Introduction
Digenea is a class of Trematoda that comprises a large group of organisms with significant medical and veterinary interest. Over 100 species of digenetic trematodes have been reported infecting humans, many of them transmitted through food. Food-borne trematodiases constitute one of the most neglected tropical diseases group and includes liver flukes, lung flukes and intestinal flukes. Commonly, these parasitic infections have been ignored both in terms of research funding and presence in the public media. More than 40 million people are currently infected and about 10% of the world's population live at risk of infection (Keiser and Utzinger, Reference Keiser and Utzinger2009; Sripa et al., Reference Sripa, Kaewkes, Intapan, Maleewong and Brindley2010; Toledo et al., Reference Toledo, Alvárez-Izquierdo, Muñoz-Antoli and Esteban2019). In the past, these infections were limited for the most part in populations living in low-income countries, particularly in southeast Asia, and were associated with poverty. However, the geographical limits and the population at risk are currently expanding and changing in relation to factors such as growing international markets, improved transportation systems and demographic changes.
The present review focuses on two intestinal food-borne trematodiases of importance: echinostomiasis, caused by members of the family Echinostomatidae, and gastrodiscoidiasis produced by the amphistome Gastrodiscoides hominis. These parasitic diseases are associated with practices of eating raw or insufficiently cooked molluscs, fish, crustaceans, amphibians, aquatic plants, promiscuous defecation and the use of human excrements collected from latrines for fertilization of ponds. They are aggravated by socioeconomic factors including poverty, malnutrition, a growing free-food market, a lack of supervised food inspection, poor or insufficient sanitation, other helminthiases and declining economic conditions. In this context, further studies are urgently needed to clarify the current epidemiology of these helminth infections and to identify new and specific targets for both effective diagnosis and treatments (Graczyk and Fried, Reference Graczyk and Fried1998; Chai, Reference Chai, Fried and Toledo2009; Toledo and Esteban, Reference Toledo and Esteban2016; Toledo et al., Reference Toledo, Alvárez-Izquierdo, Muñoz-Antoli and Esteban2019).
Herein, we will summarize the main features of the biology, clinical, epidemiology and current diagnostic tools of these food-borne trematode infections and the corresponding diseases they cause.
Echinostomiasis
Echinostomiasis is the parasitic disease caused by echinostomes. Under this term are included the trematodes belonging to the family Echinostomatidae. This family constitutes a heterogeneous group of hermaphroditic digeneans comprising 50 genera and more than 350 species (Yamaguti, Reference Yamaguti1971; Chai and Jung, Reference Chai and Jung2020). Adult echinostomes commonly inhabit the intestine of a wide range of birds and mammals, including humans. Eventually, some species of echinostomes have been also recorded in reptiles and fishes (Toledo and Esteban, Reference Toledo and Esteban2016; Toledo et al., Reference Toledo, Alvárez-Izquierdo, Muñoz-Antoli and Esteban2019). Echinostomes are characterized by the presence of a head collar of spines arranged in one or two circles around the oral sucker. The number and disposition of the spines constitute an important taxonomic feature (Fig. 1). However, systematics of echinostomes is very complex and a subject of continuous reviews and phylogenetic analyses (Chai et al., Reference Chai, Cho, Chang, Jung and Sohn2020a; Izrailskaia et al., Reference Izrailskaia, Besprozvannykh and Tatonova2021; Pham et al., Reference Pham, Saijuntha, Lawton and Le2022).
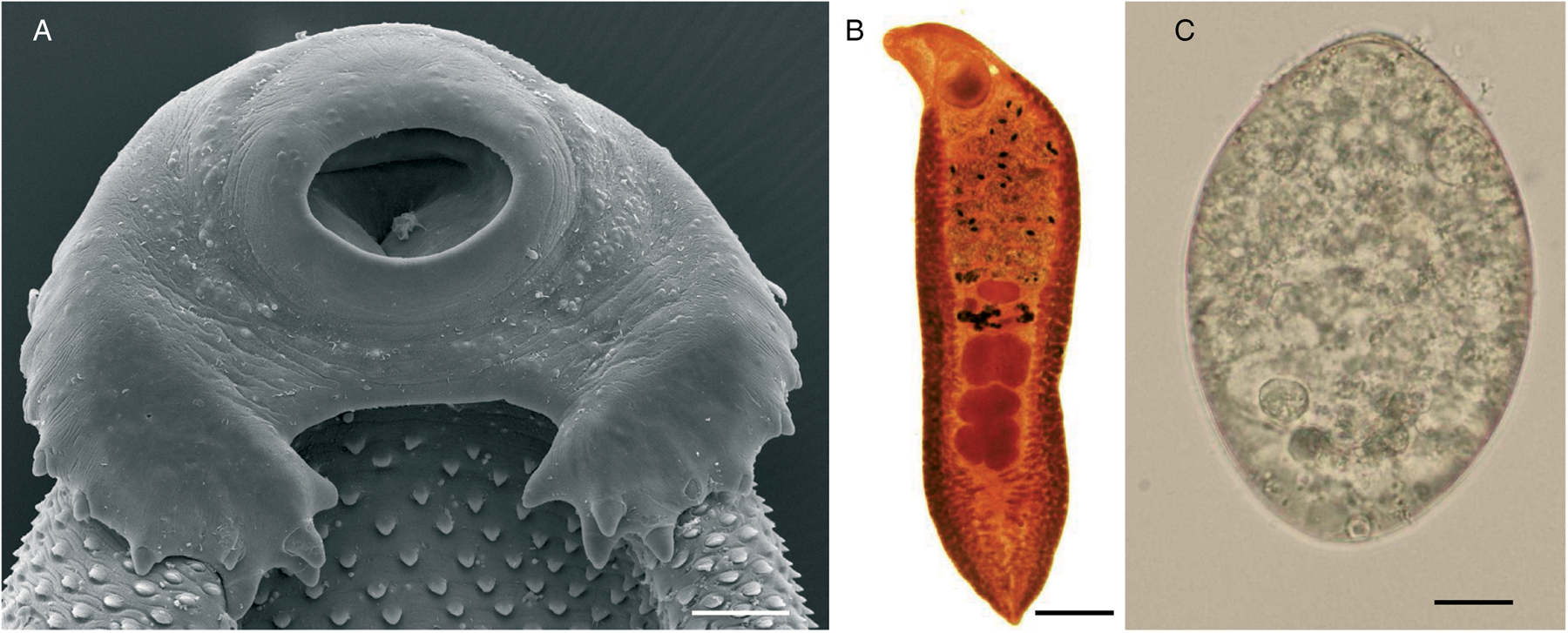
Fig. 1. Echinostoma sp.: (A) scanning electron micrographs of the cephalic collar of spines (scale bar: 100 μm); (B) adult worm (scale bar: 1 mm) and (C) unembryonated egg (scale bar: 10 μm).
Although echinostomes are distributed worldwide, human echinostomiasis mainly occurs at endemic foci in southeast Asia and Far East due to the eating habits of those areas. However, this scenario may be changing due to several factors such as changes in the eating habits in Western countries, internationalization of food markets, migratory flows and the progressive improvement of transportation systems (Graczyk and Fried, Reference Graczyk and Fried1998; Chai, Reference Chai, Fried and Toledo2009; Toledo and Esteban, Reference Toledo and Esteban2016; Toledo et al., Reference Toledo, Alvárez-Izquierdo, Muñoz-Antoli and Esteban2019).
Morphology and life cycle
Adult worms of the family Echinostomatidae are characterized by the presence of a head collar with collar spines around the oral sucker (Fig. 1A). The number and arrangement of collar spines are important features for taxonomic purposes. Considerable variation exists in size of echinostomes depending upon species, fixation procedures, definitive host and crowding effect and size ranges about 2–10 × 1–2 mm2. The spines of the cephalic collar may be arranged in one or two circles and the number of spines is constant within the species. The tegument contains scale-like spines on both dorsal and ventral surfaces, though the number and size of the spines are reduced in the posterior half of the body. The oral and ventral suckers are close to each other. The two testes, usually in tandem, are posterior to the ovary. The uterus is intercaecal and normally pre-ovarian. The vitellarium is follicular, in two lateral fields, usually in the hindbody but may extend into the forebody (Fig. 1B).
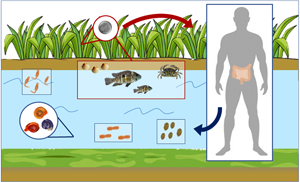
Members of the Echinostomatidae follow a three-host life cycle (Fig. 2). The first intermediate hosts are aquatic snails in which subsequently a single generation of sporocysts, two generations of rediae and, finally, cercariae develop within the second generation of rediae. After emergence of the cercariae in an aquatic environment, they infect the second intermediate host, which may be several species of snails, clams, frogs and even fishes in which cercariae encyst to form the metacercariae. The definitive host becomes infected after eating of the second intermediate host harbouring the encysted metacercariae (Huffman and Fried, Reference Huffman and Fried1990; Fried et al., Reference Fried, Graczyk and Tamang2004; Toledo et al., Reference Toledo, Esteban and Fried2009).
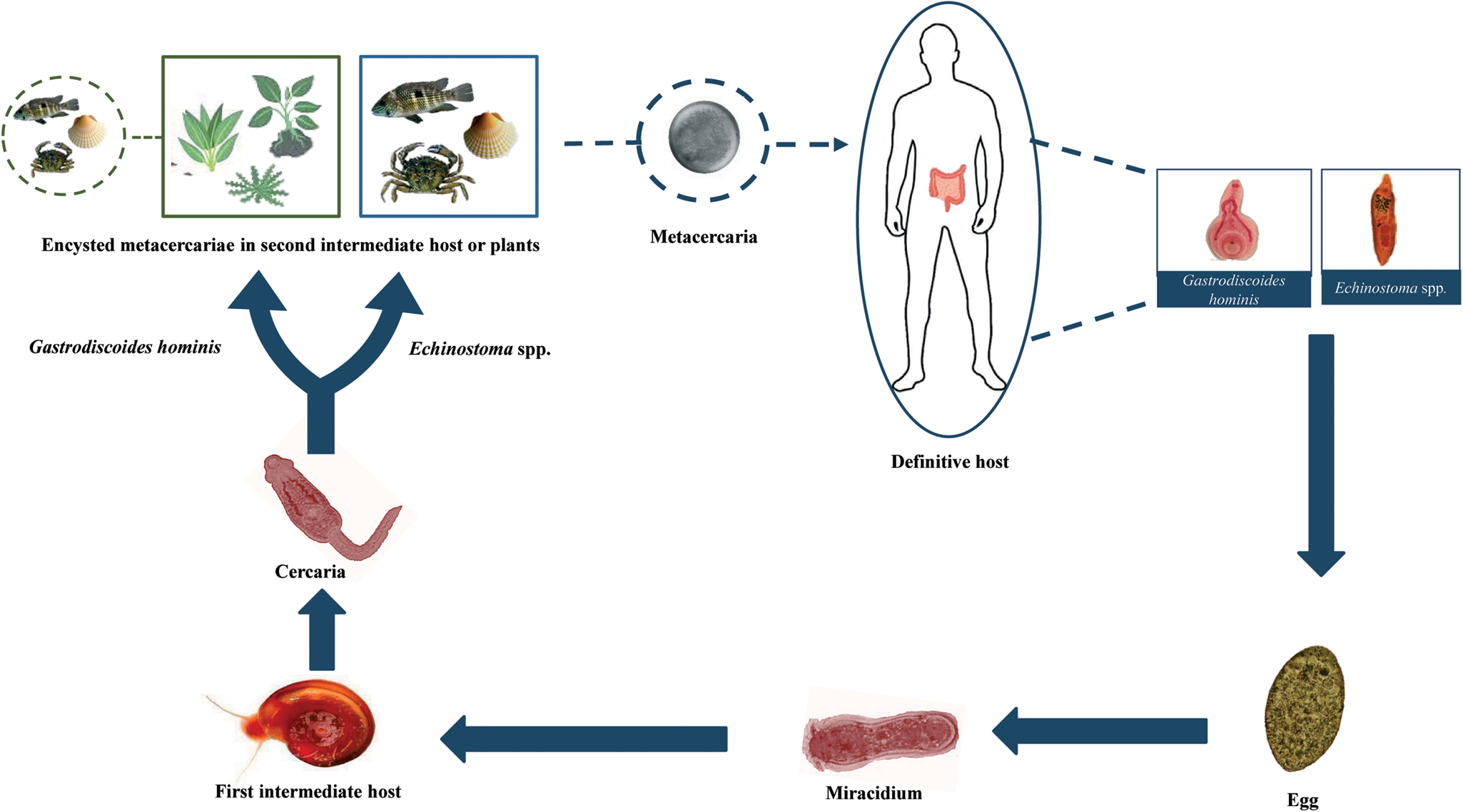
Fig. 2. Generalized life cycle of echinostomes and Gastrodiscoides hominis: adult worms inhabit the small intestine of several vertebrate hosts, including humans; eggs are voided with host feces and miracidia hatch in freshwater and actively infect the snail first intermediate host; cercariae are released by the first intermediate host and swim to locate the second intermediate host (snails, amphibians, bivalves and fishes) which they penetrate in the case of echinostomes and on aquatic plants or, alternatively, aquatic animals in the case of G. hominis; cercariae become metacercariae after encystation; metacercariae are ingested by the definitive host and excyst to become adults.
Strikingly, Miao et al. (Reference Miao, Dong, Zhou and Zhou2021) recently detected an ectopic echinostomiasis. An adult worm of Echinostoma sp. was detected in the urinary bladder of a 67-year-old man in Zhejiang (China) by cystoscopy. The patient complained of haematuria and dysuria but he did not show intestinal symptoms. The authors suggested that the metacercariae could penetrate through the intestinal wall into the urinary bladder, where the parasite attained maturity.
Epidemiology
Human echinostome infections are a common feature since ancient times (Seo et al., Reference Seo, Shim, Lee, Kim, Hong, Kim, Chai and Shin2020). Currently, human echinostomiasis is endemic in southeastern and northeastern Asia. However, the number and identity of the species involved in human infections are difficult to ascertain in relation to: (1) problematical taxonomy of the family that complicates the specific description due to continuous synonymies and misidentifications; (2) a lack of systematic surveys and (3) most of the reports are based on occasional records with uncertain, incomplete or lacking specific identifications, even today. For example, Li et al. (Reference Li, Liu, Zhou, Zhang, Wang, Ma, Chen, Zou, Cao and Li2019) detected eggs of an echinostome in the feces of a 42-year-old man from the northern area of China that was simply diagnosed as a member of Echinostomatidae. Similarly, Sah et al. (Reference Sah, Khadka, Hamal and Poudyal2018) reported the first case of human echinostomiasis in Nepal in a 62-year-old male resident in Gorkha district caused by an unidentified member of the genus Echinostoma. Consumption of insufficiently cooked snails and fishes appeared to be the cause of infection. Khanna et al. (Reference Khanna, Ashraf and Khanna2019) recorded a single case attributed to an Echinostoma species in a 3-year-old male child with severe anaemia in Karnataka (India). In fact, most recent reviews differ in the number of species causing this parasitic disease. Toledo and Esteban (Reference Toledo and Esteban2016) compiled a total of 23 species belonging to 9 different genera of Echinostomatidae. Toledo et al. (Reference Toledo, Alvárez-Izquierdo, Muñoz-Antoli and Esteban2019) listed a total of 20 species but, more recently, Chai and Jung (Reference Chai and Jung2020) considered 15 major species (having more than 6 reports each) and other 8 species (with fewer than 5 reports). Although these difficulties exist, we have listed a total of 23 species of echinostomes that have been recorded in humans, together with their main features. Table 1 compiles these species, together with the geographical locations of human infections.
Table 1. Species of Echinostomatidae involved in human infections and geographical location of cases (only those reports with specific description have been considered)

a Syn. Echinostoma malayanum.
b Syn. Episthmium caninum.
c Experimental infection.
d Syn Echinostoma echinatum.
e Imported infection.
f Syn. Echinostoma hortense.
Although echinostomes are spread worldwide, the source of infection strongly determines the geographical distribution of human echinostomiasis. Humans become infected after eating raw or undercooked freshwater or brackish water molluscs, fishes, snakes, crustaceans and amphibians (tadpoles or frogs) harbouring the infective metacercariae (Carney, Reference Carney1991; Toledo et al., Reference Toledo, Esteban and Fried2012, Reference Toledo, Alvárez-Izquierdo, Muñoz-Antoli and Esteban2019; Toledo and Esteban, Reference Toledo and Esteban2016; Chai and Jung, Reference Chai and Jung2020). Thus, echinostomiasis mainly occurs in areas where traditional cultural practices encourage ingestion of those types of foods such as Asia, mainly in southeastern and northeastern Asia. Commonly, echinostomiasis is highly focal and most of the cases have been reported in China, India, Indonesia, Korea, Malaysia, the Philippines, Russia, Taiwan and Thailand (Chai, Reference Chai, Fried and Toledo2009; Toledo and Esteban, Reference Toledo and Esteban2016). Occasional cases have also been reported in other countries (see Table 1).
The current incidence of human echinostomiasis is not known. Most of the available information is based on sporadic reports with scarce information and, in many cases, lacking specific identification as mentioned above. Moreover, microscopists may misinterpret echinostome eggs, particularly considering that eggs of different echinostome species markedly resemble making difficult specific diagnosis (Esteban et al., Reference Esteban, Muñoz-Antoli, Toledo and Ash2019; Chai et al., Reference Chai, Jung, Chang, Shin, Sohn, Eom, Yong, Min, Phammasack, Insisiengmay and Rim2020b). The only estimate available is the one made by WHO (2004) for Echinostoma hortense (currently synonym of Isthmiophora hortensis) (50 000 people infected), Echinochasmus japonicus (about 5000 cases) and Echinostoma cinetorchis and Acanthoparyphium tyosenense (with about 1000 cases each). This evidences the need for systematic epidemiological surveys, especially in areas where consumption of raw or undercooked intermediate hosts of echinostomes is a common habit.
As mentioned above, most of the cases of human echinostomiasis have been reported in Asia and commonly appear as endemic foci in areas where the presence of intermediate host and the natural definitive hosts (mainly waterfowls) coexist with the human practice of eating undercooked intermediate hosts (Toledo and Esteban, Reference Toledo and Esteban2016). Exceptionally, drinking of contaminated water harbouring metacercariae may be the source of infection (Xiao et al., Reference Xiao, Dabing, Tianping, Jianfeng, Chuang, Banju, Jingshen, Hongchuan, Mingrong, Weido, Jide and Xu1995). There are no data on the seasonality of the transmission, but it cannot be discarded as those that occurred with other trematodes (Kopolrat et al., Reference Kopolrat, Sithithaworn, Kiatsopit, Namsanor, Laoprom, Tesana, Andrews and Petney2020).
The highest number of echinostome species has been reported in China. A total of 13 species has been recorded in this country, especially in provinces of southeast China such as Fujian, Guangdong, Liaoning, Anhui, Yunnan and Hubei (Toledo et al., Reference Toledo, Alvárez-Izquierdo, Muñoz-Antoli and Esteban2019). Echinochasmus liliputanus was the first species discovered in humans from China. A prevalence of 13.4% was reported in Anhui in 1991 (Yu and Mott, Reference Yu and Mott1994) and, therefore, more than 2500 human infections have been recorded in this province (Xiao et al., Reference Xiao, Wang, Zheng, Shen and Tian2005). In Fujian province, Echinochasmus fujianensis appears to be the most common species attaining a prevalence of 1.6–7.8% among residents of 5 areas, children from 3 to 15-year-old being the most affected population segment with about two-thirds of all cases (Yu and Mott, Reference Yu and Mott1994). In some counties of Guangdong and Fujian, E. japonicus is a common human infection with prevalences of 4.9%, whereas Echinochasmus perfoliatus has been usually reported attaining prevalences of 1.8% (Yu and Mott, Reference Yu and Mott1994). Human infections with other echinostome species in China are more anecdotical. Echinostoma revolutum has been found in Yunnan and Guangdong provinces and 6 patients who had eaten raw loach were found to be infected with I. hortensis in Liaoning province (Chen et al., Reference Chen, Feng and Qian1993). Moreover, a single case of infection with Echinochasmus jiufoensis was detected during the autopsy of a 6-month-old girl in Guangzhou (Liang and Ke, Reference Liang and Ke1988).
A total of five species has been reported in humans from Indonesia, mainly in the major islands (Sumatra, Java and Sulawesi). Infections were related to the local habit of eating raw bivalves (Corbicula spp.) from lakes. An average prevalence of 43%, but attaining 96%, of Echinostoma lindoense was reported from 1937 to 1956 in residents in the lake Lindu Valley (Sulawesi) (Carney et al., Reference Carney, Sudomo and Purnomo1980). Moreover, in the area of Borneo the finding of echinostome eggs in stool specimens of residents was common (Cross and Basaca-Sevilla, Reference Cross and Basaca-Sevilla1986). The popularity of Korean and Japanese restaurants in Indonesia led to an increase in the number of cases (Kusharyono and Sukartinah, Reference Kusharyono and Sukartinah1991).
In Lao PDR, Eom et al. (Reference Eom, Yong, Sohn, Chai, Min, Rim, Jeon, Banouvong, Insisiengmay and Phommasack2014) estimated a prevalence of 0.7%. A total of 6 species of echinostomes has been found infecting humans, with special incidence in Riparian villages along the Mekong river. In Khammouane province, a prevalence of 1.1% of echinostome eggs belonging to E. revolutum, Artyfechinostomum malayanum, E. japonicus and Echinoparyphium recurvatum was detected in the feces of residents (Chai et al., Reference Chai, Sohn, Yong, Eom, Min, Hoang, Phammasack, Insisiengmay and Rim2012). Moreover, Sayasone et al. (Reference Sayasone, Tesana, Utzinger, Hatz, Akkhavong and Odermatt2009) detected three human cases of E. japonicus. Echinochasmus caninus has been recently recorded for the first time in Lao PDR infecting humans. Cases of E. japonicus were associated with eating raw or insufficiently cooked freshwater fish in a traditional food locally known as ‘lap-pa’, ‘Koi-pa’ or ‘som-pa’. A total of 11 cases in Riparian people was detected in Khammouane (Chai et al., Reference Chai, Chang, Jung, Shin, Sohn, Eom, Yong, Min, Phammasack, Insisiengmay and Rim2019). All the patients were co-infected other intestinal helminths. In Savannakhet province, Sayasone et al. (Reference Sayasone, Tesana, Utzinger, Hatz, Akkhavong and Odermatt2009) detected three cases. Recently, several cases have been additionally reported in this province. Chai et al. (Reference Chai, Sohn, Cho, Eom, Yong, Min, Hoang, Phommasack, Insisiengmay and Rim2018) recorded the first human case of Echinostoma ilocanum in Lao PDR and, moreover, Echinostoma aegyptica was found in five Riparian people for the first time in this country (Chai et al., Reference Chai, Jung, Chang, Shin, Sohn, Eom, Yong, Min, Phammasack, Insisiengmay and Rim2020b). In southern Lao PDR, a prevalence of 6% was estimated by Sayasone et al. (Reference Sayasone, Mak, Vanmany, Rasphone, Vounatsou, Utzinger, Akkhavong and Odermatt2011).
Several species of echinostomes have been discovered infecting humans in Cambodia (Table 1). A prevalence of 1% of E. ilocanum was estimated in the Oddar Meanchey province (Sohn et al., Reference Sohn, Kim, Yong, Eom, Jeong, Kim, Kang, Kim, Park, Ji, Sinuon, Socheat and Chai2011a) and of 7.5–22.4% of E. revolutum in schoolchildren from the Pursat province causing gastrointestinal symptoms (Chai et al., Reference Chai, Shin, Lee and Rim2009, Reference Chai, Sohn, Cho, Eom, Yong, Min, Hoang, Phommasack, Insisiengmay and Rim2018; Sohn et al., Reference Sohn, Chai, Yong, Eom, Yoon, Sinuon, Socheat and Lee2011b). The source of infection appeared to be the eating of undercooked snails or clams sold on the way back home from school (Sohn et al., Reference Sohn, Chai, Yong, Eom, Yoon, Sinuon, Socheat and Lee2011b). Recently, a new species (Echinostoma mekongi) has been described on the basis of adult flukes collected from Riparian people residing along the Mekong river. Parasites were detected in six Riparian people from the localities of Kratie and Takeo (Cho et al., Reference Cho, Jung, Chang, Sohn, Sinuon and Chai2020).
In Taiwan, three species of echinostomes have been recorded with a prevalence ranging from 0.11 to 0.65% in some areas (Lu, Reference Lu1982; Carney, Reference Carney1991). A total of four species has been found in Thailand, seven species in Korea, four different species in India and two in the Philippines (Table 1). However, most of these cases were occasional.
Human echinostomiasis is very uncommon outside Asia. Moreover, most of these cases were imported, occurring mainly in Asian refugees or tourists to Africa (DeGirolami and Kimber, Reference DeGirolami and Kimber1983; Poland et al., Reference Poland, Navin and Sarosi1985; Chai, Reference Chai, Murrell and Fried2007; Chunge and Chunge, Reference Chunge and Chunge2017).
Immunology
Little is known about the immunology of human echinostomiasis. Most of the current knowledge on immunology of echinostome infections is based on experimental studies in laboratory rodents. Echinostomes induce strong antibody responses both at systemic and mucosal levels (Graczyk and Fried, Reference Graczyk and Fried1994; Cho et al., Reference Cho, Ryang, Kim, Park, Im and Lee2007; Sotillo et al., Reference Sotillo, Muñoz-Antoli, Marcilla, Fried, Guillermo Esteban and Toledo2007, Reference Sotillo, Cortés, Muñoz-Antoli, Fried, Esteban and Toledo2014). However, this response markedly depends on the host species. At the serum level, energic immunoglobulin M (IgM), total IgG, IgG1 and IgG3 have been observed in mice infected with Echinostoma caproni, but, in contrast, only weak responses were observed in rats (Sotillo et al., Reference Sotillo, Muñoz-Antoli, Marcilla, Fried, Guillermo Esteban and Toledo2007). In I. hortensis-infected mice an elevation of IgG1, IgE and IgA was detected (Cho et al., Reference Cho, Ryang, Kim, Park, Im and Lee2007). At the mucosal level, intense responses of IgM, IgA, IgG1 and IgG2a were reported in E. caproni-infected mice and only elevated levels of IgG2a were observed in rats (Sotillo et al., Reference Sotillo, Muñoz-Antoli, Marcilla, Fried, Guillermo Esteban and Toledo2007). A total of four proteins (enolase, aldolase, actin and the 70 kDa heat-shock protein) was found to be the most immunogenic antigens (Sotillo et al., Reference Sotillo, Muñoz-Antoli, Marcilla, Fried, Guillermo Esteban and Toledo2007). Moreover, the glycosylation of parasite antigens appears to be essential for antibody responses, suggesting that T-independent responses are of great importance (Sotillo et al., Reference Sotillo, Cortés, Muñoz-Antoli, Fried, Esteban and Toledo2014). Strikingly, it has been shown that echinostomes are able to trap and degrade antibodies bound to their surface by secreted cathepsin L-like peptidases (Cortés et al., Reference Cortés, Sotillo, Muñoz-Antolí, Molina-Durán, Esteban and Toledo2017a, Reference Cortés, Mikeš, Muñoz-Antolí, Álvarez-Izquierdo, Esteban, Horák and Toledo2019).
Resistance to echinostome infections has been traditionally associated with the development of Th2 responses, mainly mediated by interleukin 4 (IL-4) and/or IL-13 (Brunet et al., Reference Brunet, Joseph, Dunne and Fried2000; Toledo and Fried, Reference Toledo and Fried2005; Sotillo et al., Reference Sotillo, Trelis, Cortés, Fried, Marcilla, Esteban and Toledo2011; Cortés et al., Reference Cortés, Muñoz-Antoli, Esteban and Toledo2017b; Toledo et al., Reference Toledo, Alvárez-Izquierdo, Muñoz-Antoli and Esteban2019). However, recent studies suggest that resistance depends on the ability of the host to maintain the mucosal homoeostasis in a process mediated by IL-25 (Muñoz-Antoli et al., Reference Muñoz-Antoli, Cortés, Santano, Sotillo, Esteban and Toledo2016; Álvarez-Izquierdo et al., Reference Álvarez-Izquierdo, Pérez-Crespo, Esteban, Muñoz-Antoli and Toledo2020a, Reference Álvarez-Izquierdo, Esteban, Muñoz-Antoli and Toledo2020b). Resistance is associated with hyperplasia of local tuft cells and production of IL-25 which operates by facilitating the maintenance of the epithelial homoeostasis that could be ultimately responsible for parasite rejection (Muñoz-Antoli et al., Reference Muñoz-Antoli, Cortés, Santano, Sotillo, Esteban and Toledo2016; Cortés et al., Reference Cortés, Muñoz-Antoli, Esteban and Toledo2017b; Álvarez-Izquierdo et al., Reference Álvarez-Izquierdo, Pérez-Crespo, Esteban, Muñoz-Antoli and Toledo2020a, Reference Álvarez-Izquierdo, Esteban, Muñoz-Antoli and Toledo2020b).
Echinostoma spp. also induces changes at the cellular level and in the expression of certain glycoconjugates in the intestinal mucosa (Toledo et al., Reference Toledo, Esteban and Fried2006a). Mastocytosis, eosinophilic infiltration and increase in the goblet cells and mast cell populations have been observed (Fujino et al., Reference Fujino, Fried and Tada1993, Reference Fujino, Fried, Ichikawa and Tada1996a, Reference Fujino, Fried, Ichikawa and Tada1996b; Fujino and Fried, Reference Fujino and Fried1993, Reference Fujino and Fried1996; Kim et al., Reference Kim, Im, Lee and Ryang2000; Park et al., Reference Park, Lee and Kim2005; Toledo et al., Reference Toledo, Monteagudo, Espert, Fried, Esteban and Marcilla2006b; Muñoz-Antoli et al., Reference Muñoz-Antoli, Sotillo, Monteagudo, Fried, Marcilla and Toledo2007; Ryang et al., Reference Ryang, Yang, Kim, Lee, Sung, Kim and Kim2007). Moreover, alterations of the terminal sugar of the mucins produced by goblet cells may regulate the worm expulsion (Fujino and Fried, Reference Fujino and Fried1993, Reference Fujino and Fried1996; Park et al., Reference Park, Lee and Kim2005).
Clinical manifestations
In heavy infections, echinostomes may cause more severe symptoms than other intestinal trematodes. Commonly, major clinical symptoms of human echinostomiasis may include unspecific symptoms such as diarrhoea, loss of body weight, epigastric and abdominal pain or easy fatigue. However, additional symptoms may include local eosinophilia, anaemia, oedema and anorexia (Graczyk and Fried, Reference Graczyk and Fried1998; Toledo et al., Reference Toledo, Esteban and Fried2006a, Reference Toledo, Alvárez-Izquierdo, Muñoz-Antoli and Esteban2019; Toledo and Esteban, Reference Toledo and Esteban2016). Recently, severe anaemia has been associated with echinostomiasis in a 3-year-old male child concomitantly infected with Echinostoma sp., Trichuris trichiura and Ascaris lumbricoides in India (Khanna et al., Reference Khanna, Ashraf and Khanna2019). In I. hortensis-infected patients, nausea and vomiting, headache, acid belching and urinary incontinence were observed (Chang et al., Reference Chang, Sohn, Ryu, Kang and Hong2005). Mucosal damage may include signs of chronic gastritis, infiltration of inflammatory cells, mucosal erosion and bleeding and lesions similar to adenocarcinoma (Chang et al., Reference Chang, Sohn, Ryu, Kang and Hong2005).
In ectopic locations such as the urinary bladder, infection was characterized by haematuria with 305 red blood cells per μL (normal 0–13), 297 white blood cells per μL (normal 0–9) and a positive result of 2+ for occult blood in the urine. Moreover, haematuria was accompanied by urgency and dysuria (Miao et al., Reference Miao, Dong, Zhou and Zhou2021).
Diagnosis and treatment
Laboratory diagnosis of echinostomiasis is based on the finding of eggs in feces. The eggs are yellow-brown and thin-shelled with an operculum, and a slight thickening of the shell at the abopercular end. They are unembryonated when passed in feces. The size of human-infecting echinostome eggs ranges 66–145 μm × 43–90 μm (Esteban et al., Reference Esteban, Muñoz-Antoli, Toledo and Ash2019). However, differentiation of echinostome species, and even from other trematodes, on the basis of the egg morphology is difficult and recovery and identification of adult worms if possible is recommended. The similarity among the eggs of other trematodes must be taken into account to avoid confusion, especially with those that are carcinogenic such as Opisthorchis viverrini (Crellen et al., Reference Crellen, Sithithaworn, Pitaksakulrat, Khuntikeo, Medley and Hollingsworth2021). Occasionally, human echinostomiasis has been revealed by gastroduodenal endoscopy performed in relation to severe epigastric symptoms and ulcerative lesions in the stomach and duodenum (Lee and Hong, Reference Lee and Hong2002; Cho et al., Reference Cho, Tak, Kweon, Kim, Choi, Kong and Chung2003; Park and Kim, Reference Park and Kim2006; Jung et al., Reference Jung, Lee, Kim, Kim, Na and Sohn2014).
Praziquantel is the drug of choice for intestinal fluke infections although it is not included in the US product labelling for these infections. A single dose of 25 mg kg−1 of praziquantel is recommended for treatment of intestinal fluke infections. Echinostome infections can be treated successfully with slightly lower – single, oral 10–20 mg kg−1 praziquantel (Chai et al., Reference Chai, Shin, Lee and Rim2009). Praziquantel is 90% absorbed after ingestion and then rapidly metabolized in the liver and the hydroxylated and conjugated products are excreted mainly in the urine within 24 h. Side-effects are minimal and include abdominal pain, nausea, headache and dizziness (WHO, 1995).
Gastrodiscoidiasis
Gastrodiscoidiasis is a plant-borne parasitic disease caused by the intestinal amphistome G. hominis. Adult worms are pyramidal in shape and measure 8–14 × 5.5–7.5 mm2 (Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2005, Reference Mas-Coma, Bargues and Valero2006). Morphologically, they are characterized by a dorsoventrally flattened body with an anterior part short and cylindrical and the posterior portion larger and discoidal. The most characteristic features of G. hominis include a subterminal pharynx, tandem, lobed testes, a post-testicular ovary, an ascending uterus and a ventral genital pore (Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2005, Reference Mas-Coma, Bargues and Valero2006).
Life cycle
Life cycle of G. hominis is not well known (Fig. 2). Adult flukes inhabit the caecum and colon of several mammals, where it remains attached to the mucosa (Fig. 3A). Pigs appear to be the normal definitive host (Dutt and Srivastava, Reference Dutt and Srivastava1972), but it also has been reported in monkeys, mice, rats, wild boars, muskrats and humans (Easwaran et al., Reference Easwaran, Reghu and Pillai2003; Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2005, Reference Mas-Coma, Bargues and Valero2006). Unembryonated eggs (Fig. 3B) are laid in freshwater environments and a miracidium hatches and swims to infect the first intermediate host. Only the aquatic snail Helicorbis coenosus is known to act as the first intermediate host (Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2006; Toledo et al., Reference Toledo, Alvárez-Izquierdo, Muñoz-Antoli and Esteban2019). After the development of daughter and mother rediae, cercariae emerge and swim in the freshwater to reach aquatic plants, where they encyst as metacercariae. Metacercarial encystment can also occur in snails, tadpoles, frogs and crayfishes. Definitive host becomes infected when encysted metacercariae are swallowed with tainted vegetation or with animal products, such as raw or undercooked crustaceans, molluscs or amphibians (Fried et al., Reference Fried, Graczyk and Tamang2004; Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2005, Reference Mas-Coma, Bargues and Valero2006; Toledo et al., Reference Toledo, Alvárez-Izquierdo, Muñoz-Antoli and Esteban2019).
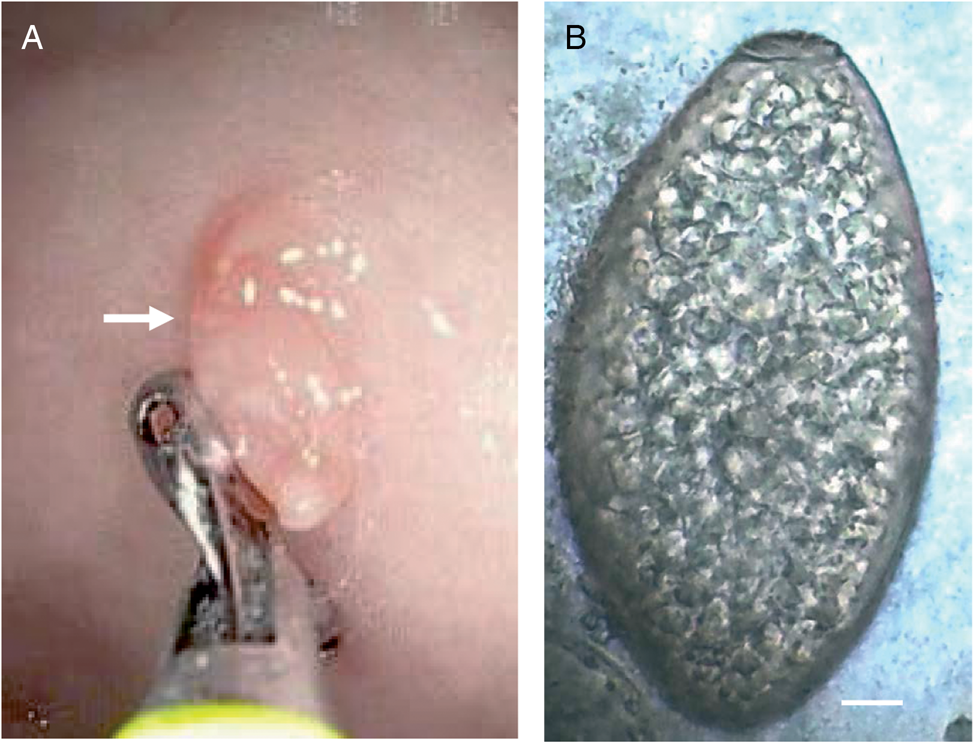
Fig. 3. Gastrodiscoides hominis: (A) image of an upper endoscopy showing a juvenile living adult worm fixed in the mucosa of a human patient (arrow) and (B) egg isolated from the stools of a human patient (scale bar: 10 μm). Photomicrographs: courtesy of Dr Ranjit Sah.
Epidemiology
First cases of human gastrodiscoidiasis were reported in India. Thereafter, human cases have been found in Burma, Nepal, Pakistan, Myanmar, Vietnam, the Philippines, Thailand, China, Kazakhstan, Indian immigrants in Guyana, Zambia, Nigeria and the Volga Delta in Russia (Yu and Mott, Reference Yu and Mott1994; Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2005; Sah et al., Reference Sah, Acosta and Toledo2019; Toledo et al., Reference Toledo, Alvárez-Izquierdo, Muñoz-Antoli and Esteban2019). Although G. hominis is not a common parasite of humans, high prevalences have been found in some areas, especially in India. Buckley (Reference Buckley1939) reported a prevalence of 41% in children from Assam and a prevalence of 27% was recorded in people from Bareilly (Dutt and Srivastava, Reference Dutt and Srivastava1972).
Most of the cases appear to be related to the consumption of tainted vegetables directly collected from ponds and rivers, which is a common practice in some Asian countries (Sah et al., Reference Sah, Acosta and Toledo2019) or even of vegetables bought in local markets. Uzairue et al. (Reference Uzairue, Ugbor, Ezeah, Eze and Nwadike2013) analysed a total of 250 samples of 7 different vegetables in markets from Ekpoma (Nigeria) and detected that 0.9% of cabbage samples were contaminated with metacercariae of G. hominis. Moreover, infection with metacercariae encysted in animal products, such as raw or undercooked crustaceans, molluscs or amphibians also may occur (Fried et al., Reference Fried, Graczyk and Tamang2004).
Clinical symptoms, diagnosis and treatment
Human gastrodiscoidiasis is commonly asymptomatic. In heavy infections, epigastric pain, abdominal discomfort, diarrhoea and headache may occur (Toledo et al., Reference Toledo, Alvárez-Izquierdo, Muñoz-Antoli and Esteban2019). Moreover, other symptoms including lymphadenopathy, hepatosplenomegaly or anaemia have been reported in individual patients (Dada-Adegbola et al., Reference Dada-Adegbola, Falade, Oluwatoba and Abiodun2004; Sah et al., Reference Sah, Acosta and Toledo2019). At the local level, inflammation at the site of attachment due to dragging with the acetabulum, surface desquamation, infiltration with eosinophils, lymphocytes and plasma cells appears in the lesions caused by the fixation of the parasites to the mucosa. Hypersecretion of mucus and necrosis of the mucous glands are also observed (Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2005, Reference Mas-Coma, Bargues and Valero2006).
Diagnosis of human gastrodiscoidiasis is performed by detection of eggs in feces. The egg is operculated, non-embryonated and measuring about 150 × 70 mm2 (Fig. 3B) (Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2005, Reference Mas-Coma, Bargues and Valero2006; Esteban et al., Reference Esteban, Muñoz-Antoli, Toledo and Ash2019; Toledo et al., Reference Toledo, Alvárez-Izquierdo, Muñoz-Antoli and Esteban2019). Praziquantel is the drug of choice. However, a single dose of 500 mg mebendazole also may be efficient (Dada-Adegbola et al., Reference Dada-Adegbola, Falade, Oluwatoba and Abiodun2004; Mas-Coma Et al., Reference Mas-Coma, Bargues and Valero2005).
Concluding remarks
Human echinostomiasis and gastrodiscoidiasis are among the most neglected intestinal trematode infections. Both parasite infections have been considered as minor diseases mainly confined to some areas of Asia. However, the real prevalence of these infections is not known, which makes necessary further efforts and systematic helminthological surveys to determine the real impact on human health. This is of particular importance considering that several factors such as growing international markets, new eating habits in developed countries or demographic changes may be expanding their risk of infection and prevalence in areas where these diseases were not known. Thus, further studies are required to elaborate new maps of risk and a more detailed follow-up may be useful to gain a better understanding of the current incidence of these diseases.
Financial support
Research at Universitat de València was supported by Ministerio de Sanidad y Consumo (Madrid, Spain) (No. RD12/0018/0013, Red de Investigación Cooperativa en Enfermedades Tropicales – RICET, IV National Program of I + D + I 2008–2011, ISCIII–Subdirección General de Redes y Centros de Investigación Cooperativa and FEDER).
Conflict of interest
The authors declare none.