Introduction
Parasitoid wasps, a diverse group within the order Hymenoptera, exhibit a unique life cycle that involves parasitizing a host and usually resulting in its death (Godfray, Reference Godfray1994; Quicke, Reference Quicke1997). The adult female parasitoid typically oviposits its eggs within or on the immature stages of a host insect or other arthropod. During this oviposition process, various parasitoid-associated factors originating from the reproductive system, including venom, polydnaviruses, virus-like particles, fibrous layer and ovarian fluids are injected (Beckage and Gelman, Reference Beckage and Gelman2004; Asgari, Reference Asgari2006). These factors create a favourable environment for the parasitoid offspring, promoting their successful development by inducing host paralysis, hindering host development and hindering the host's immune response (Huw Davies and Vinson, Reference Huw Davies and Vinson1986; Huang et al., Reference Huang, Chen, Fang, Pang, Zhou, Zhou, Pan, Zhang, Sheng, Lu, Liu, Zhang, Li, Shi, Chen and Zhan2021). Venom systems are commonly found throughout Hymenoptera, and their general structure has been thoroughly investigated in various ichneumonid and braconid wasps (Edson and Vinson, Reference Edson and Vinson1979; Edson et al., Reference Edson, Barlin and Vinson1982; Quicke et al., Reference Quicke, Tunstead, Falco and Marsh1992, Reference Quicke, Achterberg and Godfray1997; Zaldivar-Riverón et al., Reference Zaldivar-Riverón, Areekul, Shaw and Quicke2004). The structural characteristics of the venom apparatus have also been extensively investigated across other Hymenopteran families, including social bees (Bridges and Owen, Reference Bridges and Owen1984; Roat et al., Reference Roat, Nocelli and da Cruz Landim2006), wasps (Schoeters and Billen, Reference Schoeters and Billen1995a; Gnatzy and Volknandt, Reference Gnatzy and Volknandt2000), ants (Billen, Reference Billen1990; Schoeters and Billen, Reference Schoeters and Billen1995b; Billen and Al-Khalifa, Reference Billen and Al-Khalifa2018) and parasitic wasps (Edson et al., Reference Edson, Barlin and Vinson1982; Blass and Ruthmann, Reference Blass and Ruthmann1989; Wan et al., Reference Wan, Wang and Chen2006; Mao et al., Reference Mao, Tang, Tian, Shi and Chen2016) (Table 1).
Table 1. Distribution of character states from the venom apparatus characters

Note: A, number of venom gland; B, number of venom reservoir; C, number of Dufour's gland; D, the primary venom duct, 0 = no primary venom duct present, 1 = presence of primary venom duct; E, the secondary venom duct: 0 = no secondary venom duct present, 1 = presence of secondary venom duct without branching; F, the tertiary venom duct: 0 = no primary venom duct present, 1 = presence of primary venom duct.
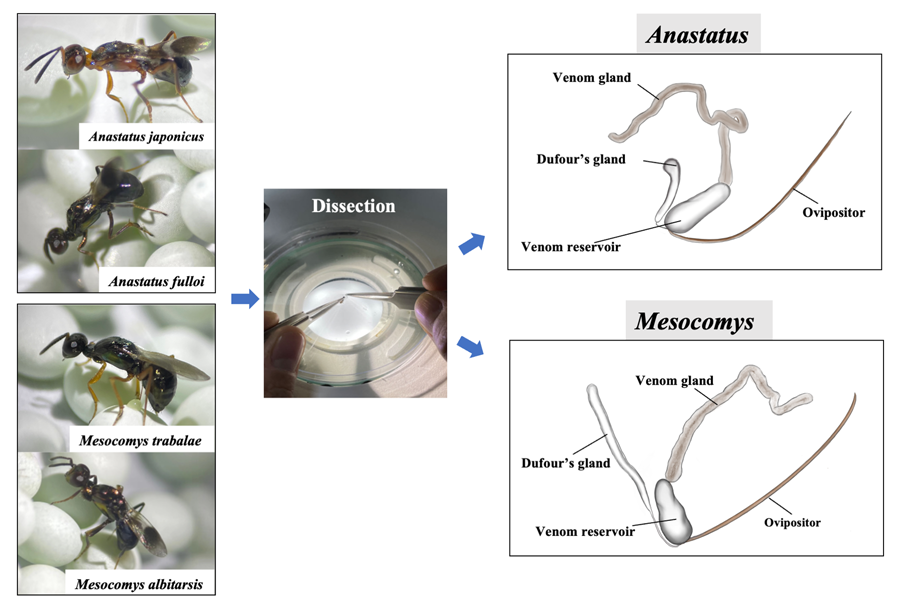
More distribution of character states from the venom apparatus characters can be found in Fig. 1. Female adult of 4 egg parasitoids and venom apparatus. (A) Anastatus japonicus. (B) Anastatus fulloi. (C) Mesocomys trabalae. (D) Mesocomys albitarsis. (E) Venom apparatus of Anastatus. (F) Venom apparatus of Mesocomys. Vg, venom gland; Dg, Dufour's gland; Vr, venom reservoir.

Figure 1. Female adult of 4 egg parasitoids and venom apparatus. (A) Anastatus japonicus. (B) Anastatus fulloi. (C) Mesocomys trabalae. (D) Mesocomys albitarsis. (E) Venom apparatus of Anastatus. (F) Venom apparatus of Mesocomys. Vg, venom gland; Dg, Dufour's gland; Vr, venom reservoir.
Parasitoid wasps are classified based on the choice of host life stage, with primary categories including egg, larval, pupal and adult parasitoids (Pennacchio and Strand, Reference Pennacchio and Strand2006). Egg parasitoids are essential to study due to their role in biological control, aiding in sustainable agriculture by suppressing pest populations prior to the pest reaching the larval feeding stage (Vinson, Reference Vinson1998; Takada et al., Reference Takada, Kawamura and Tanaka2001). These wasps deposit their eggs within the host eggs. As their offspring grow, they consume the host eggs, effectively controlling pest damage at its earliest stages. This process frequently provides vital ecosystem services by regulating host populations and contributing to the preservation of ecological equilibrium (Bragança et al., Reference Bragança, Zanuncio José, Picanço and Laranjeiro1998). Research has been conducted on the venom apparatus of both larval and larval–pupal parasitoids (Zhu et al., Reference Zhu, Ye and Hu2008; Mao et al., Reference Mao, Tang, Tian, Shi and Chen2016). However, there are limited studies on the composition of venom in egg parasitoids, and no other factors associated with these egg parasitoids have been identified (Zhao et al., Reference Zhao, Chen, Wang, Chen and Zang2023). Although most Eupelmidae are larval and pupal parasitoids, members of some genera, such as Anastatus and Mesocomys, are egg parasitoids with significant implications in the field of biological control (Chen et al., Reference Chen, Qu, Li, Iqbal, Wang, Ren, Desneux and Zang2021). These wasps predominantly target larger eggs of insects, especially those belonging to the Lepidoptera (butterflies and moths) and Hemiptera (true bugs) (Li et al., Reference Li, Liao, Zhang and Song2014; Chen et al., Reference Chen, Qu, Li, Iqbal, Wang, Ren, Desneux and Zang2021).
In previous investigations, we identified the venom protein composition of 2 Eupelmid parasitoids, Anastatus japonicus and Mesocomys trabalae (Zhao et al., Reference Zhao, Chen, Wang, Chen and Zang2023). However, the development of their venom apparatus remained unobserved and unanalysed. In this study, we investigated the developmental dynamics of the venom apparatus, including the species Anastatus fulloi and Mesocomys albitarsis. A comprehensive anatomical exploration of the venom glands of females of the 4 eupelmid egg species was conducted. Hypothesizing that the developmental changes in parasitoids might influence their physiological functionalities, we carefully documented variations in the dimensions of the venom apparatus across different developmental stages. Specifically, the length and width of the venom glands were precisely measured at various age intervals, providing insights into the dynamic growth patterns and potential functional implications during their life cycle. This research sheds light on the link between venom gland development and potential variations in venom characteristics in Eupelmid egg parasitoids.
Material and methods
Insect rearing
The egg parasitoids, including A. japonicus, A. fulloi, M. trabalae and M. albitarsis, were initially collected from fields in the Kang County, Gansu Province (105–106° E, 32.9–33.7° N) in August 2018. They were bred at the Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Guizhou University. For sustenance, the parasitoids were provided with the eggs of Antheraea pernyi as an alternative host. The incubation conditions were maintained in a temperature-controlled incubator set at 25 ± 5°C with a photoperiod of 14 h of light and 10 h of dark and a humidity level of 70 ± 5%. The duration of incubation lasted between 26 and 28 days. Post-emergence, adults were nourished with 30% honey water to promote ongoing growth and development.
Venom glands dissection
After emergence, adult female parasitoids that had fully mated (paired and observed to ensure completion of mating behaviour) were allocated into 3 distinct replicates. The dissection of the venom glands was performed using ultra-fine tweezers, specifically by detaching them from the gaster under a dissection microscope (ZEISS, Oberkochen, Baden-Württemberg, Germany). Once extracted, they were immediately immersed in a sterile 1 × Pringle's phosphate-buffered saline buffer (1 × PBS) (Biosharp, Hefei, China).
Morphometric measurements and photographic documentation of the venom apparatus
Samples of parasitoids were collected post-eclosion in intervals of 0–1, 2–3, 4–5, 6–7 and 8–9 days. Each treatment included 20 venom apparatus. The described dissection method was employed for each sampling. Photographic documentation of the venom apparatus was executed using an ultra-depth field microscope. The built-in measurement tool of the microscope was then used to determine the dimensions of the venom glands.
Data compilation, statistical analysis and illustration
The measurements pertaining to the length and width of the venom glands and reservoir across different age intervals were collected. Data analysis was conducted using IBM SPSS v23.0 software. A 2-way analysis of variance (ANOVA) with corrections for multiple comparisons was used, employing Tukey's Honestly Significant Difference (HSD). Graphical representations were executed using GraphPad v9.0 software.
Results
Morphological comparison of venom apparatus
In this study, the venom apparatus of the 4 species of parasitoids of Anastatus and Mesocomys were found to be similar. The venom apparatus of the 4 studied species consists of a venom gland and a reservoir with an associated Dufour's gland (Fig. 1E and F). The venom reservoir, a nearly transparent cylindrical organ that tapers at the tip and widens at the base, is affixed to the base of the ovipositor, acting as a storage for the venom. Attached to the base of the venom sac is Dufour's gland, which exhibits morphological distinctions between the 2 genera. In Anastatus, the gland is noticeably curved, and it is slightly widened or expanded apically (Fig. 1E). Conversely, the Dufour's gland in Mesocomys is comparatively unexpanded apically and it has negligible curvature (Fig. 1F). The venom gland, serving as the site for venom synthesis, is attached to the end of the venom reservoir opposite that of the Dufour's gland, and is a non-branched tubular light-brown filament. The venom gland in Anastatus is slightly darker in colouration (Fig. 1E), whereas that of Mesocomys is notably more transparent (Fig. 1F).
Ontogenetic morphological variation of venom apparatus
Morphological examination and comparison across various developmental stages were conducted to elucidate the developmental trajectory of the venom apparatus in the 4 species. Analysis of the imaging data revealed a significant enlargement of the venom apparatus in all 4 species as the length of the post-emergence increased. This growth was characterized by an elongation and thickening of the venom gland, coupled with an expansion in the volume of both the venom reservoir and Dufour's gland (Fig. 2). In addition, differences were observed between the 2 genera. There was a significant enlargement of the venom reservoir as developmental time increased in Anastatus, whereas the increase was relatively modest in Mesocomys.

Figure 2. Ontogenetic morphological variation of venom apparatus after emergence in 4 egg parasitoids.
Temporal changes in venom apparatus dimensions
Based on preliminary morphological assessments, it was evident that there was a temporal evolution in the dimensions of the venom apparatus. To elucidate these complex morphological dynamics, we systematically quantified the ontogenetic changes in the venom reservoir and glandular structures (essential for venom biosynthesis and storage) across the 4 species. Upon post-emergence progression, there was a significant elongation in the venom glands within all 4 species (Fig. 3A). Specifically, during this 6–7 days interval, the venom gland of A. fullio was notably longer than for those of the other 3 species (P < 0.01): M. albitarsis measured 1376.05 ± 309.98 μm, M. trabalae 1425.9 ± 334.37 μm, A. japonicus 1440.05 ± 134.57 μm and A. fullio 1636.7 ± 364.35 μm. Throughout the developmental phase, the venom gland length of M. albitarsis consistently was the shortest, whereas that of A. fullio was the longest among the observed species (Figs 2 and 3A). Regarding the width of the venom gland, the 2 Anastatus species exhibited only negligible variation throughout the developmental duration. However, there was a consistent increase in the width of the venom glands in the 2 species of Mesocomys; notably, by the 8th–9th day, the venom glands of the 2 Mesocomys species surpassed those of the Anastatus species (Fig. 3B). With regard to the length of the venom reservoirs, during the initial 3 days post-emergence, 4 species are basically the same; while from 4 to 7 days, the 2 Anastatus species had greater lengths compared to the 2 Mesocomys species; on the 8–9 days, M. trabalae arrived at the first place (Fig. 3C). However, starting from the 4th day onward, Anastatus had significantly longer venom reservoirs than for Mesocomys, surpassing them in length. For the venom width of the reservoir, all 4 species exhibited a progressive enlargement over time, with the most pronounced expansion occurring on the 8th–9th days post-emergence (Fig. 3D).

Figure 3. Temporal changes in venom apparatus dimensions (length and width) across 4 egg parasitoids. (A) The length of the venom glands (μm). (B) The width of the venom glands (μm). (C) The length of the venom reservoirs (μm). (D) The width of the venom reservoirs (μm).
Discussion
This research investigated the developmental dynamics of the venom apparatus in 4 species of Eupelmidae, A. japonicus, A. fulloi, M. trabalae and M. albitarsis. The venom apparatus of these 4 parasitoid species all consists of a venom gland, a venom reservoir and a Dufour's gland. Their structure is similar to those of 2 species of Pteromalidae, the larval–pupal parasitoid Trichomalopsis shirakii and the pupal parasitoid Pteromalus puparum, with all having unbranched venom glands. In addition, all lack primary, secondary and tertiary venom ducts and the venom glands connect directly to the venom reservoir, which in turn connects directly to the ovipositor (Zhu et al., Reference Zhu, Ye and Hu2008; Mao et al., Reference Mao, Tang, Tian, Shi and Chen2016). However, the venom reservoir of the eupelmid species does not have a distinct constriction at its centre (Fig. 2), unlike T. shirakii (Fig. 2 in Mao et al., Reference Mao, Tang, Tian, Shi and Chen2016).
The venom gland, as the organ responsible for the synthesis and secretion of venom, plays a pivotal role in the evolution of venom in parasitic wasps. It also represents the most highly differentiated organ within the venom apparatus with different taxa. Anastatus the venom gland is slightly longer than in Mesocomys, though other morphological differences are minimal, with both being unbranched, tubular glands without any swelling at the tip. Differentiation of the venom gland is more pronounced in some other parasitic wasp families. For example, some parasitic wasps possess 2 or even more venom glands (Alves et al., Reference Alves, Wanderley-Teixeira, Teixeira, Alves, Araújo, Barros and Cunha2015). Within Braconidae, some have only 1 venom gland, such as Cotesia glomerata and Yelicones delicatus (Areekul et al., Reference Areekul, Zaldivar-Riverón and Quicke2004; Arakawa et al., Reference Arakawa, Tanaka, Ishibashi, Fujii Muramatsu, Murakami and Nakashima2013), whereas others, such as Aptenobracon formicoides and Ecphylus lychii, have 2 venom glands. Each venom gland is connected to a primary duct, one end of this duct being attached to the venom sac and the other end to the ovipositor (Quicke et al., Reference Quicke, Tunstead, Falco and Marsh1992). Further, some parasitic wasps have non-branching venom glands without an enlarged tip, which is characteristic of the 4 studied species as well as C. glomerata (Arakawa et al., Reference Arakawa, Tanaka, Ishibashi, Fujii Muramatsu, Murakami and Nakashima2013). Other species are known to possess branching venom glands without an enlarged apex and in certain cases the venom glands not only branch out but also have bifurcated tips (Quicke et al., Reference Quicke, Tunstead, Falco and Marsh1992, Reference Quicke, Achterberg and Godfray1997). Within various hymenopteran parasitoid families, analogous venom gland morphologies are evident, which implies potential convergent evolutionary trajectories for these structures. In the latest study by Guiguet et al. on the venom organs of Hymenoptera, it is true that venom organs differentiate among species with different phylogenetic relationships, and this differentiation is associated with evolution (Guiguet et al., Reference Guiguet, Tooker, Deans, Mikó, Ning, Schwéger, Hines and Bond2023).
Within parasitoids, the venom reservoir appears to be a highly conserved feature, invariably being a singular entity. However, there is variation in the configuration of the ducts connecting to the reservoir and the relative positioning vs the venom gland. In certain species, such as the 4 studied herein and in T. shirakii and A. difficilis, the venom gland attaches directly to the reservoir (Quicke et al., Reference Quicke, Achterberg and Godfray1997; Mao et al., Reference Mao, Tang, Tian, Shi and Chen2016), whereas, in some taxa both the venom reservoir and the gland converge via respective ducts, ultimately joining a primary duct linked to the ovipositor (Quicke et al., Reference Quicke, Achterberg and Godfray1997). There are also visible differences in the morphology of the venom reservoir among various parasitoid wasps. Reservoir shape ranges from droplet-like and circular, to ellipsoid and tubular, with some exhibiting central constrictions (Quicke et al., Reference Quicke, Tunstead, Falco and Marsh1992).
The Dufour's gland is commonly found in Hymenoptera, though in some instances it may be degenerate or absent (Robertson, Reference Robertson1968; Quicke, Reference Quicke1997). The Dufour's gland typically has a simple structure, although occasionally it appears bilobed; commonly it is connected to the ovipositor apparatus near the juncture between the reservoir and the ovipositor, but it may attach directly to the reservoir itself (Robertson, Reference Robertson1968). The Dufour's gland is likely instrumental in facilitating egg lubrication in the majority of symphytan species and parasitic wasps and in sting lubrication in most aculeate taxa (Bender, Reference Bender1943; Bernard, Reference Bernard1951; Quicke, Reference Quicke1997). The gland has also been shown to produce alarm signals in ants and pheromones in certain ichneumonoid wasps (Guillot and Vinson, Reference Guillot and Vinson1972; Syvertsen et al., Reference Syvertsen, Jackson, Blomquist and Vinson1995). No evidence of the Dufour's gland, or any structure resembling this singular tubular gland, has been identified in any studied Cynipoidea (Vårdal, Reference Vårdal2006). The specific function of Dufour's gland in parasitic wasps remains unclear. Consequently, its morphological development was not analysed in this study, but we intend to investigate its function in future research.
To successfully parasitize, parasitic wasps need venom to assist the normal development of their offspring, hence the venom apparatus plays a crucial role. This study also found that the venom apparatus itself requires development; in the early stages after eclosion, the size of the venom apparatus increases with age. However, upon reaching a certain equilibrium point, if the venom is not utilized, for instance, by injecting into a host to parasitize, the size of the venom apparatus will no longer increase and may even decrease. This could be because the parasitic wasp enters an energy-saving mode, as producing venom is also likely an energy-consuming process.
In conclusion, venom organ morphology and development in parasitic wasps appear to be highly conserved across species. However, our study of Anastatus and Mesocomys reveals distinctive morphologic differences that distinguish them from many other parasitoids. These discoveries not only highlight the diversity within the parasitic wasps but also underscore the need for targeted research on specific wasp families to understand their unique behaviours and evolutionary trajectories.
Data availability statement
There is no other available data for this study.
Author contributions
L.-S. Z. conceived and designed the study. X. C. wrote the main manuscript text, carried out the experiments and prepared the figures. Q.-Y. Z. performed statistical analyses and carried out the sampling. Y.-M. C. and H. T. revised the manuscript. All authors reviewed the manuscript and approved the final version.
Financial support
This work was supported by the National Key R&D Program of China [grant number: 2023YFE0104800], the National Natural Science Foundation of China [grant number: 32172469] and the Foundation of Postgraduate of Guizhou Province [grant number: YJSKYJJ [2021]041].
Competing interests
None.
Ethical standards
Not applicable.