Published online by Cambridge University Press: 05 November 2021
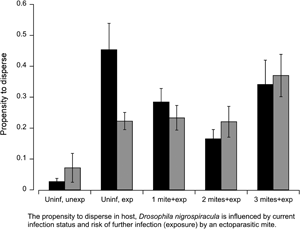
For many organisms, habitat avoidance provides the first line of defence against parasitic infection. Changes in infection status can shift the cost-benefit ratio of remaining in a given habitat vs dispersing. The aim of this study was to test the hypothesis that the propensity to disperse in Drosophila nigrospiracula is mediated by current parasite load and the risk of further infection by an ectoparasitic mite (Macrocheles subbadius). An activity monitor was used to assess dispersal propensity among infected and uninfected flies. The activity level of uninfected females increased threefold upon exposure to a mite, whereas the activity among uninfected males increased by 17-fold in the presence of a questing mite. Among infected flies, the risk of further infection also generated a change in activity, but the magnitude of the response was dependent on host sex. Current infection status influenced the probability of acquiring more parasites due to increased susceptibility to infection with mite load. The probability of acquiring additional mites among males increased more rapidly compared to female flies. Current infection status can potentially determine the risk of further infection, the host propensity and ability to disperse, with consequence for hosts and parasites at the individual, population and species level.