Published online by Cambridge University Press: 21 April 2022
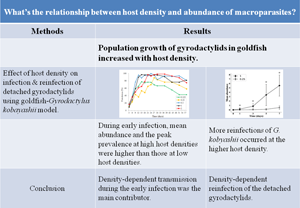
Host density is a key regulatory factor in parasite transmission. The goldfish (Carassius auratus)-Gyrodactylus kobayashii model was used to investigate effects of host density on population growth of gyrodactylids. A donor fish infected by five gravid gyrodactylids was mixed with 11 parasite-free goldfish at five host densities. There was a significant positive correlation between host density and mean abundance of G. kobayashii throughout the 58-day experiment. During early infection (days 15–24), mean abundance in medium high (0.5 fish L−1) and high host density groups (1 and 2 fish L−1) was significantly higher than that in the low host density groups (0.125 and 0.25 fish L−1). At high host density, prevalence increased more rapidly, and the peak prevalence was higher. Fitting of an exponential growth model showed that the population growth rate of the parasite increased with host density. A hypothesis was proposed that higher host density contributed to increased reinfection of detached gyrodactylids. A reinfection experiment was designed to test this hypothesis. Both mean abundance and prevalence at a host density of 1 fish L−1 were significantly higher than those at 0.25 fish L−1 on days 1 and 3, which suggested that more reinfections of G. kobyashii occurred at the higher host density. Density-dependent transmission during the early infection was an important contributor of population growth of G. kobayashii, as well as density-dependent reinfection of the detached gyrodactylids.