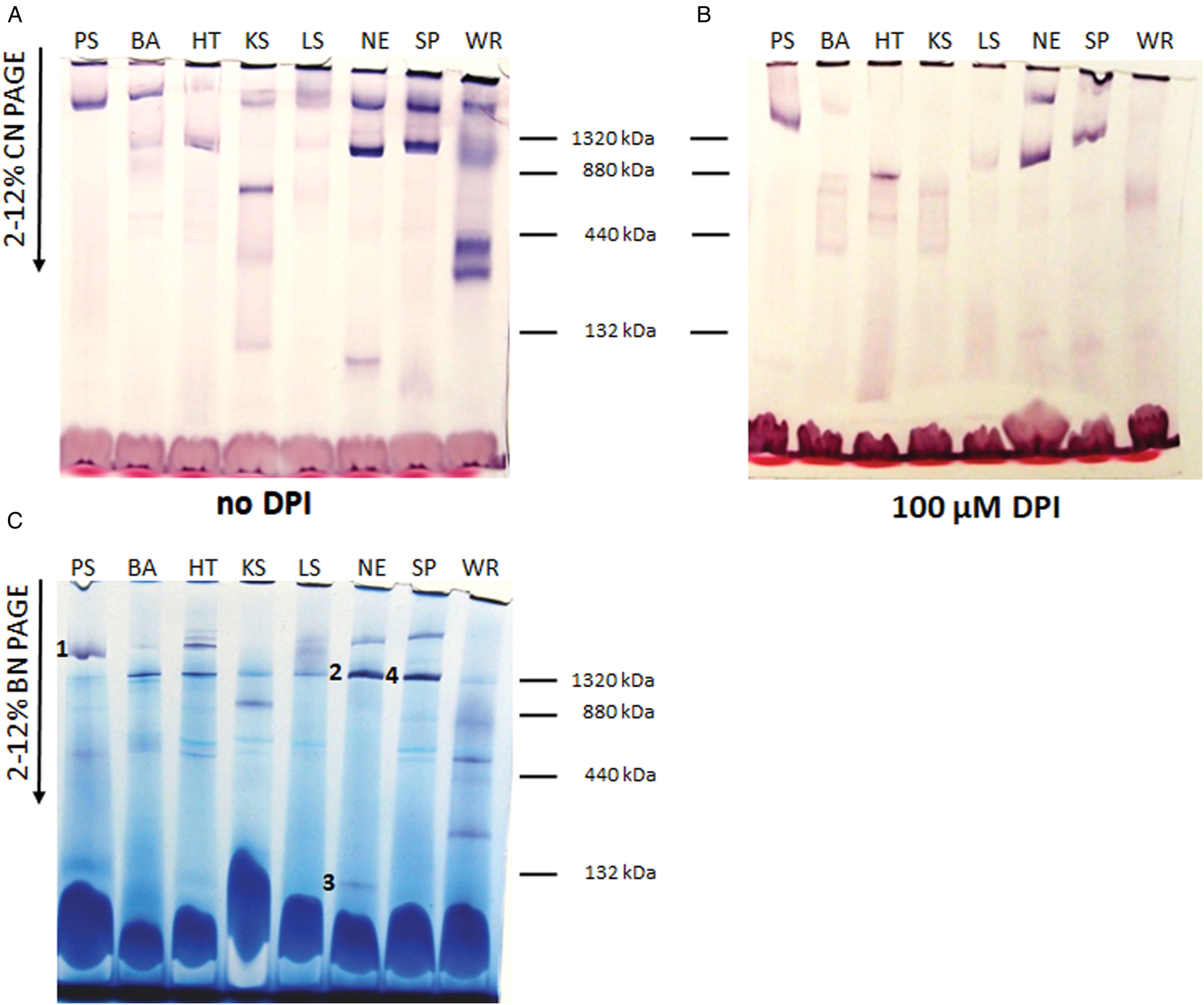
Fig. 1. In-gel NADH dehydrogenase activity staining. (A, B) Clear native and (C) blue native gradient gel; 100 μg of mitochondrial proteins from Phytomonas serpens (PS), Blechomonas ayalai (BA), Herpetomonas tarakana (HT), Kentomonas sorsogonicus (KS), Leptomonas seymouri (LS), Novymonas esmeraldas (NE), Sergeia podlipaevi (SP) and Wallacemonas raviniae (WR) were applied to each lane. The NADH dehydrogenase activity was detected without (A, C) or with (B) 100 μ m DPI. The slices with NADH dehydrogenase activity from blue native gel (C) subjected to MS analysis are marked by numbers 1–4. The positions of molecular weight markers (dimer of BSA and monomer, dimer and trimer of ferritin) are indicated.
NADH dehydrogenase activity was spectrophotometrically measured in four trypanosomatid species (selected based on either the strongest intensity of in-gel staining signal or the presence of activity in low molecular weight range – N. esmeraldas, S. podlipaevi, W. raviniae and K. sorsogonicus) in the absence or presence of specific inhibitors of the eukaryotic complex I (rotenone and capsaicin) and DPI. Our data demonstrated that contribution of the complex I and NDH2 is about equal in P. serpens (Table 2). Although the inhibitory effect of rotenone and capsaicin was comparable in this species, in all other trypanosomatids rotenone did not inhibit NADH dehydrogenase activity. In addition to P. serpens, capsaicin inhibited NADH dehydrogenase activity in N. esmeraldas and S. podlipaevi. A comparable degree of inhibition by capsaicin and DPI in all three species implies the presence of both the functional complex I and the alternative NDH2. Kentomonas sorsogonicus and W. raviniae were not sensitive to capsaicin, while sensitive to DPI, which inhibited over 80% NADH dehydrogenase activity in the W. raviniae and blocked it completely in K. sorsogonicus (Table 2). These results correlate with DPI sensitivity of NADH dehydrogenase determined in the gel (Fig. 1B) and do not indicate the presence of a fully functional complex I in the tested life stage of both W. raviniae and K. sorsogonicus.
Table 2. Specific NADH dehydrogenase activity with and without inhibitors

NADH dehydrogenase activity was measured in the mitochondrial lysates of P. serpens, K. sorsogonicus, N. esmeraldas, S. podlipaevi and W. raviniae in the absence or presence of 10 μ m rotenone, 300 μ m capsaicin and 100 μ m DPI. Average values and s.d. of activities and their inhibition (in %) from at least three independent biological replicated (each measured in triplicates) are presented. One unit (U) of NADH dehydrogenase activity catalyses the oxidation of 1 nmol NADH per minute. Specific activity is calculated as U mg−1 of mitochondrial proteins.
Protein composition of the NADH dehydrogenase complex
Four native gel's strips in the high molecular weight range of P. serpens, N. esmeraldas and S. podlipaevi and around 130 kDa of N. esmeraldas (Fig. 1C) were subjected to LC-MS analysis (Supplementary Table 2). Most of the returned hits were hypothetical proteins, yet we were able to identify 29 nuclear-encoded subunits of the P. serpens complex I and 22 and 23 subunits of this complex in N. esmeraldas and S. podlipaevi datasets, respectively (Table 3). All the identified subunits were localized to the complex I modules: the N-module forming the peripheral arm, the Q-module binding the ubiquinone, and ND1, ND4 ND5 modules forming the membrane part of the complex I. The only part, from which no subunit has been identified, is the ND2 module. The acyl-carrier protein NDUFAB1 (that is not part of any module) was also detected (Fig. 2, Table 3). In addition to the subunits orthologues to those in other organisms, we also recognized some additional trypanosomatid-specific complex I subunits (Duarte and Tomás, Reference Duarte and Tomás2014) and a few other proteins that are annotated in the TriTrypDB as NADH dehydrogenase subunits without detailed specification. In addition to the nuclear DNA-encoded complex I subunits, we have also detected several proteins encoded in mitochondrial DNA: ND8, ND7 and ND1. To the best of our knowledge, this is the first experimental detection of mitochondrial DNA-encoded subunits of the complex I at the protein level in trypanosomatids. Moreover, our analysis revealed the presence of the MURF2 (mitochondrial protein with unknown function) in a high molecular weight signals range of NADH dehydrogenase activity. Its detection in all three examined species suggests that this protein may be a part of the trypanosomatids complex I.
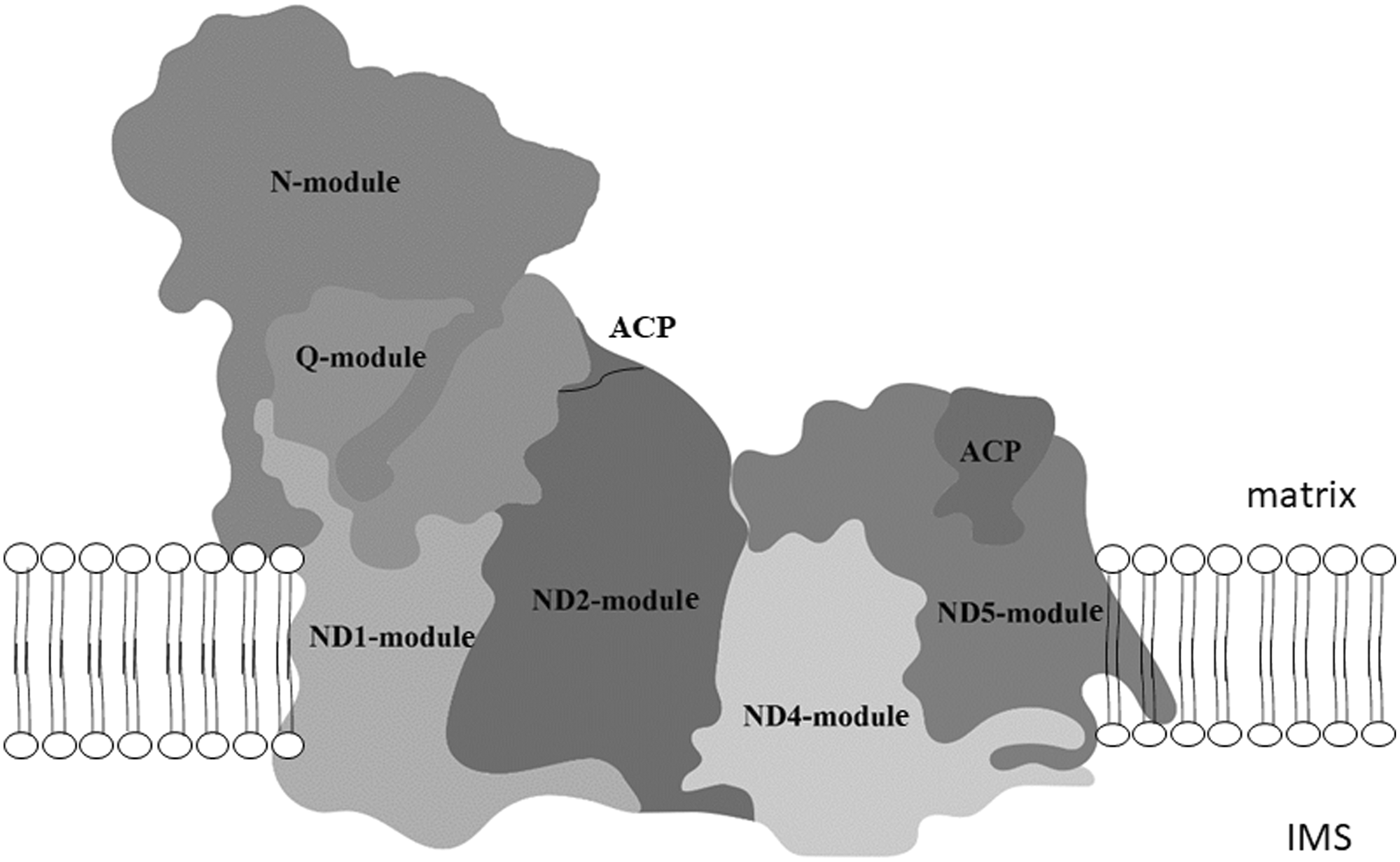
Fig. 2. Modular composition of the complex I. The different modules: N-module, Q-module, P- module (composed of ND1, ND2, ND4 and ND5) and acyl carrier protein (ACP) are shown superimposing the structure of bovine complex I. The matrix and intermembrane space (IMS) site of inner mitochondrial membrane are indicated. Adapted from Stroud et al. (Reference Stroud, Surgenor, Formosa, Reljic, Frazier, Dibley, Osellame, Stait, Beilharz, Thorburn, Salim and Ryan2016).
Table 3. Subunits of mitochondrial complex I detected by mass spectrometry analysis
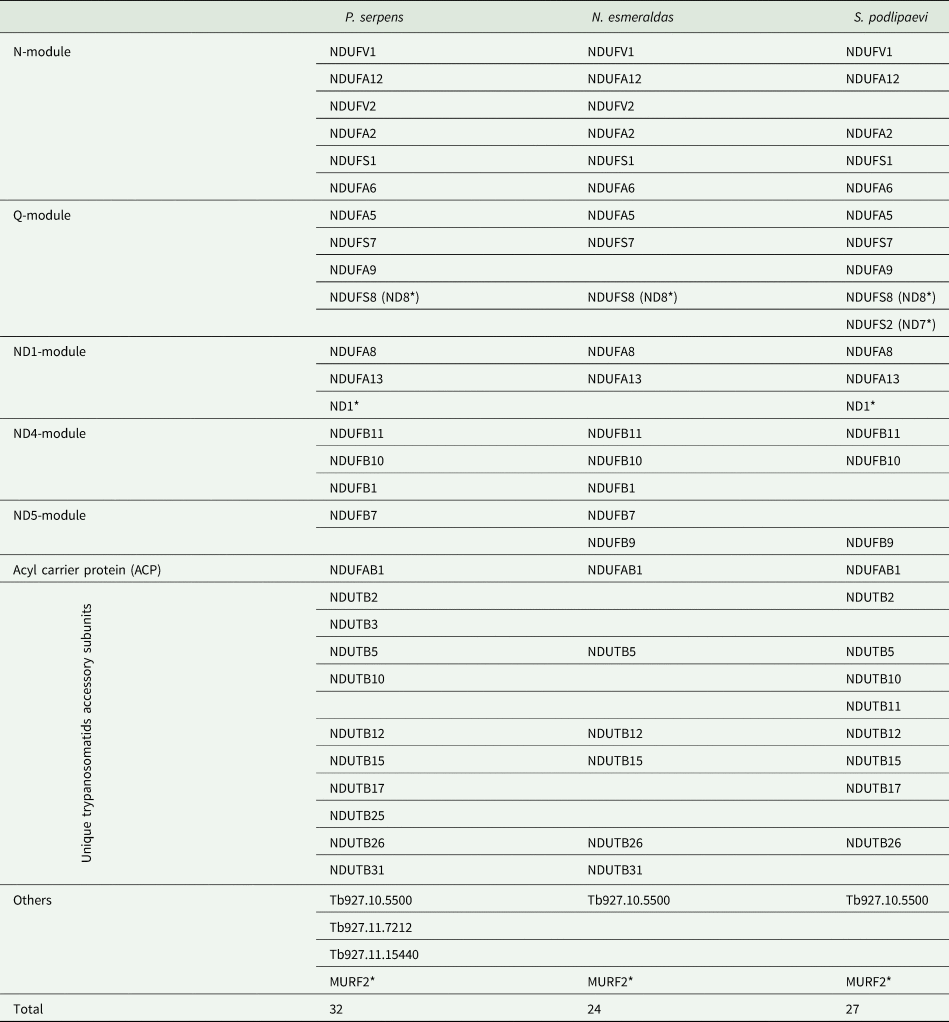
Distribution of identified subunits to the modules of complex I is indicated in the left column. Designation of H. sapiens subunits in modules and ACP rows and T. brucei subunits in other rows were used. Subunits encoded by mitochondrial DNA are marked with * (ND1, ND7, ND8 and MURF2).
In addition to the complex I proteins, we also identified nine subunits of the ATP synthase, both alternative oxidases (orthologues of Tb927.10.7090 and Tb927.10.9760) and one component of the 2-oxoglutarate dehydrogenase complex (orthologue of Tb927.11.16730) in P. serpens; eight subunits of the ATP synthase, six subunits of the cytochrome c oxidase including mitochondrial DNA-encoded COII and COIII, three subunits of the cytochrome c reductase including apocytochrome b and two subunits of the succinate dehydrogenase in S. podlipaevi; 18 subunits of the ATP synthase including mitochondrial DNA-encoded A6, six subunits of the cytochrome c oxidase, two subunits of the cytochrome c reductase and ten subunits of the succinate dehydrogenase, NDH2 and two components (E2 and E3) of the 2-oxoglutarate dehydrogenase complex in N. esmeraldas (Supplementary Table 2).
Discussion
As was already mentioned above long-term cultivated trypanosomatids L. tarentolae, and C. fasciculata have lost ability to edit some of complex I subunits and do not possess active form of this enzyme (Sloof et al., Reference Sloof, Arts, van den Burg, van der Spek and Benne1994; Thiemann et al., Reference Thiemann, Maslov and Simpson1994). It was shown for T. brucei that complex I is not essential for its bloodstream life form (Surve et al., Reference Surve, Heestand, Panicucci, Schnaufer and Parsons2012, Reference Surve, Jensen, Heestand, Mazet, Smith, Bringaud, Parsons and Schnaufer2017). Complex I contributes up to 20% of the electron flux of the respiratory chain in the procyclic form of T. brucei but it is also not essential and does not pump protons across the inner mitochondrial membrane (Verner et al., Reference Verner, Čermáková, Škodová, Kriegová, Horváth and Lukeš2011). The main pathways of the electrons entry into the respiratory chain appear to be the complex II (Turrens, Reference Turrens1989; Denicola-Seoane et al., Reference Denicola-Seoane, Rubbo, Prodanov and Turrens1992) and/or the alternative enzyme NDH2 (Verner et al., Reference Verner, Škodová, Poláková, Ďurišová-Benkovičová, Horváth and Lukeš2013). Although some authors conclude that NDH2 is matrix-oriented (Surve et al., Reference Surve, Jensen, Heestand, Mazet, Smith, Bringaud, Parsons and Schnaufer2017), our previous results strongly suggest that NDH2 is oriented into the intermembrane space and therefore cannot regenerate NAD+ in the matrix (Verner et al., Reference Verner, Škodová, Poláková, Ďurišová-Benkovičová, Horváth and Lukeš2013). Within the mitochondria, mitochondrial NADH-dependent fumarate reductase, which converts fumarate to succinate, utilized by complex II, may have this function (Coustou et al., Reference Coustou, Besteiro, Rivière, Biran, Biteau, Franconi, Boshart, Baltz and Bringaud2005). The only known exception to date was P. serpens, in which the complex I was demonstrated to be not only fully functional, but also the only proton pump in the respiratory chain (Nawathean and Maslov, Reference Nawathean and Maslov2000; Gonzalez-Halphen and Maslov, Reference Gonzalez-Halphen and Maslov2004; Čermáková et al., Reference Čermáková, Verner, Man, Lukeš and Horváth2007). Our in silico analysis confirmed the presence of the genes encoding the complex I subunits and the alternative dehydrogenase NDH2 in the genomes of all analysed species (B. ayalai, L. seymouri, N. esmeraldas and W. raviniae). Most genes are present only in one copy, with the exception of W. raviniae, where some subunits are encoded by several genes. However, the mere presence of the genes encoding the complex I subunits is not equal to the functional enzymatic activity. Leishmania tarentolae and C. fasciculata, for example, also possess all the complex I subunit genes in their genomes, and yet their enzymes are not active because the subunits encoded by mitochondrial DNA are not edited (Sloof et al., Reference Sloof, Arts, van den Burg, van der Spek and Benne1994; Thiemann et al., Reference Thiemann, Maslov and Simpson1994). Procyclic form of T. brucei has essentially no direct contribution of complex I to the mitochondrial membrane potential (Verner et al., Reference Verner, Čermáková, Škodová, Kriegová, Horváth and Lukeš2011).
Significant differences in NADH dehydrogenase activity within the examined trypanosomatids confirm the statement that complex I is the most controversial enzyme of these parasites (Opperdoes and Michels, Reference Opperdoes and Michels2008; Duarte and Tomás, Reference Duarte and Tomás2014). Regardless of the strong intensity of some bands, most of them were sensitive to 100 μ m DPI, similarly to the case of T. brucei (Verner et al., Reference Verner, Čermáková, Škodová, Kriegová, Horváth and Lukeš2011, Reference Verner, Čermáková, Škodová, Kováčová, Lukeš and Horváth2014). In addition to the expected resistance to DPI of the 2.2 MDa complex of P. serpens (Čermáková et al., Reference Čermáková, Verner, Man, Lukeš and Horváth2007), we documented a similar phenomenon only in N. esmeraldas and S. podlipaevi. However, in contrast to P. serpens, these species have also the DPI-resistant activity in the range of about 1.3 MDa (Fig. 1B). It appears that this lower molecular weight complex is even more stable under conditions of native electrophoresis, as its activity was shown to be slightly stronger than that of the upper band under clear native conditions (Fig. 1A) and much stronger in the blue native gel (Fig. 1C). It also differs from the lower P. serpens bands (~600 kDa, DPI-sensitive) in our previous studies, which were suggested to be incomplete forms of the complex I (Čermáková et al., Reference Čermáková, Verner, Man, Lukeš and Horváth2007; Verner et al., Reference Verner, Čermáková, Škodová, Kováčová, Lukeš and Horváth2014).
It has been suggested that the 2-oxoglutarate dehydrogenase complex may be responsible for the detected NADH dehydrogenase activity in T. brucei, as up to four proteins of this enzyme were localized to the activity band (Panigrahi et al., Reference Panigrahi, Ziková, Dalley, Acestor, Ogata, Anupama, Myler and Stuart2008; Acestor et al., Reference Acestor, Zíková, Dalley, Anupama, Panigrahi and Stuart2011). Our analysis revealed only one 2-oxoglutarate dehydrogenase subunit in P. serpens, two in N. esmeraldas and none in S. podlipaevi together with the complex I subunits. Therefore, we concluded that 2-oxoglutarate dehydrogenase does not contribute to the NADH dehydrogenase activity in the bands that we have analysed.
In this study, we detected a NADH dehydrogenase signal in the low molecular weight range (around 130 kDa) for the first time in trypanosomatids (K. sorsogonicus and N. esmeraldas). In the yeast Yarrowia lipolytica, the signal in the corresponding range comes from an alternative dehydrogenase (Čermáková et al., Reference Čermáková, Verner, Man, Lukeš and Horváth2007). Our results confirm that this is also the case of N. esmeraldas, as we have detected the NDH2 protein in this area by LC-MS analysis. Interestingly, we have also identified NDH2 in the high molecular range along with the complex I subunits in this species. This could suggest that NDH2 functions in association with other proteins. Nevertheless, we revealed it with the complex I only in N. esmeraldas, but not in P. serpens or S. podlipaevi. We explain this discrepancy by either species-specific peculiarities, transient nature of this protein complex, or inconsistencies in databases used for downstream analysis. For example, we used proteome of the exact species N. esmeraldas for Novymonas but had to rely on data from P. serpens isolate Hart1 for the analysis of our model strain, 9T.
Spectrophotometric measurement of enzyme activities is more accurate and quantifiable than in-gel staining. Among the analysed trypanosomatids, sensitivity of the complex I activity to the low and high concentrations of rotenone has been previously documented only for P. serpens (Moyses and Barrabin, Reference Moyses and Barrabin2004; Čermáková et al., Reference Čermáková, Verner, Man, Lukeš and Horváth2007) and T. brucei (Beattie and Howton, Reference Beattie and Howton1996; Fang et al., Reference Fang, Wang and Beattie2001), respectively. However, high concentrations of this inhibitor were shown to evoke non-specific effects (Hernandez and Turrens, Reference Hernandez and Turrens1998). It was later demonstrated that lower rotenone concentrations do not affect the NADH dehydrogenase activity of procyclic T. brucei, probably because the complex I is incomplete in this organism (Verner et al., Reference Verner, Čermáková, Škodová, Kriegová, Horváth and Lukeš2011, Reference Verner, Čermáková, Škodová, Kováčová, Lukeš and Horváth2014). In our experiments, rotenone inhibited the NADH dehydrogenase only in P. serpens. This can imply that none of the tested trypanosomatids have the P. serpens-like complex I. However, our experiments with capsaicin (which is another specific inhibitor of the complex I) led a different conclusion. The effect of capsaicin on NADH dehydrogenase activity in P. serpens was comparable to that of rotenone and inversely proportionally correlated with the effect of DPI in four other investigated species. Capsaicin was not effective in K. sorsogonicus and W. raviniae, while DPI inhibited their NADH dehydrogenase activity by 80% or more. The effects of DPI and capsaicin were similar in N. esmeraldas, S. podlipaevi and P. serpens. The resistance to rotenone in N. esmeraldas and S. podlipaevi may be explained by possible amino acid substitutions in NDUFS2, as has been described in other organisms, i.e. a substitution Tyr144Phe leads to 4× lower sensitivity to rotenone in Y. lipolytica (Tocilescu et al., Reference Tocilescu, Fendel, Zwicker, Drose, Kerscher and Brandt2010; Angerer et al., Reference Angerer, Nasiri, Niedergesass, Kerscher, Schwalbe and Brandt2012). Taken together, our data strongly indicate the presence of a fully functional complex I in N. esmeraldas and S. podlipaevi.
MS analysis of the high molecular weight NADH dehydrogenase activity bands in P. serpens identified 32 subunits of the complex I (29 nuclear and 3 mitochondrial DNA-encoded) (Table 3). The total number of identified subunits is much closer to that of Bos taurus (45 subunits) (Carroll et al., Reference Carroll, Fearnley, Skehel, Shannon, Hirst and Walker2006) or Y. lipolytica (42 subunits) (Abdrakhmanova et al., Reference Abdrakhmanova, Zickermann, Bostina, Radermacher, Schagger, Kerscher and Brandt2004) than to over 60 predicted subunits for trypanosomatids (Duarte and Tomás, Reference Duarte and Tomás2014). Nevertheless, the complex I of Y. lipolytica migrates at about 880 kDa, which differs from the migration at over 2 MDa for P. serpens (Čermáková et al., Reference Čermáková, Verner, Man, Lukeš and Horváth2007) and 1.3 MDa for N. esmeraldas and S. podlipaevi (this study). This can be explained by a higher number of the involved complex I subunits in trypanosomatids, or their significantly higher molecular weight. For example, the NDUFA6 subunit in most eukaryotes is about 15 kDa, whereas its predicted size in trypanosomatids varies from 77 to 83 kDa (Čermáková et al., Reference Čermáková, Verner, Man, Lukeš and Horváth2007).
There could be several reasons why we did not detect all the complex I proteins in our analysis: (i) we used protein databases of the related species; (ii) we could not identify unique subunits, similarly to the case of trCOIV subunit of the complex IV (Maslov et al., Reference Maslov, Zíková, Kyselová and Lukeš2002; Perez et al., Reference Perez, Lapaille, Degand, Cilibrasi, Villavicencio-Queijeiro, Morsomme, Gonzalez-Halphen, Field, Remacle, Baurain and Cardol2014) and (iii) some predicted proteins were too short (Duarte and Tomás, Reference Duarte and Tomás2014) or hydrophobic. A smaller number of subunits identified in N. esmeraldas and S. podlipaevi samples reflects the lower molecular weight form used for the MS analysis. This complex may be depleted of some weaker-bound subunits.
Importantly, we also detected several proteins of complex I encoded by mitochondrial DNA. This is the first experimental evidence for their existence in trypanosomatids. So far, only subunits of the complexes III, IV and V have been detected (Horváth et al., Reference Horváth, Berry and Maslov2000a, Reference Horváth, Kingan and Maslov2000b, Reference Horváth, Nebohacova, Lukeš and Maslov2002; Acestor et al., Reference Acestor, Zíková, Dalley, Anupama, Panigrahi and Stuart2011; Škodová-Sveráková et al., Reference Škodová-Sveráková, Horváth and Maslov2015a). We identified the ND8 subunit in three analysed species, ND1 in two and ND7 only in S. podlipaevi. We also detected the MURF2 – a mitochondrial protein of unknown function (Blum and Simpson, Reference Blum and Simpson1990). Its co-occurrence with other subunits of the complex I in all analysed species strongly suggests that it could be another subunit of this enzyme.
Comparison of bioenergetic metabolism in several trypanosomatid species suggests that these parasites have retained all the essential genes during evolution. Their expression depends on the specific living conditions – the availability of food and host–parasite relationships (Škodová-Sveráková et al., Reference Škodová-Sveráková, Verner, Skalický, Votýpka, Horváth and Lukeš2015b). Data obtained in this study indicate that the same rules apply to the complex I. Its loss is not only induced by the prolonged cultivation in vitro, but also may be influenced by natural conditions in different trypanosomatid species.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182020002425
Acknowledgements
We thank Dr Jan Votýpka (Charles University, Prague) for providing cultures of S. podlipaevi and W. raviniae, and Dr Kristína Záhonová (Institute of Parasitology, České Budějovice and Charles University, Prague) for help in analysis of unannotated genomes of N. esmeraldas and W. raviniae and Katarína Behinská (Comenius University, Bratislava) for participating in the measurement of the inhibitory effect of capsaicin.
Financial support
This study was supported by the Scientific Grant Agency of the Slovak Ministry of Education and the Academy of Sciences (1/0387/17), Slovak Research and Development Agency grants (APVV-0286-12 and APVV-15-0654) to AH, Grant Agency of Czech Republic (20-07186S) and the European Regional Funds (project ‘Centre for Research of Pathogenicity and Virulence of Parasites’ CZ.02.1.01/16_019/0000759) to VY, and by grant ITMS 26240220086 supported by the Research & Development Operational Programme funded by the ERDF. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Ethical standards
Not applicable.








