Article contents
Differential drivers of intraspecific and interspecific competition during malaria–helminth co-infection
Published online by Cambridge University Press: 11 May 2021
Abstract
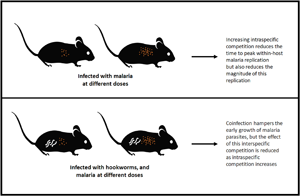
Various host and parasite factors interact to determine the outcome of infection. We investigated the effects of two factors on the within-host dynamics of malaria in mice: initial infectious dose and co-infection with a helminth that limits the availability of red blood cells (RBCs). Using a statistical, time-series approach to model the within-host ‘epidemiology’ of malaria, we found that increasing initial dose reduced the time to peak cell-to-cell parasite propagation, but also reduced its magnitude, while helminth co-infection delayed peak cell-to-cell propagation, except at the highest malaria doses. Using a mechanistic model of within-host infection dynamics, we identified dose-dependence in parameters describing host responses to malaria infection and uncovered a plausible explanation of the observed differences in single vs co-infections. Specifically, in co-infections, our model predicted a higher background death rate of RBCs. However, at the highest dose, when intraspecific competition between malaria parasites would be highest, these effects of co-infection were not observed. Such interactions between initial dose and co-infection, although difficult to predict a priori, are key to understanding variation in the severity of disease experienced by hosts and could inform studies of malaria transmission dynamics in nature, where co-infection and low doses are the norm.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press
References
- 3
- Cited by



