Introduction
Hydatid disease, or echinococcosis, is a zoonosis caused by infection with the larva of Echinococcus parasites in visceral organs of human beings and other animals. The disease has become a global public health problem and has reached epidemic proportions in China, especially in the Northwestern livestock farming region. Livestock farming is one of the main economic activities in the Northwestern region of China. The nomadic and semi-nomadic lifestyle is one of the most important risk factors for the prevalence of echinococcosis (Jiang, Reference Jiang2002). The increasing incidence of echinococcosis threatens the health and safety of farmers and herdsmen in the region. Surgical lesion removal together with albendazole/mebendazole treatment is lifesaving (Li et al., Reference Li, Wen and Zhao2010). However, complete removal of lesions is very difficult and cases are often only diagnosed at advanced stages of the disease. Therefore, it is of great importance to develop more effective drugs to safeguard the life of farmers and herdsmen, and thus promote the development of animal farming in the region.
Albendazole and mebendazole are the most important benzimidazole drugs that exhibit good efficacy and a wide range of parasiticidal activity against intestinal helminthes and other parasites (Meng et al., Reference Meng, Geng, Zhou and Gan2009; Li and Zhang, Reference Li and Zhang2013). These two drugs exhibit similar mechanisms, with which they bind to microtubulin and interfere with the normal assembly of microtubules, resulting in a metabolic disorder. Benzimidazole drugs are fatal for parasites, as they cause denaturation of cytoplasmic microtubules in intestinal cells, which interferes with intracellular transportation (Jasmer et al., Reference Jasmer, Yao, Rehman and Johnson2000; Mottier et al., Reference Mottier, Alvarez, Ceballos and Lanusse2006). Mebendazole and albendazole are the only two drugs recommended by the WHO for echinococcosis treatment (Teggi et al., Reference Teggi, Lastilla and De Rosa1993), even though they exhibit inadequate solubility and absorbance (bioavailability) in humans. Therefore, there is a need to make these drugs more effective by improving their solubility and thus bioavailability (Liu and Zhang, Reference Liu and Zhang2009).
To date, several new formulations have been developed. Phospholipid-based liposomes can be double-molecule carriers that bind the anti-echinococcosis drug albendazole (liposomal albendazole), which increases the fat solubility and stability of albendazole (Ao Qier et al., Reference Ao Qier, Wen and Yang2003). In addition, great progress has been made in highly effective albendazole emulsions that exhibit increased absorption without increasing the toxicity of albendazole (Chai et al., Reference Chai, Menghebat, Jiao, Sun, Liang, Shi, Fu, Li, Mao, Wang, Dolikun, Guliber, Wang, Gao and Xiao2001).
Modification of certain groups is another way to increase benzimidazole solubility. A typical example is the introduction of a highly hydrosoluble phosphonooxymethyl group to triclabendazole (a benzimidazole derivative), which increases solubility 50 000-fold compared with triclabendazole and results in good faciolicidal activity in vitro and in vivo (Flores-Ramos et al., Reference Flores-Ramos, Ibarra-Velarde, Hernández-Campos, Vera-Montenegro, Jung-Cook, Cantó-Alarcón, Del Rivero and Castillo2014). Our present study has demonstrated that the introduction of an epoxy group to mebendazole not only improves its solubility but also increases its efficacy against E. multilocularis protoscoleces and metacestodes. This work offers new insights into developing novel benzimidazole derivatives against echinococcosis.
Materials and methods
Animal and parasite infection
Four-week-old female mice (Kunming strain) were purchased from SLAC Laboratory Animal Center (Shanghai, China). They were maintained in cages in a P2 animal room at 23–25 °C with a 12 h light/12 h dark cycle. Litter was cleaned weekly. They were provided with food and water ad libitum. An infected mouse was sacrificed and protoscoleces (Qinghai strain) were purified and collected. The experimental mice were inoculated with 2000 protoscoleces intraperitoneally for about 3 months for block collection of protoscoleces and metacestodes.
Media and biochemical
Unless stated otherwise, all culture media and reagents were purchased from Gibco-BRL (New York, NY, USA) or Sigma (St. Louis, MO, USA).
Compound synthesis and solubility confirmation
The epoxy group was introduced to mebendazole by a reaction with epichlorohydrin and produced two isoforms, M-C1 and M-C2 (Fig. 1). Compounds were prepared at a concentration of 1 mm in 10% DMSO (dimethyl sulfoxide) solution [dissolved in PBS (phosphate buffered saline)] at 25 ± 2 °C and strongly shook for 30 s every 5 min. After 30 min, the solubility was assessed and was regarded as completely dissolved if there were no visible particles or droplets. The solubility degree was ranked with ‘+’ and ‘−’, where 5 ‘+’ denoted complete dissolution (Fig. 2).
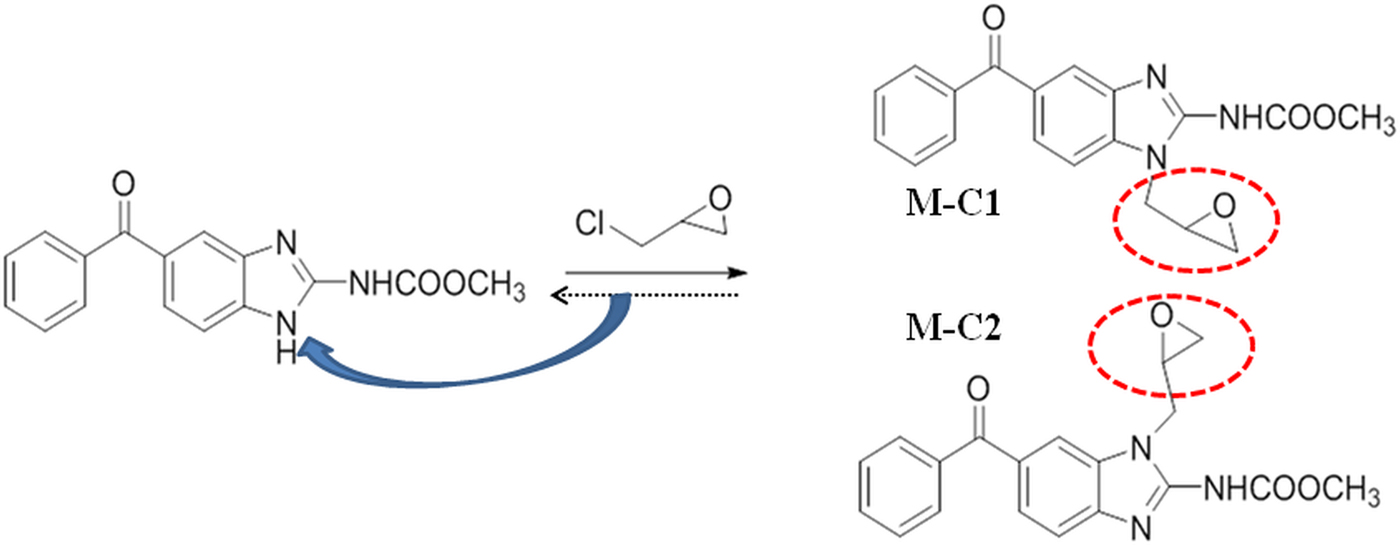
Fig. 1. (Colour online) Scheme of the synthesis of mebendazole compounds.
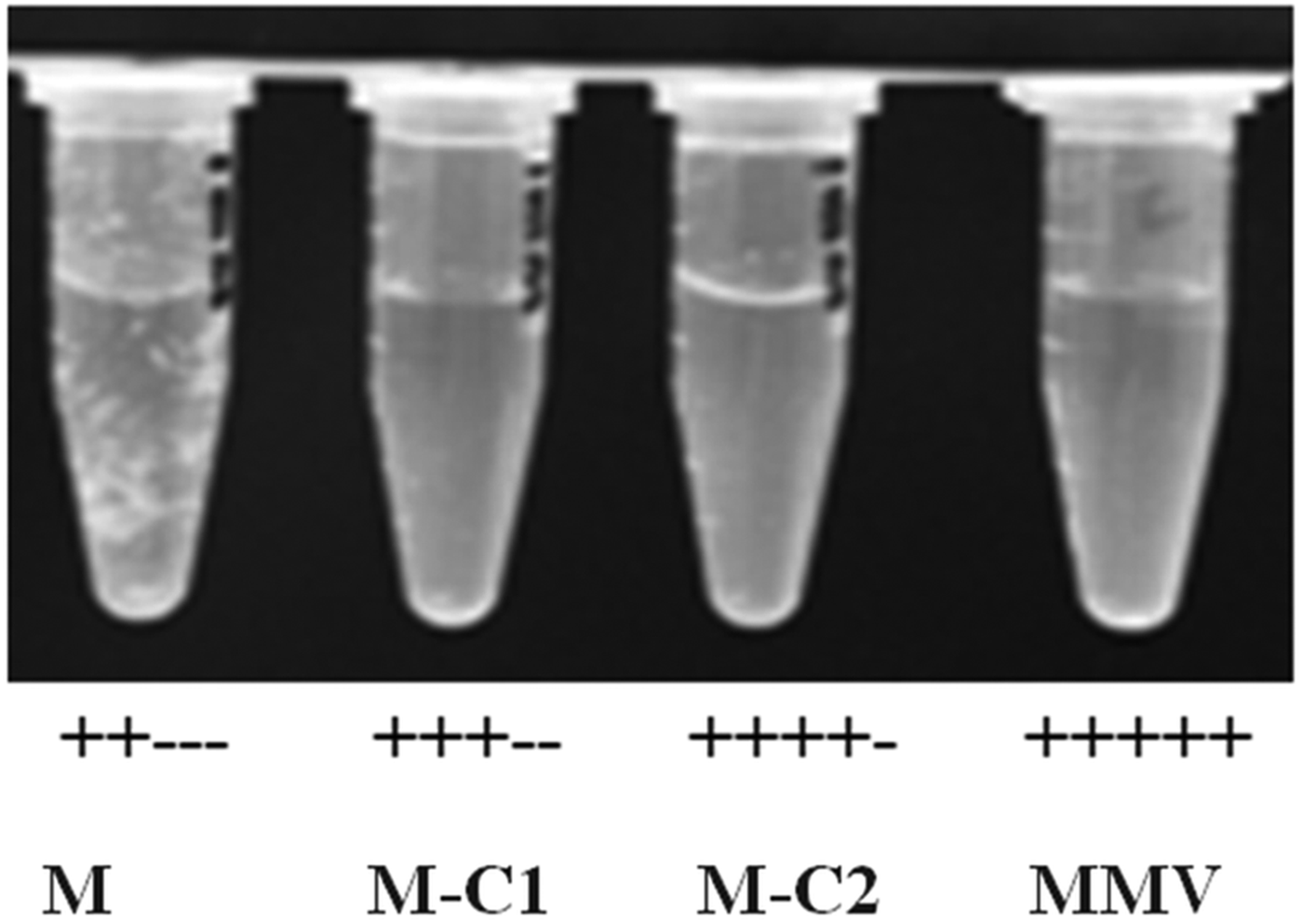
Fig. 2. Solubility of mebendazole compounds. M, mebendazole. Compounds were dissolved in DMSO-PBS (1:9) at a concentration of 1 mm. No visible particles or droplets were regarded as completely dissolved (scored as ‘ + + + + + ’).
In vitro culture of E. multilocularis protoscoleces and metacestodes
Echinococcus multilocularis were cultured as previously described (Spiliotis and Brehm, Reference Spiliotis and Brehm2009). In brief, metacestodes blocks from an infected KM mouse were cut and then crushed through a metal tea strainer. Metacestodes were washed three times with PBS. Protoscoleces were purified and stained with Trypan blue to confirm the survival rate (Liu et al., Reference Liu, Zhang, Yin and Hu2015). Protoscoleces were cultured with 1066 medium [supplemented with 10% fetal bovine serum (FBS), 100 U mL−1 penicillin G and 100 µg mL−1 streptomycin sulphate]. Metacestodes were co-cultured with rat hepatocytes (5 × 106) in DMEM medium (Dulbecco Modified Eagle's Medium, supplemented with 10% FBS, 100 U mL−1 penicillin G and 100 µg mL−1 streptomycin sulphate). Both protoscoleces and metacestodes were incubated in culture flasks at 37 °C, 5% CO2 with medium changes once a week. The splitting of metacestod cultures was performed when the total metacestod volume exceeded 20 mL, and metacestodes were used for experimental procedures when they reached a diameter of 2−4 mm (Küster et al., Reference Küster, Stadelmann, Hermann, Scholl, Keiser and Hemphill2011).
In vitro drug treatment of E. multilocularis protoscoleces
Treatments were performed in 24-well plates containing 2 mL of 1066 medium and 2000 protoscoleces were added to each well. M-C1 and M-C2 were added to each plate separately at final concentrations of 1, 10, 20 and 30 µ m. Praziquantel and mebendazole were used as experimental controls, whereas 10% DMSO was used as a negative control. Protoscoleces were incubated at 37 °C, 5% CO2 without medium changes for a period of 15 days. About 100 protoscoleces (in 100 µL medium) were taken from each well and the mortality of protoscoleces was assessed using Trypan blue stain and visualized on an inverted microscope (Yuan et al., Reference Yuan, Luo, Xin, Gao, Zhang and Jing2016).
In vitro drug treatment of E. multilocularis metacestodes
Echinococcus multilocularis metacestodes were collected after 1−2 months in culture and were washed three times in PBS. Drug treatments were performed as described by Stadelmann et al. (Küster et al., Reference Küster, Stadelmann, Hermann, Scholl, Keiser and Hemphill2011; Hemer and Brehm, Reference Hemer and Brehm2012; Stadelmann et al., Reference Stadelmann, Aeschbacher, Huber, Spiliotis, Müller and Hemphill2014). Briefly, 1 mL of medium without phenol red (DMEM, 100 U mL−1 penicillin G, 100 µg mL−1 streptomycin sulphate and 2 mm L-glutamine) was added to the same volume of metacestodes and distributed to 24-well plates (25–35 vesicles well−1). The compounds were prepared as stocks of 100 mm in DMSO. Firstly, compounds were diluted with DMSO to a concentration of 1 mm and were then added to the culture medium with final concentrations of 1, 10, 20 and 30 µ m. DMSO was used as a negative control. As a positive control, MMV (Medicines Malaria Venture) was applied. The effects of different compounds (1, 10, 20 and 30 µ m) on E. multilocularis metacestodes after 5 days were assessed by visual damage of metacestodes, which were classified into the following four levels: 0 (<10%), 1 (10–50%), 2 (50–90%) and 3 (>90%).
Morphological investigation of mebendazole C2-treated metacestodes
Echinococcus multilocularis metacestodes incubated with 30 µ m M-C2 were collected, washed once with PBS and fixed in 2.5% glutaraldehyde in 100 mm sodium cacodylate buffer (pH 7.2) for 2 h. This was followed by post-fixation in 2% OsO4 in 100 mm sodium cacodylate buffer for 2 h. Subsequently, specimens were washed in distilled water, treated with 1% uranyl acetate for 30 min, washed again in water and dehydrated by sequential incubations in ethanol (30–50–70–90–100%). For SEM (Scanning Electron Microscopy) analysis, dehydrated specimens were finally immersed in hexamethyl-disilazane and dried under a fume hood. They were then sputter-coated with gold and inspected with a scanning electron microscope operating at 25 kV (Stadelmann et al., Reference Stadelmann, Küster, Scholl, Barna, Kropf, Keiser, Boykin, Stephens and Hemphill2011, Reference Stadelmann, Rufener, Aeschbacher, Spiliotis, Gottstein and Hemphill2016).
Cytotoxicity assay
The in vitro cytotoxicity of M-C1 and M-C2 on rat hepatoma (RH) cell lines, after 72 h incubation at different concentrations (1, 10, 20 and 30 µ m, respectively), was investigated in comparison with mebendazole under identical conditions with the CCK-8 (Cell Counting Kit-8) assay. RH cells were seeded onto a 96-well microtitre plate at a density of 5000 cells well−1. Cells were cultured in DMEM without phenol red (supplemented with 1% L-glutamine, 10% FCS, 50 U mL−1 penicillin G and 50 g mL−1 streptomycin) for 72 h at 37 °C, 5% CO2. Then 10 µL of CCK-8 solution was added to each well and incubated for 1 h in the incubator. The absorbance was measured at 450 nm using a microplate reader (Küster et al., Reference Küster, Kriegel, Boykin, Stephens and Hemphill2013).
Results
Solubility improved by introduction of epoxy group
When dissolved in DMSO-PBS (1:9) solution, mebendazole formed an opaque liquid, with an emulsion floating (‘++−−−’), while M-C1 and M-C2 were relatively clear solutions with only a few tiny particles (‘+++−−’ and ‘++++−’, respectively). MMV completely dissolved without any visible particles or droplets (‘+++++’) (Fig. 2).
Effects of mebendazole compounds on protoscoleces in vitro
Trypan blue stain indicated that the mortality of protoscoleces was about 70–80% on day 7 post-exposure to mebendazole or M-C2, and M-C2 showed higher parasiticidal effects than mebendazole (P > 0.05). The parasiticidal activity of both mebendazole and M-C2 were higher than praziquantel at corresponding concentrations and time points (Fig. 3). The parasiticidal effect of M-C1 was low, and even at a concentration of 30 µ m, the mortality of protoscoleces was not more than 40%. DMSO and PBS exhibited no significant effects on protoscoleces. Significant morphological changes (disruption) of protoscoleces were observed on day 11 post-treatment with praziquantel, mebendazole and M-C2 (Fig. 4) compared with DMSO and PBS control groups.
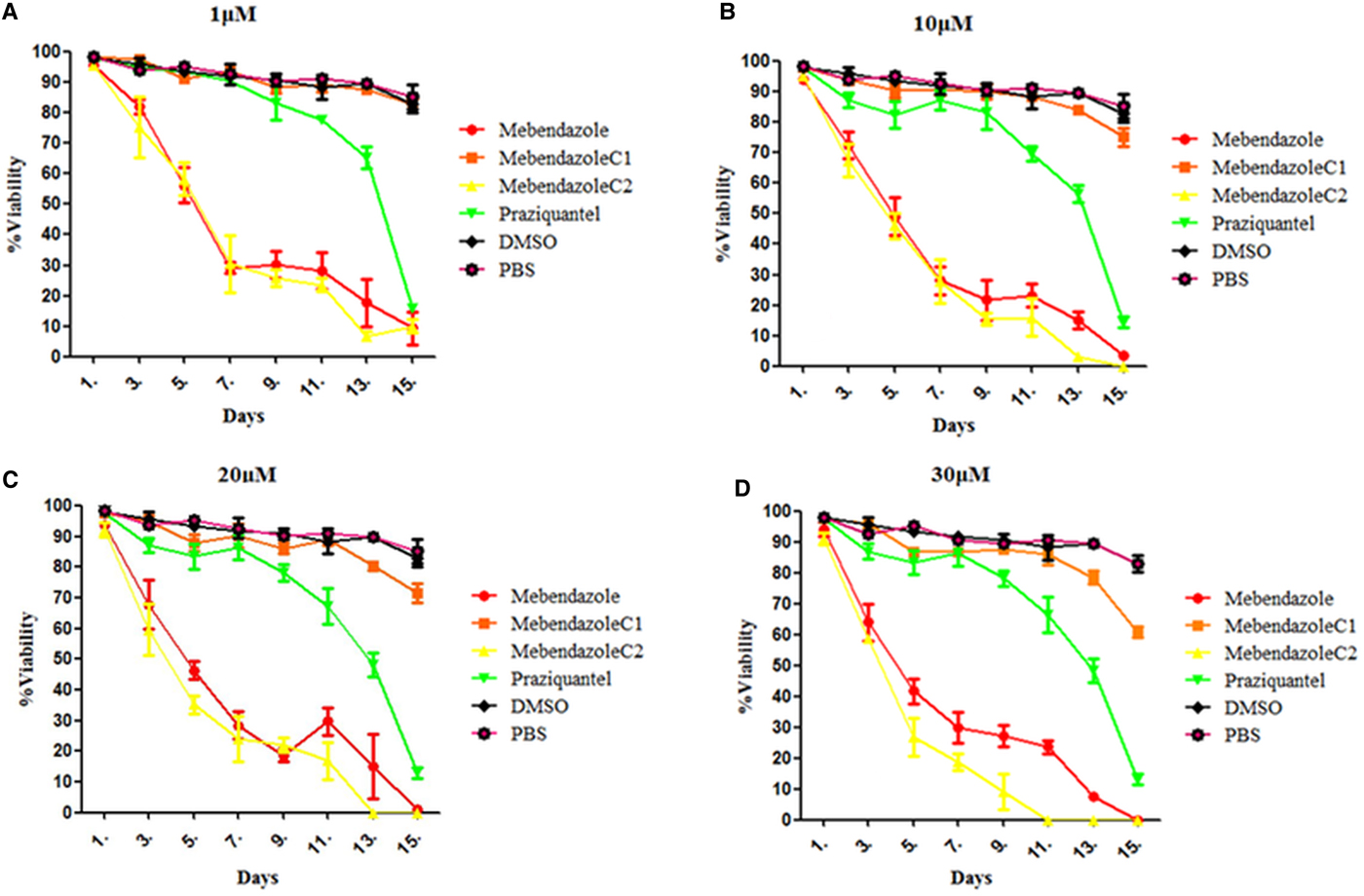
Fig. 3. (Colour online) Effects of compounds to protoscoleces following in vitro treatment with compounds at different concentrations. (A) 1 µ m, (B) 10 µ m, (C) 20 µ m and (D) 30 µ m. Mean values were derived from three biological repeats.
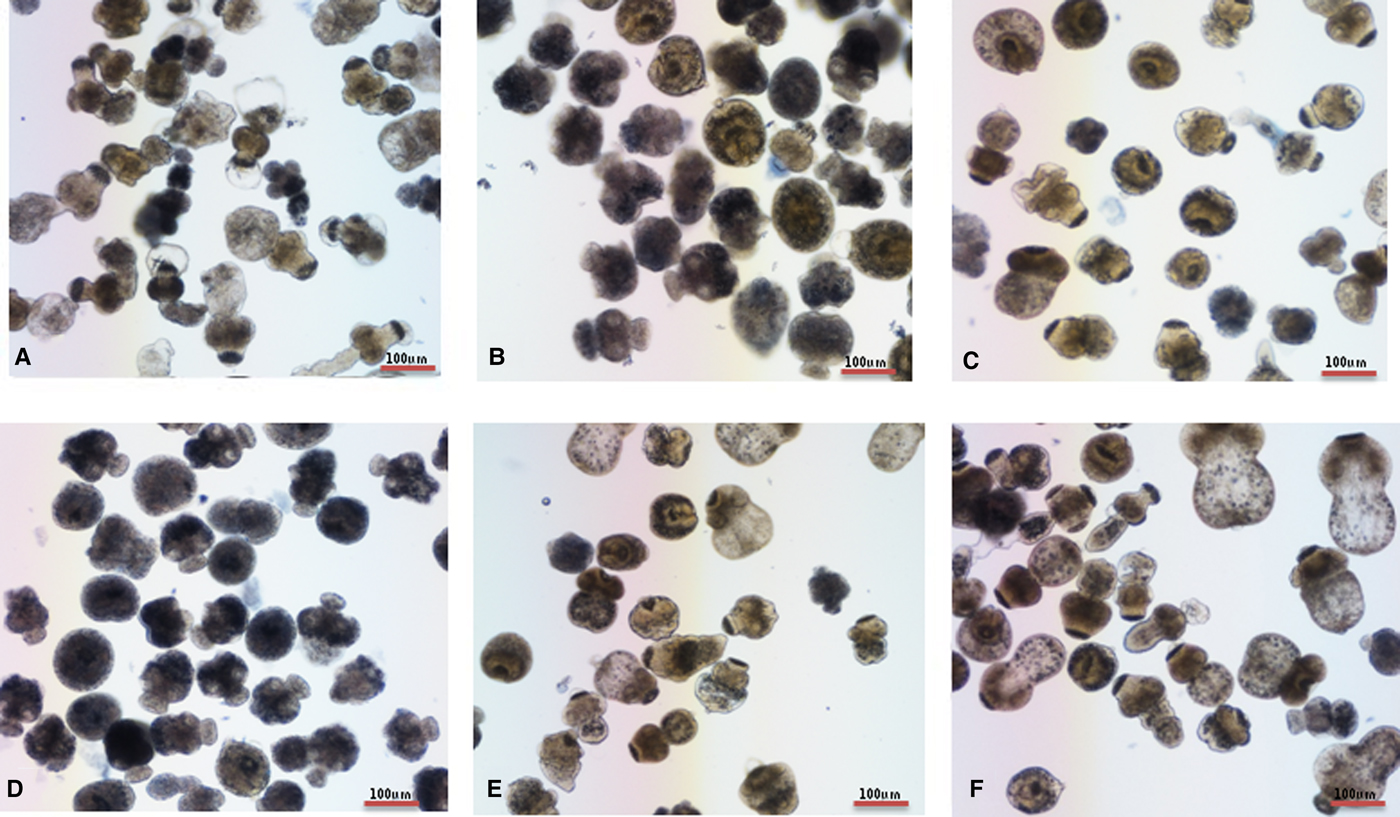
Fig. 4. (Colour online) In vitro activities of compounds on E. multilocularis protoscoleces. The effects of compounds on protoscoleces were assessed by Trypan blue every each day. Protoscoleces of E. multilocularis were exposed to (A) praziquantel, (B) mebendazole, (C) M-C1, (D) M-C2, (E) DMSO and (F) PBS 11 days post-treatment at a concentration of 30 µ m.
Effects of mebendazole compounds on metacestodes in vitro
In vitro effects of mebendazole compounds on E. multilocularis metacestodes were assessed (Table 1). The results demonstrate that the percentage of damaged metacestodes treated with mebendazole and M-C2 at different concentrations is similar. M-C1, DMSO and PBS had almost no effects on metacestodes. The basic trend of the effects of used compounds on metacestodes was similar to that on protoscoleces.
Table 1. In vitro activity of compounds on E. multilocularis metacestods
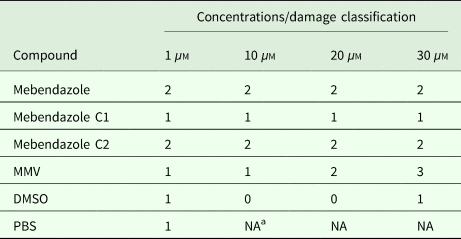
a NA, not available.
Effects on E. multilocularis metacestodes incubated with compounds (1, 10, 20 and 30 µ m, respectively) for 5 days were accessed by visual damaged percentage of metacestodes (0, <10%; 1, 10–50%; 2, 50–90%; 3, >90%).
Morphological analysis with light microscopy revealed that in the DMSO-treated group, no observable collapse of the germinal layer from the laminated layer was observed, while in M-C2- and MMV-treated groups, germinal layers detached from laminated layers and germinal cells accumulated in the centre of metacestodes and formed a circular rim (Fig. 5).
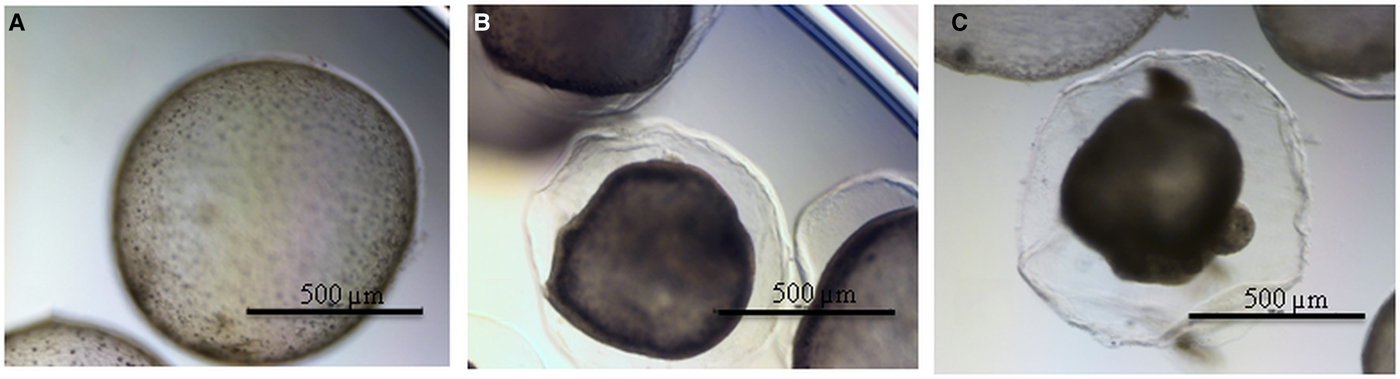
Fig. 5. (Colour online) Morphological changes of E. multilocularis metacestodes treated with compounds. (A) DMSO-treated metacestodes, (B) M-C2-treated metacestodes and (C) MMV-treated metacestodes.
Morphological study under SEM revealed that metacestodes treated with PBS and DMSO showed intact/slight damage in the germinal layer. Ultrastructural changes in metacestodes treated with M-C2 were significant-overall damage of germinal cells and thus the collapse of the germinal layer (Fig. 6).
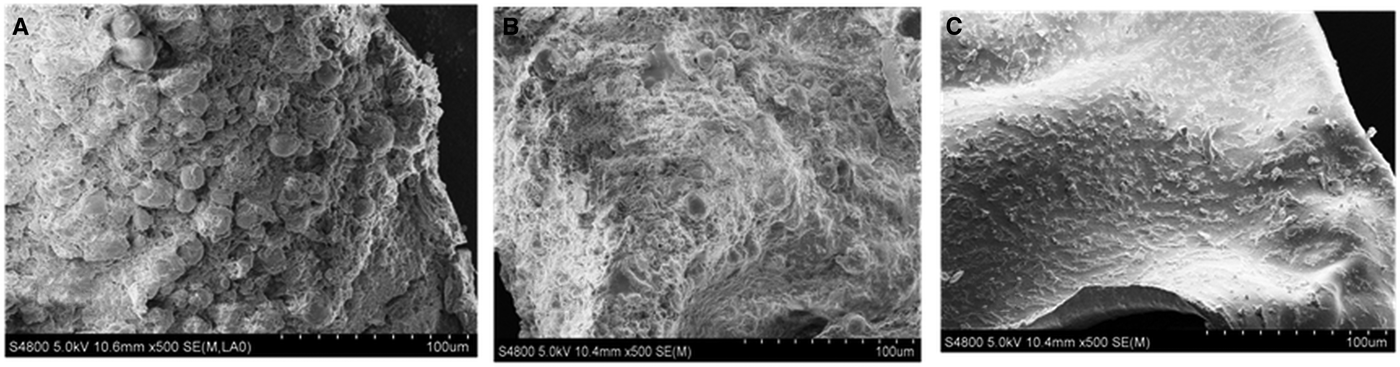
Fig. 6. Ultrastructural changes of E. multilocularis metacestodes treated/untreated with M-C2. (A) PBS-treated metacestodes, (B) DMSO-treated metacestodes and (C) M-C2-treated metacestodes.
Cytotoxicity of different compounds in RH cells
The introduction of the epoxy group not only improved the solubility of mebendazole but also reduced the cytotoxicity of mebendazole in RH cells. The cytotoxicity of M-C1 was lower than M-C2, and MMV exhibited the lowest toxicity (Fig. 7).
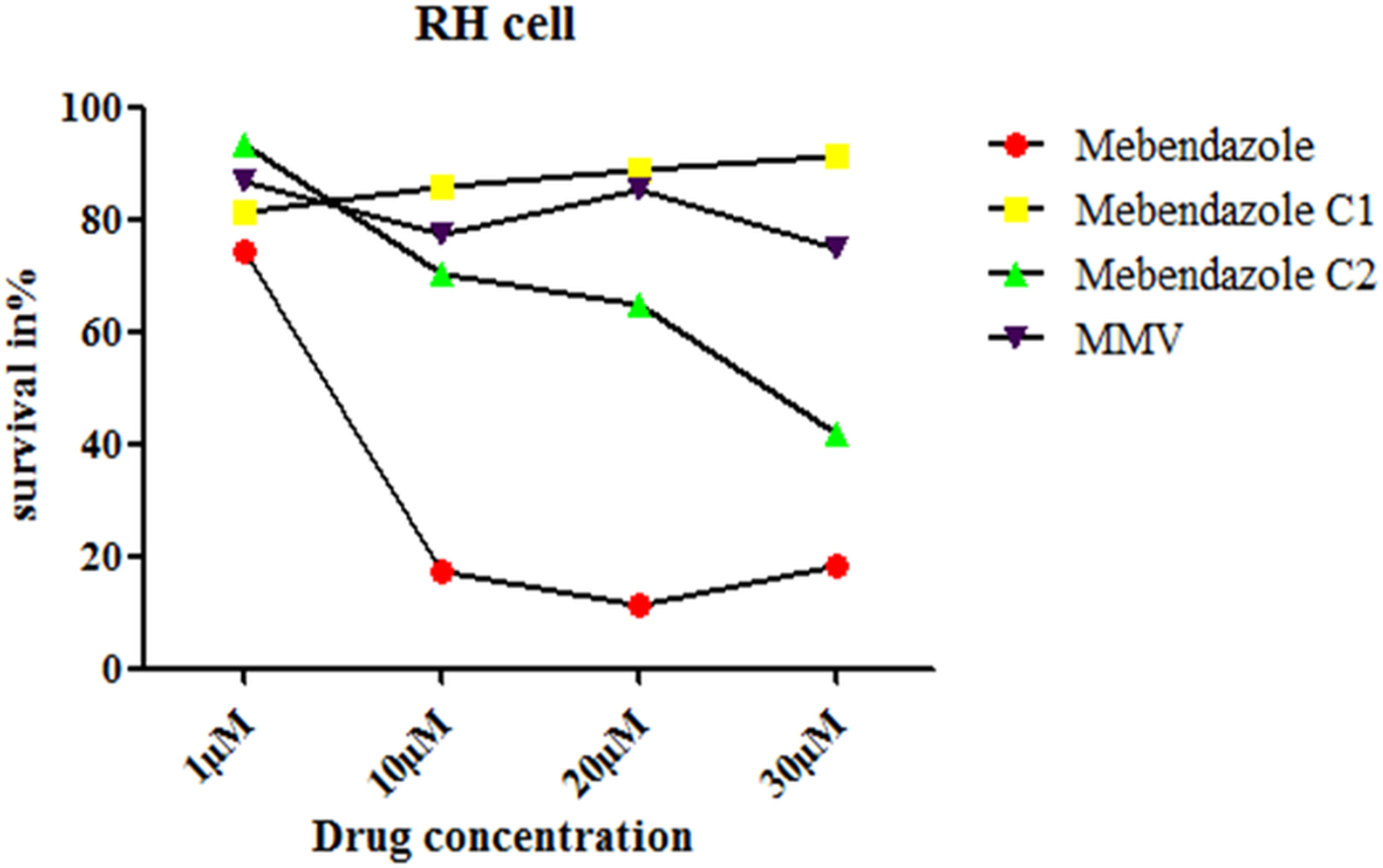
Fig. 7. (Colour online) Survival rate of RH cells incubated in different compounds at different concentrations. The cytotoxicity to RH cell was examined by CCK-8 assay. The term ‘survival in %’ in Fig. 7 relates to the fluorescence measured at 450 nm.
Discussion
Benzimidazoles are a class of compounds with a broad biological activity and are widely applied in medical fields to treat cancer (Abdel-Mohsen et al., Reference Abdel-Mohsen, Ragab, Ramla and El Diwani2010) as well as bacterial (Patel et al., Reference Patel, Patel, Kumari, Rajani and Chikhalia2012), viral (Luo et al., Reference Luo, Yao, Yang, Feng, Tang, Wang, Zuo and Lu2010) and parasitic infections (Meng et al., Reference Meng, Geng, Zhou and Gan2009; Hernández-Luis et al., Reference Hernández-Luis, Hernández-Campos, Castillo, Navarrete-Vázquez, Soria-Arteche, Hernández-Hernández and Yépez-Mulia2010; Saify et al., Reference Saify, Azim, Ahmad, Nisa, Goldberg, Hussain, Akhtar, Akram, Arayne, Oksman and Khan2012; Li and Zhang, Reference Li and Zhang2013). To date, mebendazole and albendazole are the main drugs used to treat echinococcosis (Teggi et al., Reference Teggi, Lastilla and De Rosa1993). However, their poor intestinal absorption results in low parasiticidal effects and thus there is an urgent need to develop new formulations that will increase bioavailability and therapeutic efficacy (Liu and Zhang, Reference Liu and Zhang2009).
A previous study demonstrated that the introduction of phosphonooxymethyl to albendazole N1 resulted in both high solubility and stability in water (Chassaing et al., Reference Chassaing, Berger, Heckeroth, Ilg, Jaeger, Kern, Schmid and Uphoff2008). In our present study, the introduction of an epoxy group to mebendazole N1 or N3 also greatly improved its solubility (Fig. 2). These studies suggest that the introduction of certain chemical groups to certain sites of mebendazole may change its hydrophilicity and thus improve its solubility.
Both M-C1 and M-C2 exhibited higher solubility than mebendazole, while only M-C2 exhibited higher parasiticidal activity on protoscoleces than mebendazole 7 days post-treatment (Figs 3 and 4). Mebendazole and M-C2 are more effective (P < 0.05) than praziquantel (which was used as a positive control) at corresponding concentrations and time points (Fig. 3). Although the solubility of M-C1 is better than that of M-C2, its parasiticidal effect is not as good as M-C2. The number of damaged metacestodes in the M-C2-treated group was greater than in the M-C1-treated group (Table 1). These results indicate that the improvement of drug solubility by the introduction of a chemical group to mebendazole will not necessarily increase the parasiticidal effect, and the impact on its effective conformation is also one of the most important factors that should be considered. Further studies are needed to elucidate how the introduction of an epoxy group affects the conformation of mebendazole and thus its efficacy.
Significant ultrastructural changes were observed in metacestodes treated with M-C2, namely an overall damage of germinal cells and thus collapse of the germinal layer. This is because the compounds penetrated the external protective laminated layer of metacestodes, which protects the parasite against host attack and immune responses (Díaz et al., Reference Díaz, Casaravilla, Allen, Sim and Ferreira2011, Reference Díaz, Casaravilla, Barrios and Ferreira2016; Díaz, Reference Díaz2017). Such drug penetration is fatal, as it directly effects the normal development and metabolism of germinal cells on the laminated layer.
The introduction of an epoxy group to mebendazole not only improved its solubility but also reduced its cytotoxicity in RH cells (Fig. 7). Compared with M-C2, the toxicity of M-C1 was lower. M-C1 kept a non-concentration-dependent trend, while for M-C2, the cell inhibition rate increased from 1 to 30 µ m. Further studies are needed to determine the side-effects (including cytotoxicity to different organs) and appropriate concentrations of these compounds in vivo.
Conclusions
This study has evaluated the parasiticidal effects of mebendazole derivatives with improved solubility and examined their cytotoxicity in RH cells. The new derivative M-C2 exhibits both improved solubility and strong parasiticidal activity against E. multilocularis protoscoleces and metacestodes in vitro. Additionally, M-C2 exhibited lower cytotoxicity in RH cells. Medical applications of mebendazole have been restricted, due to its low solubility and thus low bioavailability in the human body. Therefore, the improvement of mebendazole solubility is one of the key strategies to improve its effectiveness in treating echinococcosis. This study indicates that M-C2 may be a potent drug candidate for further research on echinococcosis treatment.
Author ORCIDs
Fuqiang Huang, 0000-0002-3613-2350; Yumin Zhao, 0000-0001-5378-0328
Acknowledgements
We would like to thank all study participants and the medical staff who took part in the study. We also thank Mrs Li Zhen for project and fund management.
Financial support
This study was financially supported by the Technical Reserve Research Fund for Prevention and Control (CB-16001), National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention.
Conflict of interest
None.
Ethical standards
Animal care and all animal procedures were carried out in compliance with the Guidelines for the Care and Use of Laboratory Animals produced and approved by the Ethics Committee of the Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention (No.IPD-2016-21).
Consent for publication
Not applicable.
Availability of data and material
The datasets supporting the conclusions of this article are included within the article.











