Introduction
Protozoa are common inhabitants of the mammalian gut and an integral part of the mammalian gut microbiome (Filyk and Osborne, Reference Filyk and Osborne2016; del Campo et al., Reference del Campo, Bass and Keeling2020), but are often overlooked in host-microbiome studies in favour of prokaryotic taxa (Laforest-Lapointe and Arrieta, Reference Laforest-Lapointe and Arrieta2018). The protozoa of the mammalian gut can be arranged in 5 meta-groups: the Amoebozoa (e.g. Entamoeba, Endolimax), Apicomplexa (e.g. Eimeria, Cryptosporidium), Ciliophora (e.g. Balantidium, Entodinium), Metamonada (e.g. Giardia, Trichomonas), and Stramenopiles (e.g. Blastocystis) (Parfrey et al., Reference Parfrey, Walters and Knight2011; Ruggiero et al., Reference Ruggiero, Gordon, Orrell, Bailly, Bourgoin, Brusca, Cavalier-Smith, Guiry and Kirk2015; Adl et al., Reference Adl, Bass, Lane, Lukeš, Schoch, Smirnov, Agatha, Berney, Brown, Burki, Cárdenas, Čepička, Chistyakova, del Campo, Dunthorn, Edvardsen, Eglit, Guillou, Hampl, Heiss, Hoppenrath, James, Karnkowska, Karpov, Kim, Kolisko, Kudryavtsev, Lahr, Lara, Le Gall, Lynn, Mann, Massana, Mitchell, Morrow, Park, Pawlowski, Powell, Richter, Rueckert, Shadwick, Shimano, Spiegel, Torruella, Youssef, Zlatogursky and Zhang2019; Langda et al., Reference Langda, Zhang, Zhang, Gui, Ji, Deji, Cuoji, Wang and Wu2020; Guzzo et al., Reference Guzzo, Andrews and Weyrich2022). Gut protozoa exist across the entire parasitism – mutualism continuum, thus ranging from disease-causing parasites to long-term residents of the gut providing benefits to their host (Lukeš et al., Reference Lukeš, Stensvold, Jirků-Pomajbíková and Parfrey2015; Dubik et al., Reference Dubik, Pilecki and Moeller2022), with them having both direct and indirect effects.
Mutualistic gut protozoa that provide nutritional benefits to their hosts are well-documented in ruminants (Williams et al., Reference Williams, Thomas, McEwan, Rees Stevens, Creevey and Huws2020; Solomon and Jami, Reference Solomon and Jami2021). For example, the protozoa Eudiplodinium maggii and Polyplastron multivesiculatum contribute to enzymatic degradation of plant polysaccharides in sheep (Béra-Maillet et al., Reference Béra-Maillet, Devillard, Cezette, Jouany and Forano2005). Gut protozoa can also positively contribute to host disease resistance (Lukeš et al., Reference Lukeš, Stensvold, Jirků-Pomajbíková and Parfrey2015; Leung et al., Reference Leung, Graham and Knowles2018; Dubik et al., Reference Dubik, Pilecki and Moeller2022). For example, Tritrichomonas musculus indirectly protects host mice against Salmonella infection by inducing inflammasome-driven IL-18 release (Chudnovskiy et al., Reference Chudnovskiy, Mortha, Kana, Kennard, Ramirez, Rahman, Remark, Mogno, Ng, Gnjatic, Amir, Solovyov, Greenbaum, Clemente, Faith, Belkaid, Grigg and Merad2016). Furthermore, Blastocystis subtype 4 can directly induce oxidative stress in the prokaryote Bacteroides vulgatus, decreasing its growth (Deng and Tan, Reference Deng and Tan2022).
Negative interactions between gut protozoa and the host can result in gastrointestinal disease (Huh et al., Reference Huh, Moon and Lim2009). Some, e.g. Giardia and Cryptosporidium, can directly cause disease by damaging and inflaming the gut epithelium (Savioli et al., Reference Savioli, Smith and Thompson2006). Gut protozoa can also indirectly affect host health and disease state by changing the wider species composition of the gut microbiome (Burgess et al., Reference Burgess, Gilchrist, Lynn and Petri2017). For example, the presence of Blastocystis is associated with a lower abundance of beneficial prokaryotes (for example Bifidobacterium) whose presence normally limits infections by potential pathogens (Russell et al., Reference Russell, Ross, Fitzgerald and Stanton2011; Yason et al., Reference Yason, Liang, Png, Zhang and Tan2019; Caudet et al., Reference Caudet, Trelis, Cifre, Soriano, Rico and Merino-Torres2022).
Despite clear examples of parasitic and mutualistic effects of gut protozoa, it can be difficult to categorize species as either beneficial or harmful because their effects on the host can be context-dependent (Parfrey et al., Reference Parfrey, Walters and Knight2011; Lukeš et al., Reference Lukeš, Stensvold, Jirků-Pomajbíková and Parfrey2015; Sardinha-Silva et al., Reference Sardinha-Silva, Alves-Ferreira and Grigg2022). For example, host diet, age, immune status, microbiome, and genotype, as well as protozoa genotype, can all influence the nature and strength of the interaction between a protozoan species and its host (Thompson and Monis, Reference Thompson, Monis, Rollinson and Hay2012; Ryan et al., Reference Ryan, Fayer and Xiao2014; Lepczyńska et al., Reference Lepczyńska, Białkowska, Dzika, Piskorz-Ogórek and Korycińska2017; Dubik et al., Reference Dubik, Pilecki and Moeller2022). For example, Blastocystis can shift from being mutualistic, to becoming pathogenic when the host immune system is compromised (Scanlan et al., Reference Scanlan, Stensvold, Rajilić-Stojanović, Heilig, De Vos, O'Toole and Cotter2014).
Gut protozoa predominately have faecal – oral routes of transmission among hosts, typically through coprophagy or faecal contamination of food and / or water (Dehority, Reference Dehority1986; Burgess et al., Reference Burgess, Gilchrist, Lynn and Petri2017). Some gut protozoa, for example members of the Ciliophora meta-group, are dependent on the rapid faecal-oral transmission of infective stages (Michaiowski, Reference Michaiowski, Holzapfel, Naughton, Pierzynowski, Zabielski and Salek2005). In contrast, other species, such as Giardia and Cryptosporidium, form environmentally resistant cysts or oocysts that can persist in the environment for long periods of time allowing for more sustained transmission (Dumètre et al., Reference Dumètre, Aubert, Puech, Hohweyer, Azas and Villena2012).
Host behaviour contributes to the chance of a host encountering and acquiring infective stages of protozoa (Kołodziej-Sobocińska, Reference Kołodziej-Sobocińska2019), with more social individuals with comparatively greater social interactions having a greater chance of being exposed to protozoa (Ezenwa et al., Reference Ezenwa, Archie, Craft, Hawley, Martin, Moore and White2016). For example, a meta-analysis showed that male vertebrates with a higher social status (and thus increased mating) have an overall higher parasite risk, compared to those with a lower social status (Habig et al., Reference Habig, Doellman, Woods, Olansen and Archie2018). Similarly, increased parent–offspring interactions will increase the exposure of offspring to the parents’ existing protozoa community, which is seen with Ciliophora meta-group infections in ruminants (Michaiowski, Reference Michaiowski, Holzapfel, Naughton, Pierzynowski, Zabielski and Salek2005).
The demographics of a host population will also affect protozoa transmission in a number of ways. As host density increases this will increase the chance of protozoa transmission (Ostfeld and Mills, Reference Ostfeld, Mills, Wolff and Sherman2008; Ebert, Reference Ebert2013), but increases in host density will also affect hosts' social organization and home ranges, thus also altering individuals' risk of exposure (Bertolino et al., Reference Bertolino, Wauters, De Bruyn and Canestri-Trotti2003; Brei and Fish, Reference Brei and Fish2003; Sanchez and Hudgens, Reference Sanchez and Hudgens2019). Other aspects of host biology, such as foraging behaviour, can affect transmission; for example, foraging on the ground, compared to arboreal and aerial foraging, can increase exposure to environmentally-transmitted protozoa, as is seen with Entamoeba in baboons and Isospora in birds (Dolnik et al., Reference Dolnik, Dolnik and Bairlein2010; Barelli et al., Reference Barelli, Pafčo, Manica, Rovero, Rosà, Modrý and Hauffe2020).
An individual's diet, immune state, and pre-existing microbiome (both prokaryotic and eukaryotic) can also influence the chance of a protozoan successfully establishing in the gut (Thursby and Juge, Reference Thursby and Juge2017; Kołodziej-Sobocińska, Reference Kołodziej-Sobocińska2019; Coyte et al., Reference Coyte, Rao, Rakoff-Nahoum and Foster2021). Host diet can alter nutrient availability, allowing the establishment and maintenance of different gut protozoan communities (Zhang et al., Reference Zhang, Wei, Yang, Huang, Li, Yu, Qi, Liu, Loor, Wang and Zhang2022). For example, the relative abundance of Entodinium in sheep rumen fluid changes in response to different diets (Henderson et al., Reference Henderson, Cox, Ganesh, Jonker, Young and Janssen2015; Zhang et al., Reference Zhang, Wei, Yang, Huang, Li, Yu, Qi, Liu, Loor, Wang and Zhang2022). Host immune state can affect the initial establishment and subsequent persistence of protozoa in the gut (Evering and Weiss, Reference Evering and Weiss2006; Sardinha-Silva et al., Reference Sardinha-Silva, Alves-Ferreira and Grigg2022). Long-term co-evolution of protozoa with their hosts has allowed many protozoa to evolve to be either tolerated by and / or evade the host immune response (Zambrano-Villa et al., Reference Zambrano-Villa, Rosales-Borjas, Carrero and Ortiz-Ortiz2002; Macpherson et al., Reference Macpherson, Geuking and McCoy2005; Schmid-Hempel, Reference Schmid-Hempel2009; Tanoue et al., Reference Tanoue, Umesaki and Honda2010; Sardinha-Silva et al., Reference Sardinha-Silva, Alves-Ferreira and Grigg2022). A host's pre-existing microbiome can also affect subsequent establishment of other taxa (Coyte et al., Reference Coyte, Rao, Rakoff-Nahoum and Foster2021). For example, some Ciliophora species in the livestock rumen microbiome require a pre-established prokaryotic community for their survival (Michaiowski, Reference Michaiowski, Holzapfel, Naughton, Pierzynowski, Zabielski and Salek2005). Furthermore, there is often an obligate pattern of succession in establishment; for example, in many ruminants Entodinia spp. is the primary colonizer after which other Ciliophora species establish (Michaiowski, Reference Michaiowski, Holzapfel, Naughton, Pierzynowski, Zabielski and Salek2005). Competition among microbial species for nutrients and other resources results in the generation of niches within the gut, controlling the diversity of protozoa that can establish (Pereira and Berry, Reference Pereira and Berry2017). For example, Tritrichomonas musculus competes with prokaryotic taxa for dietary fibre, a resource essential for T. musculus colonization (Wei et al., Reference Wei, Gao, Kou, Meng, Zheng, Liang, Sun, Liu and Wang2020). Prokaryotic taxa can produce molecules that limit the establishment of protozoa; for example, Lactobacillus reuteri and L. acidophilus-derived factors can inactivate Cryptosporidium oocysts (Foster et al., Reference Foster, Glass, Courtney and Ward2003).
Most of what is known about gut protozoa of mammals comes from studies of people, livestock, and laboratory animals. In contrast, there are limited studies describing the diversity of gut protozoa in wild mammals, and what drives variation in protozoa composition. The gut microbiomes of laboratory and domesticated animals are likely to be quite distinct from those of their wild counterparts (Prabhu et al., Reference Prabhu, Wasimuddin, Kamalakkannan, Arjun and Nagarajan2020; Bowerman et al., Reference Bowerman, Knowles, Bradley, Baltrūnaitė, Lynch, Jones and Hugenholtz2021), so there is a need to study wild animals in greater detail. The Rodentia are a highly speciose order of mammals (Fabre et al., Reference Fabre, Hautier, Dimitrov and Douzery2012), but their gut protozoa are not well described. As with most mammals, the majority of described gut protozoa in wild rodents are parasitic, rather than mutualist (Parfrey et al., Reference Parfrey, Walters, Lauber, Clemente, Berg-Lyons, Teiling, Kodira, Mohiuddin, Brunelle, Driscoll, Fierer, Gilbert and Knight2014). In part, this may be because there has been a focus on parasitic protozoa of rodents, given their potential as sources of zoonotic infection (Meerburg et al., Reference Meerburg, Singleton and Kijlstra2009; Han et al., Reference Han, Schmidt, Bowden and Drake2015). There has been limited effort to describe the mutualistic gut protozoa of wild rodents, except in those species with comparatively enhanced digestive efficiency, e.g. the capybara, Hydrochoerus hydrochaeris (Borges et al., Reference Borges, Dominguez-Bello and Herrera1996).
To further our understanding of mammalian gut protozoa we have systematically reviewed records of protozoa present in the gut microbiome of wild rodents. This, as far as we are aware, has not been done before. After describing the protozoa known to infect the gut of wild rodents, we then sought to understand how the prevalence of their infection varies among different protozoa and among different hosts, and how aspects of host biology affect this.
Materials and methods
Literature search
We searched the Web of Science for articles describing gut protozoa infections of wild rodents, following PRISMA guidelines (Moher et al., Reference Moher, Liberati, Tetzlaff and Altman2009; Page et al., Reference Page, McKenzie, Bossuyt, Boutron, Hoffmann, Mulrow, Shamseer, Tetzlaff, Akl, Brennan, Chou, Glanville, Grimshaw, Hróbjartsson, Lalu, Li, Loder, Mayo-Wilson, McDonald, McGuinness, Stewart, Thomas, Tricco, Welch, Whiting and Moher2021). We used 2 independent searches: the first in March 2020, using the 4 search terms ‘infection rodent protozoa gut’, ‘gut protozoa rodent’, ‘parasite rodent gut’ and ‘eukaryotic microbiome rodent’, where each term was searched for simultaneously in ‘Topic’; the second in April 2020, performed as above but using the search term ‘protozoa wild rodent’, with an additional 7 search terms (wild-type, ‘wild type’, model, and the 4 search terms used in March 2020) using the ‘NOT’ command. This second search was used to avoid articles reporting studies on laboratory rodents while excluding any potential duplicate articles from the first search. In all, this resulted in retrieving 6852 articles, which were then screened and reduced to 2018 articles that were carried forward for full-text screening (Fig. 1), where we retained articles that reported naturally occurring protozoa infections of the gut of a wild rodent. We excluded articles that did not give the location of the wild rodent, as too those that did not identify the rodent host or the protozoan parasite to the genus level. Once data were extracted, their reference lists were searched to identify any additional potential articles not identified in the literature search; this identified a further 112 articles, from which data were also extracted.

Figure 1. PRISMA diagram showing the source of articles and the subsequent screening stages used to generate the data records used in the meta-analysis.
Data extraction
We categorized articles as either (i) a report of the presence of a protozoan (henceforth ‘presence’ article) or (ii) a report of the protozoan prevalence (henceforth ‘prevalence’ article). We created data records by extracting the following data from articles: host species, protozoa species, geographical location (as continent, country, and latitude and longitude (if provided)), diagnostic technique and year sampled. A single article could produce multiple data records. We recorded protozoa prevalence from prevalence articles, where necessary calculating this from reported data. We used median prevalence when prevalence ranges were reported; we used mean prevalence when different prevalence values were reported for host sub-species and species complexes; if multiple prevalence values were reported for con-generic protozoan species, a mean protozoa genus prevalence was calculated. Weighted means were calculated based on the sample size of the individual reports. For articles that used multiple diagnostic techniques for the same rodents we recorded either (i) the combined prevalence from the multiple diagnostic techniques reported in the article or (ii) if the combined prevalence was not given, then we calculated the average prevalence of the multiple diagnostic techniques, and then reported the diagnostic technique for these records as ‘Mixed’.
From these data we generated a meta-table recording the presence of different protozoa in the gut of wild rodents, with data recorded at the genus level for protozoa, and at species level for the host. Rodent host taxonomy was after the Handbook of the Mammals of the World (Wilson et al., Reference Wilson, Mittermeier and Lacher2017). Protozoa genera were assigned to 1 of 5 meta-groups: Amoebozoa; Apicomplexa; Ciliophora; Metamonada and Other (Adl et al., Reference Adl, Bass, Lane, Lukeš, Schoch, Smirnov, Agatha, Berney, Brown, Burki, Cárdenas, Čepička, Chistyakova, del Campo, Dunthorn, Edvardsen, Eglit, Guillou, Hampl, Heiss, Hoppenrath, James, Karnkowska, Karpov, Kim, Kolisko, Kudryavtsev, Lahr, Lara, Le Gall, Lynn, Mann, Massana, Mitchell, Morrow, Park, Pawlowski, Powell, Richter, Rueckert, Shadwick, Shimano, Spiegel, Torruella, Youssef, Zlatogursky and Zhang2019). A generalized linear model (GLM) with a Poisson error distribution (Zuur and Ieno, Reference Zuur and Ieno2016) was used to determine if the number of protozoa genera identified in a rodent species was dependent on the surveying effort (i.e. the number of records) for that rodent species.
Analysis of protozoa prevalence
Our aim was to explore the causes of variation in protozoa prevalence in the gut of wild rodents. The records for which an average prevalence was calculated were removed, but the average prevalence record was kept (Fig. 1). This was to ensure that there was no pseudo-replication of the data. Each data record was assigned an article ID and a unique record number (URN). We used the metafor package within RStudio to conduct all meta-analyses (v2.4.0, Viechtbauer, Reference Viechtbauer2010). Our general strategy was: (i) create a base restricted maximum likelihood estimator (REML) model with only random effects that would be used throughout the following data analyses, (ii) investigate if there was variation in the prevalence of protozoa across different rodent host species, (iii) identify variables contributing to variation in protozoa prevalence, and (iv) investigate any potential publication and methodological biases in the dataset.
The base REML model listed article ID, URN, diagnostic technique and host phylogeny as random factors. Host phylogeny accounted for potential variation in prevalence due to hosts' shared evolutionary history (Koricheva et al., Reference Koricheva, Gurevitch and Mengersen2013). The phylogeny was created using the Open Tree of Life (OTL) database (Hinchliff et al., Reference Hinchliff, Smith, Allman, Burleigh, Chaudhary, Coghill, Crandall, Deng, Drew, Gazis, Gude, Hibbett, Katz, Laughinghouse, McTavish, Midford, Owen, Ree, Rees, Soltis, Williams and Cranston2015) and the rotl R package (v3.0.14, Michonneau et al., Reference Michonneau, Brown and Winter2016). Some species were not present in the OTL and so these were manually added to the tree. Grafen's method was used to compute branch lengths using the ape R package (Grafen, Reference Grafen1989; Paradis et al., Reference Paradis, Claude and Strimmer2004). The final phylogenetic tree is available in Supplementary Figure 1. Diagnostic technique was included as a random factor to account for potential variation in prevalence due to the diagnostic technique used. In all models, the dependent variable was double-arcsine transformed prevalence (Wang, Reference Wang2023), with this transformation fitting the assumptions of normality required for meta-analyses (Barendregt et al., Reference Barendregt, Doi, Lee, Norman and Vos2013). Recent work has recommended not using double-arcsine transformation in meta-analyses (Lin and Xu, Reference Lin and Xu2020; Röver and Friede, Reference Röver and Friede2022), and so we completed all analyses on both double-arcsine and single-arcsine transformed data, finding that for all models the results and conclusions drawn were identical (Hunter-Barnett, Reference Hunter-Barnett2023). To test whether various factors significantly affect protozoan prevalence we added these factors as a fixed effect (henceforth called a moderator) to the base model.
We used the rma.mv function in the base model to calculate the overall double-arcsine transformed prevalence, with this result back-transformed to obtain the summary percentage prevalence and 95% confidence intervals (CI) (Wang, Reference Wang2023). The number of records included in the model (k) was also recorded. Heterogeneity of prevalence was examined using the I 2 statistic, which is the proportion of variance in effect sizes that is not attributable to sampling (i.e. error) variance (Higgins et al., Reference Higgins, Thompson, Deeks and Altman2003). The proportion of I 2 attributable to differences in article ID, URN, diagnostic technique, and host phylogeny was calculated using the i2_ml function in the orchaRrd R package (Nakagawa et al., Reference Nakagawa, Lagisz, O'Dea, Rutkowska, Yang, Noble and Senior2021).
To investigate how gut protozoa prevalence varied among different host taxa we performed 2 meta-regressions of gut prevalence, incorporating host family or host species as the moderator. The moderator ‘protozoa genus’ and the subsequent interaction terms with the host family and host species were also included in the models, but only incorporating either where there were at least 10 records, thus guarding against bias caused by small sample sizes (Lin, Reference Lin2018). Significant moderators indicated that they affected mean protozoa prevalence; significance was defined by examining the Q M statistic and marginal R 2 values were calculated to establish how much heterogeneity in prevalence was described by the moderators, using the r2_ml function in the orchaRd R package (Nakagawa and Schielzeth, Reference Nakagawa and Schielzeth2013; Nakagawa et al., Reference Nakagawa, Lagisz, O'Dea, Rutkowska, Yang, Noble and Senior2021).
When we found significant effects of interactions between protozoa and host, we examined these further by dividing the host family or host species into subgroups and running separate meta-regressions for each subgroup, with protozoa genus as the moderator. Only the host subgroups that had at least 2 protozoa genera, with at least 10 records per protozoa genus, were tested. If there was a significant effect of protozoa genus we conducted pairwise comparisons between protozoa genera, using Tukey post hoc comparisons, which was done by re-running the meta-regression and excluding the intercept, and using the multcomp R package to compare combinations of protozoa genera (Hothorn et al., Reference Hothorn, Bretz and Westfall2008). We used the holm method to correct for multiple testing (Holm, Reference Holm1979). Finally, the average double-arcsine transformed prevalence for each subgroup within each moderator was obtained by using the subset function within the rma.mv model. Orchard plots (including 95% CIs and 95% prediction intervals) were used to show differences in prevalence among subgroups (Nakagawa et al., Reference Nakagawa, Lagisz, O'Dea, Rutkowska, Yang, Noble and Senior2021). Prediction intervals represent the range of prevalence in which the prevalence of a new observation would fall (IntHout et al., Reference IntHout, Ioannidis, Rovers and Goeman2016). Precision, as the inverse of the standard error for each record, was used in these plots, where a larger precision equates to a larger sample size.
To investigate if geographical differences were contributing to variation in protozoa prevalence, 3 geographical moderators were included: longitude, latitude and continent. Latitude and longitude were converted from degrees, minutes and seconds format to the decimal degrees format using OSMscale (v0.5.1, Boessenkool, Reference Boessenkool2017), so generating a continuous variable. In this model, the interactions of latitude and longitude with continent were also included as moderators. Additionally, protozoa genus and its interactions with each of the 3 geographical moderators were also included, to account for variation stemming from different protozoa genera.
To investigate if host behaviour was contributing to variation in protozoa prevalence, host behaviour moderators were created for each host species. A single resource was used to extract behavioural information (Wilson et al., Reference Wilson, Mittermeier and Lacher2017), forming eight moderators that we hypothesized may affect interactions between rodent hosts, so affecting protozoa transmission (Ostfeld and Mills, Reference Ostfeld, Mills, Wolff and Sherman2008; Sarkar et al., Reference Sarkar, Harty, Johnson, Moeller, Archie, Schell, Carmody, Clutton-Brock, Dunbar and Burnet2020). The 8 moderators were: (i) host density, (ii) host home range (i–ii extracted as quantitative values), (iii) host dispersal distance (and then made into <1 and >1 km categories), (iv) typical social grouping (solitary or group-living), (v) typical mating system (monogamous or polygamous), (vi) development type (altricial or precocial) (iv–vi recorded as categorical data), (vii) social system (with 11 sub-groups), and (viii) typical lifestyle (general behaviour, locomotion and morphology) (Derrickson, Reference Derrickson1992; Wilson et al., Reference Wilson, Mittermeier and Lacher2017). If behavioural information was not available for a species, family characteristics were used but only if this characteristic applied to all species in that family. These 8 moderators were tested separately in a meta-regression, each with protozoa genus included and the relevant interaction term.
To investigate if diagnostic technique affected reported gut protozoan prevalence, diagnostic technique was added as a moderator in a meta-regression. This model removed diagnostic technique from the random effects. Post hoc tests were completed as described above. A second meta-regression was conducted, with precision as a moderator, to determine if sample size affected protozoa prevalence. A funnel plot was used to visualize publication bias, with an asymmetrical plot indicating missing effect sizes, potentially from publication bias (Koricheva et al., Reference Koricheva, Gurevitch and Mengersen2013; Shi and Lin, Reference Shi and Lin2019). A trim-and-fill test (Duval and Tweedie, Reference Duval and Tweedie2000) was used to detect missing effect sizes and predict the average effect size if these were to be included in the analysis.
Results
Protozoa and host records
A total of 344 suitable articles were identified from the literature search, published between 1915 and 2020 (Supplementary Table 1). From these, 2245 data records of 44 genera of protozoa, across 69 countries (Supplementary Table 2), encompassing all 5 protozoa meta-groups (Amoebozoa 95 records, 4 genera; Apicomplexa 1725, 12; Ciliophora 38, 14; Metamonada 368, 11; Other 19, 2 (Blastocystis and Pharyngomonas)), were recorded in the gut of wild rodents. The most data records were of Apicomplexa and Metamonada protozoa, and the most common protozoan genera for which there were data records were Cryptosporidium, Eimeria and Giardia. 275 rodent host species were identified from 110 genera and 21 families, with large variation in the number of data records generated for each host species, with the most common data records for Apodemus, Microtus and Rattus.
From the 2245 data records, there were 1886 records of gut protozoa in wild rodents. Of the 275 host species, 228 had a confirmed protozoan in the gut (combining both presence and prevalence articles) (Table 1; Supplementary Table 3). In total 44 genera of protozoa are present in the gut of wild rodents, though genera were highly variable in the number of host species from which they have been reported. Only 7 protozoa genera (Chilomastix, Cryptosporidium, Eimeria, Entamoeba, Giardia, Isospora, Trichomonas, from Apicomplexa, Metamonada and Amoebozoa) were recorded in the gut of more than 10 host species. Eimeria was recorded as the most widely host-distributed distributed protozoa genus, identified in 194 (85% of 228) host species. In comparison, 27 protozoa were reported from only one host species, including 13 (of 14) Ciliophoran genera.
Table 1. Protozoa found in the gut of wild rodents

Protozoa are grouped by meta-group, and then alphabetically, with the number in parentheses showing the number of host species from which that protozoa had been identified. ‘Cilio’ are the ciliophora mega-group. Rodent taxa are shown by rodent families; the same data for rodent species are shown in Supplementary Table 3.
The number of protozoa genera identified in the gut of each wild rodent host species was highly variable. Nineteen host species had 5 or more protozoa, with most of these belonging to the Muridae and Cricetidae. The greater capybara (H. hydrochaeris) had the most (17), followed by the brown rat (13, Rattus norvegicus) and the black rat (11, R. rattus). Most (145, 64% of 228) rodent species had just a single protozoan recorded, and these host species were from 14 rodent families. The number of different protozoa identified in rodent species was linked to how intensively that host species was surveyed; specifically, there was a significant, positive relationship between the number of data records for a rodent host and the number of different protozoa identified (GLM: F 1,226 = 145.5, P < 0.001).
Protozoa prevalence
A total of 1237 (of 2245) data records (after the removal of pseduoreplicated data records and presence records) were used to investigate variation in the prevalence of protozoa in the wild rodent gut. A total of 255 rodent species were surveyed across 289 articles, from 102 host genera and 21 host families, and 36 protozoa genera were used in the meta-analysis.
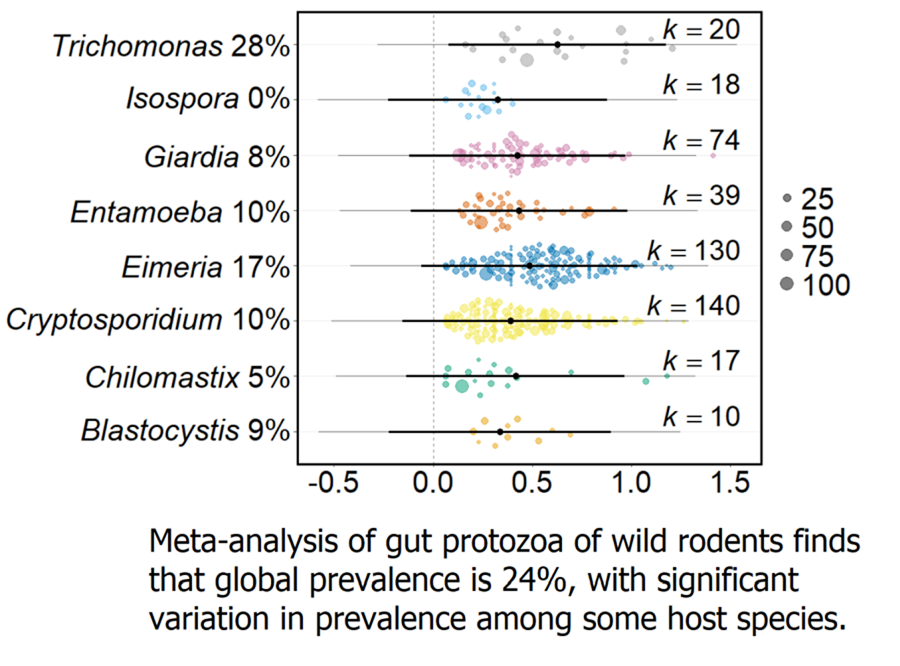
Across all wild rodents, the average prevalence of gut protozoa infection was predicted to be 23.7% (95% CI 4.8–48.5, k = 1237). However, the trim-and-fill test detected asymmetry in the funnel plot, with 187 missing effect sizes being added above the mean. Adding these 187 effect sizes adjusted the overall protozoa prevalence to 32.9% (CI 30.6–35.1, k = 1424). There was no change in prevalence over the time period of the records (Q M = 0.023, P = 0.880, k = 1015).
There was substantial variation in the prevalence of protozoa infection in the dataset (I 2 = 97.8%), with much of this variation stemming from differences among individual data records (32.3%) and differences attributed to the article ID of the data record (32.0%). However, host phylogeny explained 26.9% of the variation in protozoa prevalence, and diagnostic techniques 6.5%.
Host species differed significantly in their prevalence of gut protozoa (host species moderator Q M = 41.7, P < 0.001; interaction protozoan genus Q M = 122.4, P < 0.001, k = 538; Figure 2A; Supplementary Table 4). We examined 7 host species (Apodemus agrarius, A. flavicollis, A. sylvaticus, Mus musculus, Myodes glareolus, Ondatra zibethicus and R. rattus) more closley, which showed that protozoan genus was only a significant moderator of prevalence for the muskrat (O. zibethicus), such that it had a higher prevalence of Giardia (64.2%) compared with Cryptosporidium prevalence (29.2%); for the other 6 host species there was no effect of protozoan genus on prevalence. The prevalence of Giardia in the muskrat was significantly higher compared to hosts Castor canadensis, M. musculus and R. rattus (Q M = 18.8, P < 0.001, k = 65, Fig. 2B).

Figure 2. The prevalence of (A) protozoa in 7 host species, (B) Giardia in 4 host species, and (C) protozoa in the rodent families Cricetidae, Muridae and Sciuridae. In all, prevalence, shown on the x-axis, is double-arcsine transformed; the x-axis differs among panels. The black point indicates the estimated average prevalence, with the bold lines showing 95% CIs, and thin lines showing 95% prediction intervals. The size of the points are scaled to precision (shown on the scale on the right-hand side of each panel), and k indicates the number of records for that protozoan. The back-transformed predicted prevalence percentage is provided next to the protozoa genus label.
There was no significant difference in the predicted prevalence of protozoa infection among different rodent families, though there were significant differences in interactions between protozoa genus and host family (host family moderator Q M = 1.5, P = 0.59, interaction protozoan genus Q M = 107.6, P < 0.001, k = 1111); thus, host families had different prevalence of gut protozoa infection for certain genera of protozoa. We investigated this further by analysing different rodent families separately, finding that 3 host families – Cricetidae, Muridae and Sciuridae – had at least 2 protozoa genera, with at least 10 records per protozoa genera, and protozoa genus was a significant moderator of prevalence in all (Fig. 2C; Q M = 33.2, P < 0.001, k = 448, Q M = 46.2 P < 0.001, k = 360, Q M = 42.0, P < 0.001, k = 142 for Cricetidae, Muridae and Sciuridae, respectively).
Factors affecting prevalence of infection
Variation in host lifestyle – arboreal, fossorial, semi-aquatic, semi-fossorial and terrestrial – did not significantly affect protozoa prevalence. However, there was a significant interaction between host lifestyle and protozoan genus (lifestyle moderator Q M = 1.06, P = 0.983, interaction protozoan genus Q M = 57.3, P = 0.003, k = 988). We examined this further, finding that for arboreal, fossorial and terrestrial host lifestyles, protozoa genus had a significant effect on prevalence (Q M = 33.8, P < 0.001, k = 62, Q M = 15.9, P = 0.001, k = 76, Q M = 26.3, P < 0.001, k = 547 for arboreal, fossorial and terrestrial lifestyles, respectively). Specifically, Eimeria had a significantly higher prevalence in the gut of arboreal and fossorial rodents (82.9% and 40.8%) compared with other protozoa (Fig. 3A). Eimeria was also significantly more prevalent in terrestrial rodents compared to Cryptosporidium (26.8% and 15.0%, respectively); Trichomonas was significantly more prevalent in terrestrial rodents (28.5%), compared to Entamoeba (8.9%) and Cryptosporidium (15.0%). Different protozoa genera did not have a significantly different prevalence in either semi-aquatic or semi-fossorial rodents.

Figure 3. The average prevalence of protozoa (A) across 5 different host lifestyles and (B) according to method of diagnosis. In all, prevalence, shown on the x-axis, is double-arcsine transformed; the x-axis differs among panels. The black point indicates the estimated average prevalence, with the bold lines showing 95% CIs and thin lines showing the 95% prediction intervals. The size of the points are scaled to precision (shown on the scale on the right-hand side of each panel) and k indicates the number of records for the specified protozoa or diagnostic method. The back-transformed predicted prevalence percentage is provided next to the protozoa genus name or diagnostic method. In B, the p values for post hoc comparisons between the following diagnostic techniques with significant differences are: PCR: flotation <0.001; PCR: microscopy 0.017; PCR: mixed 0.038; PCR: staining 0.024.
There was no evidence that geographical location nor rodent host sociality as measured by 7 variables (home range size; dispersal distance; density; social system; binary social system; development type; and mating system) affected protozoa prevalence.
Methodological effects
The use of eight different diagnostic techniques were recorded from the articles. The most common were flotation (550 records), staining (185) and PCR (120). There was significant variation in protozoa prevalence according to the diagnostic technique used (Q M = 23.62, P < 0.001, k = 1,225, Fig. 3B). Post hoc comparisons showed that PCR-based diagnoses found a significantly lower prevalence of protozoa (13.2%) compared to microscopy, flotation and staining methods (38.3%, 37.5% and 32.4% respectively). Using multiple diagnostic techniques did not increase the report of protozoa prevalence compared with using any single diagnostic method, except PCR.
A meta-regression did not detect a significant relationship between study precision and protozoa prevalence (Q M = 0.920, P = 0.338, k = 1,237), indicating that across the whole dataset, larger sample sizes did not reveal a higher prevalence of protozoa.
Discussion
This work found that 44 genera of protozoa from all 5 mega-groups have been recorded from the gut of wild rodents. Some genera – Cryptosporidium, Eimeria, Entamoeba, Giardia – occurred commonly, in 29 rodent host species, consistent with their wide host range among vertebrates more generally (Appelbee et al., Reference Appelbee, Thompson and Olson2005; Ryan et al., Reference Ryan, Fayer and Xiao2014; Duszynski, Reference Duszynski2021; Zanetti et al., Reference Zanetti, Malheiros, de Matos, dos Santos, Battaglini, Moreira, Lemos, Castrillon, da Costa Boamorte Cortela, Ignotti and Espinosa2021). Isospora also had a wide rodent host range, being recorded from 22 species, contrasting with previous suggestions that rodents are not its natural hosts (Trefancová et al., Reference Trefancová, Mácová and Kvičerová2019). However, Blastocystis was found in only 8 rodent species, therefore contrasting with reports of its wide host range (Alfellani et al., Reference Alfellani, Taner-Mulla, Jacob, Imeede, Yoshikawa, Stensvold and Clark2013). Other protozoa appear to have a much more narrow host range: Balantidium was found in only 2 rodent host species, consistent with them acting as potential carriers while its infection predominates in pigs and primates (Schuster and Ramirez-Avila, Reference Schuster and Ramirez-Avila2008). Many studies of wild rodents have likely focussed on protozoa that are parasites, and so there may be an under representation of mutualistic species of protozoa.
These records of infection require accurate identification of the protozoan taxa, which is not always straightforward, and can be further complicated by changes to taxonomic names and reclassification. For example, Trichomonas was reported from 21 rodent species, despite being commonly associated with the digestive tract of birds and the human vagina (Malik et al., Reference Malik, Brochu, Bilic, Yuan, Hess, Logsdon and Carlton2011), suggesting that overall it has a wide vertebrate host range. However, some Trichomonas spp. are synonymous with Tritrichomonas spp. (Burr et al., Reference Burr, Paluch, Roble, Lipman, Suckow, Stevens and Wilson2012), with Tritrichomonas being described from the laboratory rodent gut microbiome (Escalante et al., Reference Escalante, Lemire, Cruz Tleugabulova, Prescott, Mortha, Streutker, Girardin, Philpott and Mallevaey2016), but was only reported in one wild rodent species in the present study. Combining the presence records of the synonymous Trichomonas and Tritrichomonas spp. then shows that it has a wider rodent host range. Similarly, the protozoa Spironucleus muris is known to colonize the gut of many laboratory rodents (Jackson et al., Reference Jackson, Livingston, Riley, Livingston and Franklin2013) but was only reported from 3 wild rodent species. However, Spironucleus spp. are often misidentified as Hexamita spp. and reclassifications are common (Jørgensen and Sterud, Reference Jørgensen and Sterud2007; Jackson et al., Reference Jackson, Livingston, Riley, Livingston and Franklin2013). Hexamita, is better known for infecting fish and birds (Uldal and Buchmann, Reference Uldal and Buchmann1996; Cooper et al., Reference Cooper, Charlton, Bickford and Nordhausen2004), but has records in 4 rodent species. Combining Spironueclus and Hexamita presence records leads to the conclusion that it has a wider rodent host range. Clarifying and stabilizing protozoa taxonomy would help improve our understanding of the host range of gut protozoa of wild rodents.
Three protozoa genera – Adelina, Klossia, Monocystis – reported from wild rodents in the present study are also known to infect arthropods and earthworms (Field and Michiels, Reference Field and Michiels2005; Bekircan and Tosun, Reference Bekircan and Tosun2021; Zeldenrust and Barta, Reference Zeldenrust and Barta2021). While these rodent records could be true infections of rodents, it is also possible that these records are actually because rodents ate invertebrates harbouring these protozoa. Furthermore, Acanathomoeba spp. and Amoeba spp. are typically considered to be free-living (Rodríguez-Zaragoza, Reference Rodríguez-Zaragoza1994) but were each identified from one rodent species, and these putative rodent infections are more likely transient infections. Similarly, the genus Pharyngomonas (originally Trichomastix) was recorded in the naked mole rat, Heterocephalus glaber, though it is a halophilic protozoan (Park and Simpson, Reference Park and Simpson2015) and so it unlikely to be a natural resident of this rodent.
Meta-analysis of these data found that the global protozoa prevalence of wild rodents is 23.7%, which is slightly higher than previous estimates for individual protozoa genera in wild rodents e.g. 18%, 19.8% and 20.1% for Blastocystis, Cryptosporidium and Giardia, respectively (Li et al., Reference Li, Wang, Wang and Zhang2017; Zhang et al., Reference Zhang, Fu, Li and Zhang2021; Barati et al., Reference Barati, KarimiPourSaryazdi, Rahmanian, Bahadory, Abdoli, Rezanezhad, Solhjoo and Taghipour2022). It is important to note that this global estimate may be conservative since many studies included in this meta-analysis sought particular protozoa taxa, rather than any protozoa taxa.
We found that rodent host species differed significantly in the prevalence of protozoa infection, but that protozoa genera did not differ in their prevalence within a host species. This, combined with no evidence of geographical effects on protozoa prevalence, suggests that the rodent species-level effect on prevalence applies widely to different protozoa, perhaps driven by host species-specific traits or wider demographic effects. The exception to this finding was the muskrat, Ondatra zibethicus, where Giardia had a significantly higher prevalence than Cryptosporidium. Giardia cysts are detected in water more frequently than Cryptosporidium, which may explain the higher Giardia prevalence in the semi-aquatic muskrat (Cacciò et al., Reference Cacciò, Thompson, McLauchlin and Smith2005; Ganoe et al., Reference Ganoe, Brown, Yabsley, Lovallo and Walter2020). There were no differences in protozoa prevalence among different rodent families. For some rodent families – Cricetidae, Muridae, Sciuridae – there were protozoa-level effects, which warrants further investigation into the underlying cause and mechanism.
The meta-analysis found no effect of host sociality on protozoa prevalence, which is interesting given that there are rodent species-level effects and an increasing awareness of the importance of social interactions affecting transmission of gut microbes (Grieneisen et al., Reference Grieneisen, Livermore, Alberts, Tung and Archie2017; Raulo et al., Reference Raulo, Allen, Troitsky, Husby, Firth, Coulson and Knowles2021). However, other work focussed on parasitic taxa has shown that there is no relationship between rodent sociality and endoparasite load (e.g. Bordes et al., Reference Bordes, Blumstein and Morand2007; Hillegass et al., Reference Hillegass, Waterman and Roth2008). Our analyses also found no evidence for an effect of host population density or home range size on protozoa prevalence, despite evidence that both are associated with the chance of incidental transmission of gut microbes in wild mammals (Li et al., Reference Li, Qu, Li, Li, Lin and Li2016; Sarkar et al., Reference Sarkar, Harty, Johnson, Moeller, Archie, Schell, Carmody, Clutton-Brock, Dunbar and Burnet2020; Wikberg et al., Reference Wikberg, Christie, Sicotte and Ting2020). Together, this suggests that other rodent species-level traits not considered here are important in affecting the prevalence of protozoa infection. These data do not include any information on hosts' immune responses or immune state, and this could affect the amount of detectable infection in host species.
Our analyses also found no effect of host lifestyle on protozoa prevalence, which contrasts with previous suggestions that arboreal and semi-arboreal lifestyles disfavour faecal-oral protozoa transmission, potentially leading to a comparatively lower protozoa prevalence in animals with such lifestyles (Gilbert, Reference Gilbert1997; Barelli et al., Reference Barelli, Pafčo, Manica, Rovero, Rosà, Modrý and Hauffe2020). However, our analyses did find that for arboreal, fossorial, and terrestrial lifestyles there were protozoa-level effects. Specifically, Eimeria was comparatively more prevalent in arboreal and fossorial rodents; Trichomonas and Eimeria were comparatively more prevalent in terrestrial rodents. However, it is important to note that these findings may be driven by protozoa-level effects within the Sciuridae, Muridae and Cricetidae. Specifically, (i) Eimeria was comparatively more prevalent in the Sciuridae, and many Sciuridae species were classed as either arboreal or fossorial and (ii) Trichomonas and Eimeria were comparatively more prevalent in the Muridae and Cricetidae and many Muridae and Cricetidae species were classed as terrestrial rodents. Thus, it is probable that the protozoa-levels effects seen within the arboreal, fossorial and terrestrial rodents may be confounded by rodent family-level taxonomic effects. Furthermore, the meta-analysis did not include data on other environmental factors known to impact transmission of gut microbes in wild mammals, such as habitat type and seasonality (Kołodziej-Sobocińska, Reference Kołodziej-Sobocińska2019; Barelli et al., Reference Barelli, Pafčo, Manica, Rovero, Rosà, Modrý and Hauffe2020). Thus, the impact of these traits on transmission, and therefore protozoa prevalence, were not addressed in this meta-analysis.
Concerning diagnosis of infection, we found that PCR reported comparatively lower prevalence of infection. This result is perhaps unexpected because PCR is typically highly sensitive (McHardy et al., Reference McHardy, Wu, Shimizu-Cohen, Couturier and Humphries2014; Compton, Reference Compton2020). However, this PCR effect may be due to difficulties in extracting DNA from protozoa (oo)cysts, whereas (oo)cysts are often readily detected (and diagnosed) by microscopical examination (Hawash, Reference Hawash2014). Furthermore, the taxonomic tight-specificity of PCR diagnosis contrasts with the other diagnostic methods that can detect a broader range of taxa (den Hartog et al., Reference den Hartog, Rosenbaum, Wood, Burt and Petri2013; Compton, Reference Compton2020). In the future metagenomic sequencing may be beneficial to get a more broad-based measure of the protozoa community in animal guts.
Publication bias was detected in the dataset, driven by a lack of studies reporting high prevalence of infection. Publication bias normally arises from a tendency to not publish studies with less significant results and / or smaller sample sizes (Shi and Lin, Reference Shi and Lin2019); instead, one may expect publication bias in favour of reporting high protozoa prevalence. Therefore, the comparative rarity of reports of high prevalence suggests that high protozoa prevalence is actually rare. Our meta-analysis has also highlighted how taxonomic reclassifications and revisions of protozoa make it hard to define, even at the genus level, which protozoa can colonize the rodent gut.
In summary, this analysis is the first, of which we are aware, synthesizing information about the gut protozoa of wild rodents, estimating the global prevalence of gut protozoa, and identifying host species-level effects on protozoa prevalence. To investigate these patterns further new studies will be required that, for example, generate data on individual- and population-level traits of hosts to understand the context-specific role of host behaviour on protozoa infection. Given the current focus on parasitic gut protozoa, future studies should also seek to include putative mutualistic protozoa, so furthering our understanding of the gut eukaryome of wild rodents.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182024000556.
Data availability
The data we have analysed are available from within our manuscript; the source data are in the papers included in our meta-analysis.
Acknowledgements
We would like to thank Liam Dougherty and Jane Hurst for help and advice. SHB was supported by a NERC ACCE studentship.
Author contributions
The study was conceived by SHB and MV; the literature search and analysis was done by SHB; the paper was written by SHB and MV.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Competing interests
The authors declare there are no conflicts of interest.
Ethical standards
Not applicable.