Introduction
Cystic and alveolar echinococcosis (CE & AE) are considered the second and third most significant foodborne parasitic diseases worldwide (FAO and WHO, 2014). Echinococcus granulosus sensu lato (s.l.), the causative agent of CE, is found throughout the world, whereas AE, caused by E. multilocularis, is generally limited to the Northern Hemisphere (Deplazes et al., Reference Deplazes, Rinaldi, Alvarez Rojas, Torgerson, Harandi, Romig, Antolova, Schurer, Lahmar, Cringoli, Magambo, Thompson, Jenkins, Thompson, Deplazes and Lymbery2017; Massolo et al., Reference Massolo, Simoncini and Romig2022, Fig. 1). The highest AE disease burden is in Asia, especially China, while that for CE is more widely distributed in parts of Eurasia, Africa and South America (Deplazes et al., Reference Deplazes, Rinaldi, Alvarez Rojas, Torgerson, Harandi, Romig, Antolova, Schurer, Lahmar, Cringoli, Magambo, Thompson, Jenkins, Thompson, Deplazes and Lymbery2017). The mortality rates in humans are higher for AE (2–5% in Western and Central Europe and North America, 10–30% in Eastern Europe, and around 100% elsewhere) than for CE (1–2%) (Torgerson et al., Reference Torgerson, Devleesschauwer, Praet, Speybroeck, Willingham, Kasuga, Rokni, Zhou, Fèvre, Sripa, Gargouri, Fürst, Budke, Carabin, Kirk, Angulo, Havelaar and de Silva2015). Advances during recent decades have led to a better diagnosis and treatment of echinococcosis notably long term albendazole therapy strongly associated to very low mortality rate but the accessibility to them is still remaining disparate between countries (Torgerson et al., Reference Torgerson, Schweiger, Deplazes, Pohar, Reichen, Ammann, Tarr, Halkik and Müllhaupt2008). One study estimates an annual combined figure of 200 000 new human cases of either CE or AE (Kern, Reference Kern2010; Torgerson et al., Reference Torgerson, Devleesschauwer, Praet, Speybroeck, Willingham, Kasuga, Rokni, Zhou, Fèvre, Sripa, Gargouri, Fürst, Budke, Carabin, Kirk, Angulo, Havelaar and de Silva2015). Most research in the past has been focused on detection in definitive and intermediate hosts (DHs & IHs), including dead-end hosts such as humans, mainly for the purpose of determining and describing endemic areas. For instance, the diagnosis of human CE and AE is mainly based on imaging and then may be attested with human blood and tissue samples analysis. Parasitic forms (e.g. eggs, worms, metacestodes and protoscolices) from feces and tissue samples of animal hosts are used to study the epidemiology of the parasite in animals. Indeed, Echinococcus eggs, transmitted to humans through the fecal-oral route, are initially shed in DH stools. In Europe, red foxes are considered to be the main definitive host of E. multilocularis based on high population densities, high susceptibility to infection, high worm burden and high prevalence of infection reaching more than 50% in high endemic areas (Oksanen et al., Reference Oksanen, Siles-Lucas, Karamon, Possenti, Conraths, Romig, Wysocki, Mannocci, Mipatrini, La Torre, Boufana and Casulli2016; Romig et al., Reference Romig, Deplazes, Jenkins, Giraudoux, Massolo, Craig, Wassermann, Takahashi, de la Rue, Thompson, Deplazes and Lymbery2017). When the prevalence in other sympatric species notably domestic carnivores is far much lower. Despite a high susceptibility to infection of dogs, their low exposure to infected rodent and occasional deworming limit their global contribution to environmental contamination in Europe (Hegglin and Deplazes, Reference Hegglin and Deplazes2013). However, the contribution of dogs in environmental contamination in Central Asia and China is significant due to higher prevalence (Wang et al., Reference Wang, Zhong, Yu, Zhang, Budke, Liao, He, Chen, Xu, Xie, Danbazeli, Wang, Yang, Huang, Li, Yao, Giraudoux and Craig2021; Abdykerimov et al., Reference Abdykerimov, Kronenberg, Isaev, Paternoster, Deplazes and Torgerson2024). At the opposite, if the population densities of cats can be very important with frequent predation of rodents (Forin-Wiart et al., Reference Forin-Wiart, Poulle, Piry, Cosson, Larose and Galan2018), the low worm establishment and the few eggs excreted which are considered to be non-infective result to an insignificant role of cats in E. multilocularis transmission (Kapel et al., Reference Kapel, Torgerson, Thompson and Deplazes2006). Therefore, according to population densities, prevalence and biotic potential, DHs with the foremost contribution to E. multilocularis environmental contamination by eggs in rural and urban areas are foxes (81–96%), followed by dogs (4–19%) and, to a lesser extent, cats (0–0.2%) (Hegglin and Deplazes, Reference Hegglin and Deplazes2013). Thus, the biotic potential of overall of E. multilocularis is estimated at between more than 57 000 to several million eggs/km2 depending on the level of endemicity and urbanization (Hegglin and Deplazes, Reference Hegglin and Deplazes2013). Based on the number of eggs produced by E. granulosus s.l. worms (~1503 eggs per parasite during its lifetime) and the mean number of worms in infected dogs (~202), the biotic potential in DHs seems to be close between E. granulosus s.s. (~303 606 eggs) and E. multilocularis (~279 910 eggs) (Thompson and Eckert, Reference Thompson and Eckert1982; Gemmell, Reference Gemmell1990; Kapel et al., Reference Kapel, Torgerson, Thompson and Deplazes2006). Due to the high density of E. granulosus DHs (i.e. domestic and stray dogs), we could expect similar or higher values of environmental contamination by E. granulosus eggs in endemic areas than those for E. multilocularis in foxes.
The environmental contamination by Echinococcus eggs may therefore be high in endemic regions where DH prevalence is high, leading to a risk of human infection. Nevertheless, both food and environmental contamination by Echinococcus eggs has been neglected for a long time, despite the fact that the oral uptake of infective eggs from these matrices is the main source of human contamination. It is difficult to estimate the potential contribution of each matrix, such as the environment (e.g. soil, water or fomites, including DH hairs), animals or food as a vehicle for the transmission of Echinococcus eggs. The assessments obtained by meta-analysis or based on expert knowledge elicitation ultimately give an insight into the risk factors rather than information on the relative contribution of each compartment in human diseases. Indeed, the confidence intervals (CIs) are usually wide and thus uninformative. For instance, based on the WHO (2015) report, the greatest source of human contamination for E. granulosus appears to be contact with animals [52% (CI95%: 22 to 75%)], whereas it might be food for E. multilocularis [50% (CI95%: 0 to 88%)]. Meta-analysis results suggest that contaminated water could be responsible for up to 29.4% (CI95%: 12 to 52%) of CE infections in humans, while for AE, the major pathway of transmission could be contact with dogs [34.4% (CI95%: 21 to 48%)] (Torgerson et al., Reference Torgerson, Robertson, Enemark, Foehr, van der Giessen, Kapel, Klun and Trevisan2020). However, in endemic regions within Europe, the canine prevalence of E. multilocularis is clearly lower (0 to 1.5%) (Hegglin and Deplazes, Reference Hegglin and Deplazes2013) than that of E. granulosus (0 to 19%) (Carmena and Cardona, Reference Carmena and Cardona2013). On the other hand, in Asia, dogs can play an important role in the E. multilocularis life cycle, especially in China or Kyrgyzstan, where high canine prevalence and incidences of human infection have been reported (Budke et al., Reference Budke, Jiamin, Craig and Torgerson2005; Ziadinov et al., Reference Ziadinov, Mathis, Trachsel, Rysmukhambetova, Abdyjaparov, Kuttubaev, Deplazes and Torgerson2008; Abdykerimov et al., Reference Abdykerimov, Kronenberg, Isaev, Paternoster, Deplazes and Torgerson2024). Instead of attempting to evaluate the relative contribution of contamination sources, some publications have focused on potential echinococcosis risk factors. Contact with animals, especially DHs, is generally significant for CE and AE (Possenti et al., Reference Possenti, Manzano-Román, Sánchez-Ovejero, Boufana, La Torre, Siles-Lucas and Casulli2016; Conraths et al., Reference Conraths, Probst, Possenti, Boufana, Saulle, La Torre, Busani and Casulli2017; Torgerson et al., Reference Torgerson, Robertson, Enemark, Foehr, van der Giessen, Kapel, Klun and Trevisan2020). Depending on the regions and the disease, having a kitchen garden or living in a rural area may also have an impact on Echinococcus infection rates. It has also been highlighted that the possibility of ingesting eggs from food (e.g. wild fruits, raw/unwashed vegetables and fruits) or water exists, but these factors do not significantly increase the risk of human infection (Possenti et al., Reference Possenti, Manzano-Román, Sánchez-Ovejero, Boufana, La Torre, Siles-Lucas and Casulli2016; Conraths et al., Reference Conraths, Probst, Possenti, Boufana, Saulle, La Torre, Busani and Casulli2017). However, in Europe, it appears in the light of case–control studies (Torgerson et al., Reference Torgerson, Robertson, Enemark, Foehr, van der Giessen, Kapel, Klun and Trevisan2020) that food may be a major source of contamination for AE. The direct link between water or unwashed vegetables/fruits and the human disease is still unclear due to the long incubation time (2–15 years) and in the absence of scientific evidence of foodborne transmission. As a result, it is necessary to clarify these causal links in order to implement prevention plans and reduce the risk of infection.
Currently, we only have an approximate overview of matrices that play a role in the transmission of Echinococcus eggs to humans. After excretion in carnivore feces, a huge quantity of eggs must withstand harsh environmental conditions before they are finally ingested by intermediate or dead-end hosts to pursue their life cycle. Time and fluctuating or extreme field conditions may damage egg integrity and so hamper both viability and infectiousness. So how long do they actually remain viable on average in the environment or on food? What are the contamination rates for the environment and food? Answering these questions with any precision is challenging even today, because despite constant, expanding research on the parasite since the 19th century, there are still large gaps in the field (Eckert and Thompson, Reference Eckert, Thompson, Thompson, Deplazes and Lymbery2017). It is necessary to determine the presence and viability of eggs on various matrices of interest (e.g. soil, food, or water) in order to establish source attributions and risk factors. In this context, this review summarizes current knowledge on (i) the survival properties of Echinococcus spp. eggs; (ii) their capacity for dispersal in the environment; (iii) environmental and (iv) food contamination and finally, (v) gaps and future prospects in the field of interest. In light of the lack of data for Echinococcus on some topics, literature on other taeniid may be cited as a model.
Survival of Echinococcus eggs
Eggs, the only free-living stage of the parasite, are the only source of contamination for both intermediate and dead-end hosts. Eggs are infectious, and therefore viable, from their moment of excretion. However, harsh environmental conditions can damage their ability to survive. Thus, the survival capacity of Echinococcus eggs were a major focus of studies during the last century, in order firstly to find a way of making them inoperative or killing them, and secondly to propose appropriate control strategies.
Laboratory conditions
In the early days, various laboratory conditions were tested, such as the effect of temperature (negative and positive), humidity and specific chemicals. As this review focuses on the conditions favourable to egg survival in the environment, resistance to chemicals will not be addressed. Only a few authors have estimated the viability of E. multilocularis and E. granulosus eggs at different temperatures. However, a wide temperature range (−196°C to 100°C) has been applied to in vivo tests (Table 1). According to this literature, it appears that E. granulosus and E. multilocularis have a similar survival capacity regarding the temperature factor. Although the assay conditions (e.g. time of exposure, inoculation method, host species) may differ, an overall profile may be deduced. The eggs are freeze-resistant to −50°C, but they are killed by very low (<−70°C) and high (>65°C) temperatures. Among these articles, two also compared the survival of E. multilocularis eggs in water and air with various humidity rates (Veit et al., Reference Veit, Bilger, Schad, Schäfer, Frank and Lucius1995; Federer et al., Reference Federer, Armua-Fernandez, Hoby, Wenker and Deplazes2015). Eggs seem to be more resistant to positive temperatures in water than in air, even with high relative humidity (rh). For example, at 45°C, eggs remain viable 478 days in water compared with 75 days in air with 85–95% rh (Veit et al., Reference Veit, Bilger, Schad, Schäfer, Frank and Lucius1995). At 65°C, Federer et al. (Reference Federer, Armua-Fernandez, Hoby, Wenker and Deplazes2015) noted that eggs remained infectious in water up to 1 h, while they were killed after 30 minutes with 70% rh. These results show a susceptibility of Echinococcus eggs to desiccation, as already observed for Taenia species in the literature. Overall, humidity appears to be more critical to taeniid egg survival than temperature. A low humidity rate, under 34%, hampers egg survival (Jansen et al., Reference Jansen, Dorny, Gabriël, Dermauw, Johansen and Trevisan2021). A high humidity rate combined with a low or high temperature appears to affect infectiousness more than survival.
Table 1. In vivo evaluation of the resistance of E.multilocularis and E. granulosus eggs to temperature
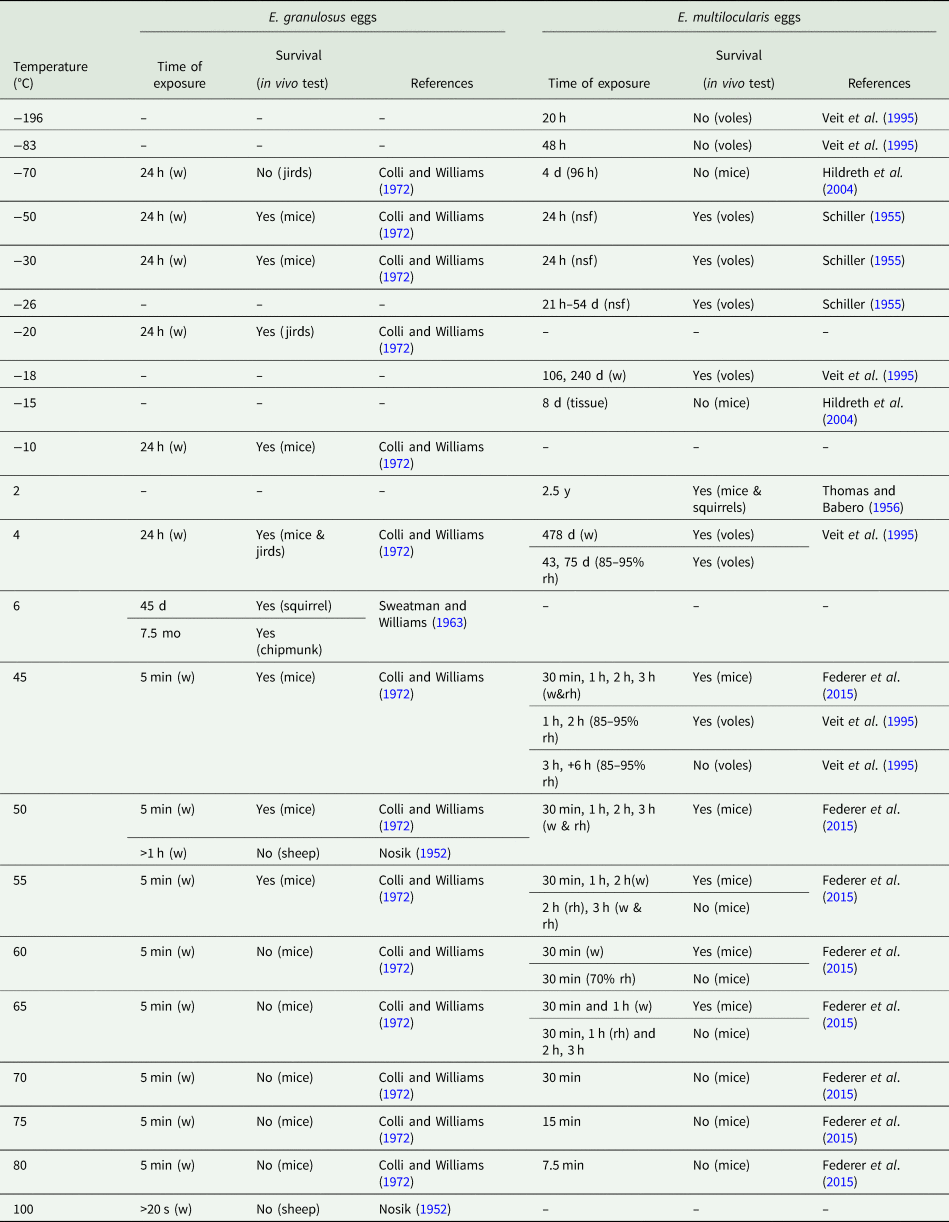
w, water; rh, relative humidity (70%); d, day; mo, month; y, year; nsf, natural semi-fluid.
Outdoor conditions
Laboratory conditions have been used to evaluate the great resistance of Echinococcus eggs under control and steady conditions. Technically, the temperature thresholds listed above allow the eggs to survive on food or in the environment and then to be transmitted to intermediate and dead-end hosts. Nevertheless, is survival possible under fluctuating outdoor conditions? To answer this question, some scientists have dropped eggs onto the ground, either in feces or nylon bags, and then checked their viability as the seasons passed via in vivo (Sweatman and Williams, Reference Sweatman and Williams1963; Abbasov, Reference Abbasov1965; Veit et al., Reference Veit, Bilger, Schad, Schäfer, Frank and Lucius1995; Thevenet et al., Reference Thevenet, Jensen, Drut, Cerrone, Grenóvero, Alvarez, Targovnik and Basualdo2005) or in vitro tests based on hook motility and the eosin exclusion dye test (Wachira et al., Reference Wachira, Macpherson and Gathuma1991). The maximum survival time of E. multilocularis and E. granulosus eggs in natural environmental conditions has thus been appraised (Table 2). Temperature ranges are very heterogeneous and vary between regions. No real trend stands out regarding the effect of seasons. Indeed, in the same temperature range (~−16°C to ~10°C), E. multilocularis eggs may survive around 3 months (96 days) in Germany (Veit et al., Reference Veit, Bilger, Schad, Schäfer, Frank and Lucius1995) but less than 1 month for E. granulosus in Azerbaijan (Abbasov, Reference Abbasov1965). However, the maximum survival time for E. multilocularis eggs observed in Germany was 240 days during the autumn/winter and 78 days in summer (Veit et al., Reference Veit, Bilger, Schad, Schäfer, Frank and Lucius1995). It is therefore very complicated to attempt to summarize or draw conclusions about the maximum survival time due to the difference in timescale, the lack of information in some old research papers and, more generally, the scarcity of studies. Nevertheless, in Kenya, Wachira et al. (Reference Wachira, Macpherson and Gathuma1991) reached the same conclusion about the harmful effect of desiccation during their field experiments in ‘open ground’, ‘house’ and ‘water hole’. Their results showed that E. granulosus eggs are viable but not motile for a longer period (19 days) in the Maasai environment than in Turkana (2 days). This may be explained by the difference in climate between these 2 localities: cool, humid conditions in Maasailand vs a semi-arid environment in Turkana. In addition, only eggs in a water hole remained viable and motile for 12 days. This tends to prove that low humidity hampers egg survival, whereas water and high humidity favour survival.
Table 2. Estimation of the survival of E. multilocularis or E. granulosus eggs under natural conditions
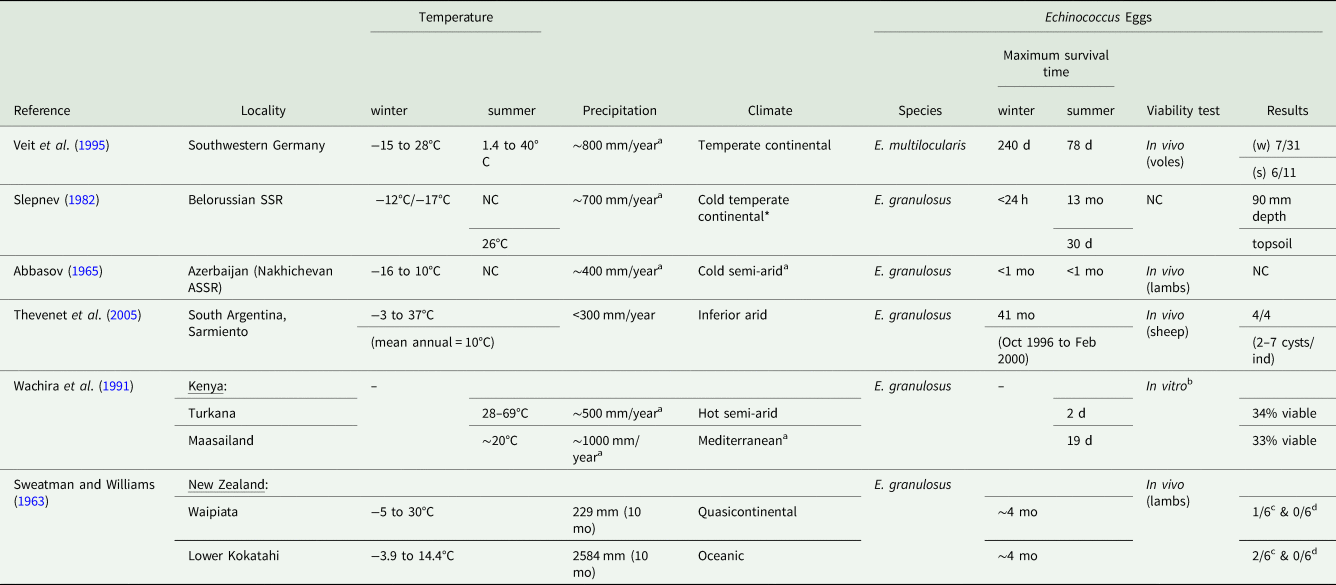
a Information not indicated in the article (pulled from climate-data.org);
b Viability was assessed using hook motility and eosin exclusion dye test;
c 4 months after eggs were sprayed over the pasture;
d 10 months after eggs were sprayed over the pasture.
d, day; mo, month; NC, not communicated in the abstract; (w), winter; (s), summer.
However, the field study in New Zealand led by Sweatman and Williams (Reference Sweatman and Williams1963) pointed out the fact that ‘heavy rainfall was a more important limiting factor in the availability and infectivity of the eggs than was meteorological dryness’. Succinctly, the survival of E. granulosus eggs was assessed in 2 localities with different climates: high temperatures and winds, very low rainfall (Waipiata) vs extreme rainfall (Lower Kokatahi, LK). Eggs were sprayed over a pasture and then, after either 4 (group 1) or 10 months (group 2), lambs were allowed to graze the pasture for several days. One of the 6 lambs from group 1 became weakly infected in Waipiata compared with 2 of the 6 lambs in LK. None of the lambs from group 2 developed cysts after 10 months. These results appear to show no significant difference between the 2 localities. Nevertheless, the heavy rainfall affected the depth dispersal of the eggs, as described below in the dispersal section.
In addition, the outdoor survival of E. granulosus eggs has been compared with some species of Taenia, including T. hydatigena (Sweatman and Williams, Reference Sweatman and Williams1963; Wachira et al., Reference Wachira, Macpherson and Gathuma1991). Their life cycles are similar, sharing livestock and dogs as intermediate and definitive hosts, respectively. The in vivo study by the aforementioned authors revealed no significant difference in the survival time between eggs of these 2 tapeworms, whatever the climate (Sweatman and Williams, Reference Sweatman and Williams1963). However, the rate of infection after 4 months was higher for T. hydatigena (100% in W and 83.3% in LK) than E. granulosus (16.7% in W and 33.3% in LK). After a year's exposure to an oceanic climate, most of the eggs seemed to be unavailable and/or were no longer infective. Even though the eggs' viability had been estimated in vitro, the results from Wachira et al. (Reference Wachira, Macpherson and Gathuma1991) appeared to lead to the same conclusion. By contrast, under various controlled humidity conditions (0–80%), Laws (Reference Laws1968) observed higher in vitro survival rates for T. hydatigena and T. ovis than for T. pisiformis and E. granulosus. Within these taeniid species, and despite a similar life cycle, there appears to be a difference in their resistance capacity. In the literature, taeniid eggs have globally the same resistance capacity (Jansen et al., Reference Jansen, Dorny, Gabriël, Dermauw, Johansen and Trevisan2021 table 1; Table 1) but egg survival rates appear to be heterogeneous and to vary according to outdoor conditions, even for the same species.
Age effect on Echinococcus egg survival
Other factors, such as the age or dispersion of eggs, play a role in the survival and infectivity capacity. For instance, Batham (Reference Batham1957) not only studied the impact of humidity on viability, but also the influence of the age of E. granulosus eggs. Briefly, one batch was kept in tap water, and the second in air, both in covered petri dishes at room temperature (between 10°C and 21°C) for 1 year. At various timepoints, the E. granulosus eggs were injected intraperitoneally into mice, which were autopsied 3 months post infestation. Unexpectedly, results tended to show that eggs kept in air, unlike eggs kept in water, could remain viable for 1 year. Although no mice developed cysts, ‘spots’ (probably indicating dead remains of cestode cysts) were observed in viscera among mice infected with eggs kept in air. In another study, a drop in egg infectivity was noticed after 41 months, as a small number of cysts (2–7/individual) had developed in the 6 sheep (Thevenet et al., Reference Thevenet, Jensen, Drut, Cerrone, Grenóvero, Alvarez, Targovnik and Basualdo2005) compared with infection using fresh eggs (24.3 cysts/individual) (Lightowlers et al., Reference Lightowlers, Jensen, Fernandez, Iriarte, Woollard, Gauci, Jenkins and Heath1999).
To conclude this part about survival of Echinococcus eggs, laboratory and field experiments tend to confirm the relative susceptibility of the eggs to desiccation. Under field conditions, egg survival may depend on specific outdoor conditions and perhaps on the Echinococcus species, even if they seem to have a similar resistance profile under stable laboratory conditions. Some papers assert that the eggs are sensitive to freezing conditions, occasionally accompanied by a drop in egg longevity (Slepnev, Reference Slepnev1982). However, agricultural practices such as irrigation and food storage could help eggs survive the critical summer months.
The comparison with other taeniid regarding the eggs' resistance capacities seems reasonable under lab conditions, but is it reasonable to use a Taenia species as a model for studying the outdoor survival of an Echinococcus species? The answer probably depends on the purpose of the study, because it is hard to compare laboratory and field conditions then draw definitive conclusions. However, the morphology of taeniid eggs is the same for all genera within the Taenidae family. Therefore, eggs from different Taenia species — particularly non-zoonotic ones — can be easily used in the study of dispersal.
Dispersal of Echinococcus eggs in the environment
As seen above in the study of Sweatman and Williams (Reference Sweatman and Williams1963), factors favouring egg survival can also lessen their infectivity by affecting availability in the environment. Indeed, the eggs were dispersed quite differently on each location, as reported for Taenia species with the same protocol. Heavy rainfall buried Taenia hydatigena and T. pisiformis eggs deeper into the soil (11–13 cm) at LK and spread them farther apart than in the near-continental climate of W (0–0.64 cm). Although other authors have studied E. granulosus, they have never estimated the eggs' burial depth. However, given the similarity between the morphology of Taenia spp. and Echinococcus spp. eggs, this observation of vertical dispersal might be relevant. Extreme rainfall can scatter taeniid eggs across the soil and even bury them. Dispersal is both boosted by a linear movement over the topsoil and hampered due to eggs being buried. At the same time, these buried eggs can be protected from harmful environmental conditions and then reemerge when the soil is tilled.
Role of insects and birds
Furthermore, among animals, invertebrates are considered as potential egg vectors. Several species of wild invertebrates have been found to carry parasitic organisms, including Taenia spp. eggs. These include cockroaches (1/820) in Thailand (Chamavit et al., Reference Chamavit, Sahaisook and Niamnuy2011), dung beetles (5/309) in Peru (Vargas-Calla et al., Reference Vargas-Calla, Gomez-Puerta, Pajuelo, Garcia and Gonzalez2018) and flies in various countries (Saelens et al., Reference Saelens, Robertson and Gabriël2022, table 4). On the other hand, no eggs were observed being carried by flies caught in either Mexico (n = 600 and n = 1187) (Keilbach et al., Reference Keilbach, de Aluja and Sarti-Gutierrez1989; Martinez et al., Reference Martinez, de Aluja and Gemmell2000) or Iran (n = 210) (Hemmati et al., Reference Hemmati, Afshar, Mohammadi, Afgar, Nasibi and Harandi2018). The most experimental studies currently available on the spreading of Echinococcus eggs by invertebrates are focused on flies, with only two on beetles (Benelli et al., Reference Benelli, Wassermann and Brattig2021, table 2). Diptera and Coleoptera from these studies have successfully transmitted ingested Echinococcus eggs to intermediate hosts, which developed infection. Eggs are mainly detected in the invertebrates' gut and occasionally on the body.
Data for taeniids such as T. hydatigena are more abundant and widespread (Gemmell, Reference Gemmell1976; Gemmell et al., Reference Gemmell, Johnstone and Boswell1978; Benelli et al., Reference Benelli, Wassermann and Brattig2021) than those on Echinococcus, but similarities include egg dispersal. In a field experiment in New Zealand, 24.3% (183/754) of wild blowflies, allowed to feed for 2 to 3 minutes on infested dog feces, contained taeniid eggs in their digestive tract (0–860 eggs/fly) (Lawson and Gemmell, Reference Lawson and Gemmell1985). Only one external attachment of an egg was observed in this study. Most of the eggs tested were still viable after their ingestion by flies, as attested by the development of cysts in naive lambs that were fed these flies (ten flies/lamb). In laboratory conditions, blowflies fed for 2 h on dog feces can ingest up to 5411 taeniid eggs per blowfly. No data are available on the resistance of taeniid eggs in the blowfly digestive tract, nor about their excretion in droppings. However, the viability of taeniid eggs ingested into beetles' digestive tract has been proved (Bílý et al., Reference Bílý, Stĕrba and Dyková1978; Gomez-Puerta et al., Reference Gomez-Puerta, Lopez-Urbina, Garcia and Gonzalez2014). Considering that taeniid eggs excreted by flies after ingestion are still viable, the impact on egg dispersal could be significant. Indeed, according to studies, the daily dispersal capabilities of blowflies in temperate and subtropical regions are 0.10–0.15 km and 1.25–2.35 km, respectively (Lee et al., Reference Lee, Dong, Yan, He, Yu, Wee and Wilson2023; Table 1). Moreover, the observation of sheep commonly infected by T. hydatigena despite the absence of a definitive host on St Kilda, an island 60 km off the Scottish coast, suggests that eggs may be transported by birds or insects from the nearest coastland (Torgerson et al., Reference Torgerson, Pilkington, Gulland and Gemmell1995). Indeed, some birds may be involved in the dispersal of tapeworm eggs, such as herring gulls for T. saginata (Crewe and Crewe, Reference Crewe and Crewe1969) or coprophagous birds like black-billed magpies for E. multilocularis (Raymond and St. Clair, Reference Raymond and St. Clair2023). Furthermore, viable helminth eggs have been found in bird droppings (Crewe, Reference Crewe1967; Crewe and Owen, Reference Crewe and Owen1978). The eating behaviour of these birds, combined with their proximity to areas inhabited by humans, may increase the contamination risk for humans.
Role of climatic factors
Wind can help invertebrates to move greater distances, but does it have a direct impact on the dispersal of Echinococcus eggs? No papers have investigated the role of wind in Echinococcus egg dispersal either in the field or in laboratory conditions. Regarding taeniid eggs, Lawson and Gemmell (Reference Lawson and Gemmell1985) placed feces contaminated by eggs of T. pisiformis 3 cm away from 4 trays filled with water (from 1 cm to 2.6 m). A fan was used (23.4 km h−1) to simulate wind action for 10 days. Four rabbits were then given the water from the trays to drink, and none became infected, failing to prove in this context a potential effect of wind. However, a mathematical model has theoretically shown that E. multilocularis eggs can be spread by wind (Siegert and Neumann, Reference Siegert and Neumann2022). The average flight distance of eggs in forest areas could vary from 1.3 m to 17 m, depending on factors such as wind speed and altitude. Another study using T. laticollis eggs as a model for Echinococcus observed dispersal of between 0 m and 12 m from fox feces to wild berries in a boreal forest in Finland (Malkamäki et al., Reference Malkamäki, Oksanen, Näreaho and Sukura2022). In Argentina, E. granulosus eggs were found 10 m from pens housing experimentally infected dogs. This dispersal distance and area matched the direction of prevailing winds (Sánchez Thevenet et al., Reference Sánchez Thevenet, Alvarez, Torrecillas, Jensen and Basualdo2020). It appears that the dispersal distance also depends on the layout of the studied site. It is reasonable to assume that the distance travelled by an egg is probably higher in open areas than in a forest.
According to the abovementioned literature, bad weather (e.g. rainfall, wind), invertebrates and birds may play a role in the scattering of eggs in the environment. Even if the contribution of each factor in the dispersal of eggs is still unclear and hard to quantify, each one increases the contamination risk for intermediate and dead-end hosts. To a lesser extent, domestic and wild animals can also increase the risk of transmission by carrying eggs on their body (Matoff and Kolev, Reference Matoff and Kolev1964; Okolo, Reference Okolo1986; Nagy et al., Reference Nagy, Ziadinov, Schweiger, Schnyder and Deplazes2011). As DHs are the original source of environmental contamination, they obviously play an important role in the dispersal of taeniid eggs through defecation at different places. The population density of DHs, their movements and behaviour are all parameters that significantly impact the distribution of eggs, so they all need to be further investigated in order to shed light on the presence of Echinococcus eggs in the environment and food.
Environmental and food contamination by Echinococcus eggs
Since the early 2000s, there has been a significant increase in the production of data on environmental and food contamination by taeniid eggs as reviewed by Saelens et al. (Reference Saelens, Robertson and Gabriël2022). The specific detection of Echinococcus species follows the same trend, with a majority of articles published in the last 10 years. Echinococcus species cannot be specifically identified by microscopic examination of taeniid eggs, so a molecular method was needed, leading to the tedious and much less sensitive microscopic observation being gradually discarded. Nowadays, environmental and food contamination by Echinococcus spp. is mainly detected by targeting parasite DNA, assumed to originate from eggs isolated after filtration and/or flotation methods. As early as Reference Craig, Macpherson, Watson-Jones and Nelson1988, Craig et al., specifically identified for the first time E. granulosus among the taeniid eggs isolated from soil and water samples from Turkana in Kenya using an immunological technique. It was not until the early 2000s that new studies on environmental contamination were published, this time systematically using molecular biology to identify Echinococcus eggs, with the exception of one study that used an immunological test (Sánchez Thevenet et al., Reference Sánchez Thevenet, Jensen, Mellado, Torrecillas, Raso, Flores, Minvielle and Basualdo2003). Investigations into environmental contamination mainly focus on 2 types of matrices: water and soil. In all, 17 studies have been published covering a wide range of geographical areas, from Asia to South America, as well as Europe and Africa, and targeting either E. multilocularis or E. granulosus.
Water
There are currently 7 studies that investigate the specific detection of Echinococcus eggs in water. Four of them focus on the detection of E. multilocularis in water, while the others (all concerning E. granulosus) took place in a broader context with other matrices such as soil and feces also being tested (Table 3). The isolation method for eggs in water typically involves filtration, though a flotation method has also been successfully used once. However, the performance of these methods using 10 mL to 50 L of water has been evaluated only for the 2 methods used by Lass et al. (Reference Lass, Szostakowska, Kontogeorgos, Korzeniewski, Karamon, Sulima and Karanis2019, Reference Lass, Ma, Kontogeorgos, Xueyong, Li and Karanis2020) resulting in high limits of detection, with 100 and 200 eggs for 10 L. According to the studies available, Echinococcus detection rates in water vary mainly between 0 and 6%. Higher values were obtained in only 2 particular situations with a very small number of samples tested: 60% (n = 5) of samples collected from open water sources in Kenya (Craig et al., Reference Craig, Macpherson, Watson-Jones and Nelson1988) and 20% (n = 10) in water samples corresponding to the washing of cattle on a farm on the Tibetan plateau (Lass et al., Reference Lass, Ma, Kontogeorgos, Xueyong, Li and Karanis2020). The marked heterogeneity of the sampling, both in terms of water type and number of samples collected, complicates the interpretation of contamination rates. However, it appears (as expected) that eggs were detected mainly in stagnant water (lakes, waterholes) rather than flowing water (rivers, canals). The presence of eggs in contaminated water can contribute to the maintenance of the parasitic life cycle, but except in certain areas of Africa and Asia, such water is not intended for human consumption. However, it may contribute to soil contamination and may also contaminate vegetables when used for irrigation, which may indirectly cause human cases. Currently there is no specific data about contamination of wastewater by Echinococcus eggs, even though the presence of taeniid eggs has already been asserted in some studies (Saelens et al., Reference Saelens, Robertson and Gabriël2022). There has been a recent increase in interest in waterborne Echinococcus detection, motivating 6 of the 7 studies carried out in the last 5 years. With this new interest, it is likely that more studies will follow, providing a better understanding of the role of water in human contamination.
Table 3. Data from studies concerning the detection of Echinococcusspecies in water
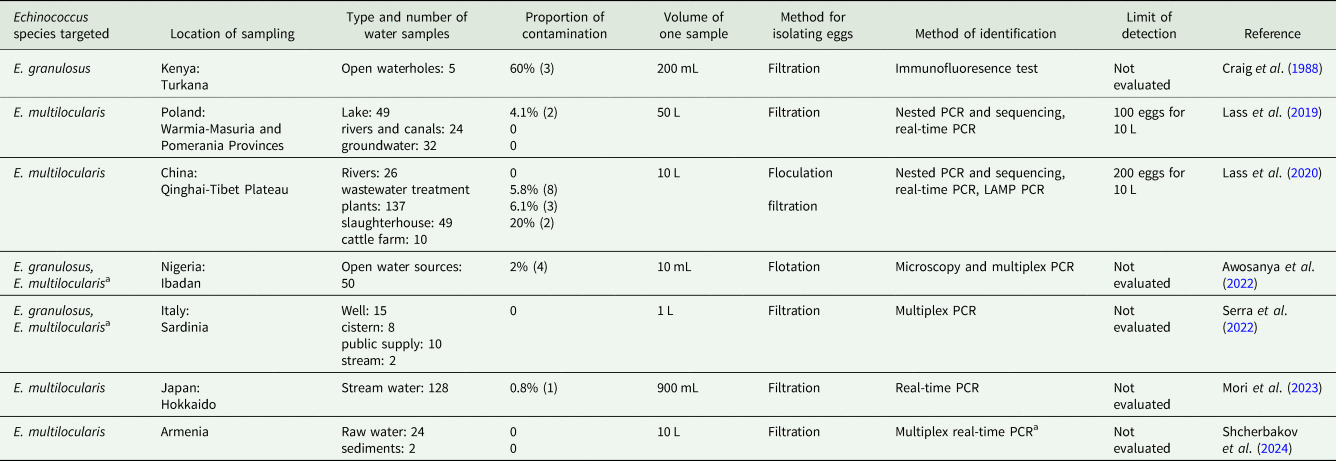
a Studies assuming the non-endemic status for E. multilocularis in the country but detection conducted in the context of a muliplex PCR including other parasite species.
Soil
Detection of Echinococcus in soil has been more frequently reported than in water. A total absence of detection was only reported for 2 of the 10 studies published (Table 4). The first of these 2 studies collected only 2 samples of 36 kg each from the car deck of ferries in order to investigate the potential transfer of E. multilocularis eggs by cars from the endemic island of Hokkaido to a bigger island in mainland Japan (Matsuo et al., Reference Matsuo, Inaba and Kamiya2003). The second one analysed 33 samples of 400 g each from educational farms on the island of Sardinia, Italy (Serra et al., Reference Serra, Masu, Chisu, Cappai, Masala, Loi and Piseddu2022), which is highly endemic for E. granulosus. For both studies, the sample mass was significantly higher than the other 8, which ranged only between 5 g and 40 g of soil. Unfortunately, the performance of the 9 different methods used by the various studies was evaluated only for 2. One of these reports an increase in the detection limit from 10 g to 20 g (Umhang et al., Reference Umhang, Bastien, Renault, Faisse, Caillot, Boucher, Hormaz, Poulle and Boué2017) with an increase in the soil quantity analysed. According to the studies available, detection rates ranged from 2.7 to 25% for E. granulosus and 10.4 to 11.7% for E. multilocularis. Contamination has also been detected both inside the huts of the Turkana community, a nomadic tribe in Kenya (Craig et al., Reference Craig, Macpherson, Watson-Jones and Nelson1988), and in vegetable gardens such as in Kazakhstan for E. granulosus (Shaikenov et al., Reference Shaikenov, Torgerson, Mathis, Deplazes, Rysmukhambetova and Massenov2004) and in Europe for E. multilocularis (Szostakowska et al., Reference Szostakowska, Lass, Kostyra, Pietkiewicz and Myjak2014; Umhang et al., Reference Umhang, Bastien, Renault, Faisse, Caillot, Boucher, Hormaz, Poulle and Boué2017; Da Silva et al., Reference Da Silva, Bastien, Umhang, Boué, Bastid, Boucher, Caillot, De Garam, Renault, Faisse, Courquet, Scalabrino, Millon, Knapp and Poulle2021), which can lead to human contamination. These results indicate relatively significant soil contamination, especially when considering it represents contamination by at least one egg from a very small amount of soil, demonstrating both the eggs' ability to disperse and their high numbers in the environment.
Table 4. Data from studies concerning the detection of Echinococcus species in soil
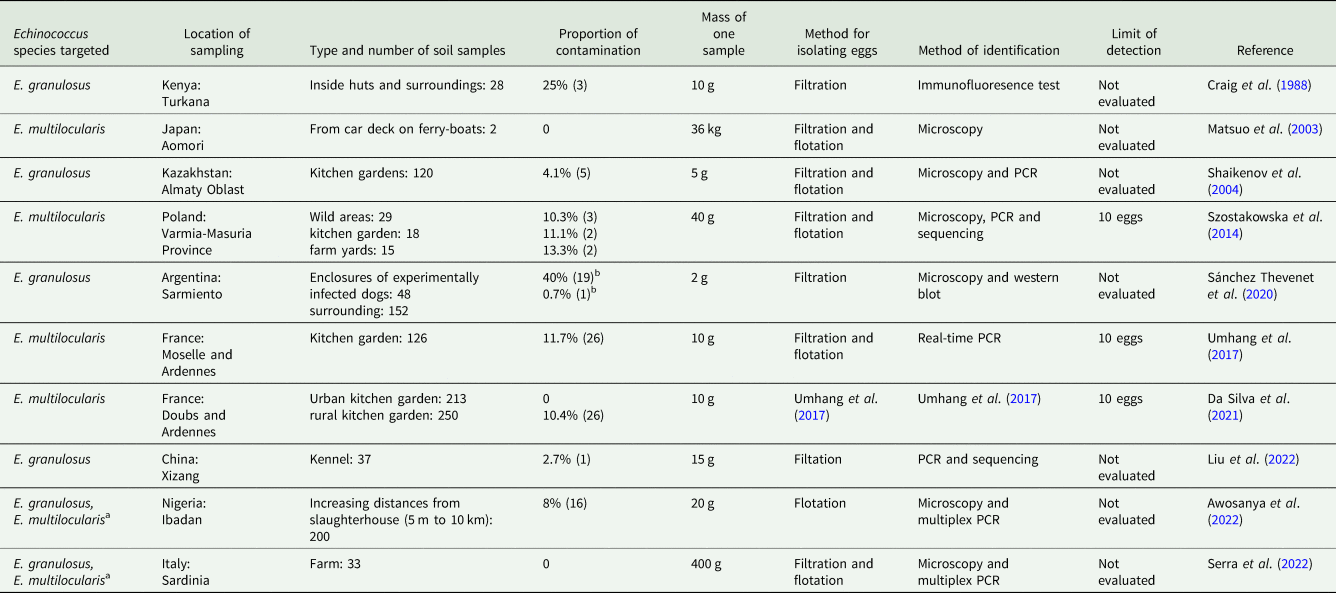
The presence of a citation for the egg isolation and identification methods means that it is the same method used in the study cited.
a A study that assumed the non-endemic status for E. multilocularis in the area but detection conducted in the context of a muliplex PCR including other parasite species.
b Data from four collections 0, 41, 70 and 84 months after the experimental infection of dogs were pooled.
Food
Echinococcus species have only recently been sought in food, despite E. multilocularis and E. granulosus being considered foodborne parasites for several decades. In Europe, the detection of Echinococcus spp. in food mainly focuses on E. multilocularis, probably due to its greater impact on human health (Devleesschauwer et al., Reference Devleesschauwer, Bouwknegt, Dorny, Gabriël, Havelaar, Quoilin, Robertson, Speybroeck, Torgerson, van der Giessen and Trevisan2017). The parasite is found on salad vegetables and berries (e.g. strawberries and blueberries), with contamination rates globally ranging from 0 to 4% and 0 to 7%, respectively (Table 5). Among the ten European endemic countries surveyed in the 9 papers concerned, E. multilocularis was identified in at least one food item from 8 countries. Contaminated food items were mainly identified in the historical geographical focus, France and Switzerland, but also in Eastern Europe with Estonia, Latvia and Poland (Lass et al., Reference Lass, Szostakowska, Myjak and Korzeniewski2015; Guggisberg et al., Reference Guggisberg, Alvarez Rojas, Kronenberg, Miranda and Deplazes2020; Umhang et al., Reference Umhang, Bastien, Cartet, Ahmad, van der Ark, Berg, Bonelli, Davidson, Deplazes, Deksne, João Gargate, van der Giessen, Jamil, Jokelainen, Karamon, M'rad, Maksimov, Oudni-M'rad, Muchaamba, Oksanen, Pepe, Poulle, Rinaldi, Samorek-Pieróg, Santolamazza, Santoro, Santucciu, Saarma, Schnyder, Villena, Wassermann, Casulli and Boué2024). Nevertheless, detections were also reported in countries considered endemic but at low rates, such as Denmark and the Netherlands (Umhang et al., Reference Umhang, Bastien, Cartet, Ahmad, van der Ark, Berg, Bonelli, Davidson, Deplazes, Deksne, João Gargate, van der Giessen, Jamil, Jokelainen, Karamon, M'rad, Maksimov, Oudni-M'rad, Muchaamba, Oksanen, Pepe, Poulle, Rinaldi, Samorek-Pieróg, Santolamazza, Santoro, Santucciu, Saarma, Schnyder, Villena, Wassermann, Casulli and Boué2024), and unexpectedly in locally produced mixed salad vegetables from southern Italy, an area not currently known to be endemic (Barlaam et al., Reference Barlaam, Temesgen, Tysnes, Rinaldi, Ferrari, Sannella, Normanno, Cacciò, Robertson and Giangaspero2021). Thus, while the contamination of food by E. multilocularis appears to mainly affect highly endemic areas, the risk of contamination cannot be ruled out for endemic areas with lower rates due to the patchy distribution of the parasite. The level of E. multilocularis contamination of lettuces from endemic areas in Europe is globally estimated to be 1% according to the 3 studies focusing on this food item (Guggisberg et al., Reference Guggisberg, Alvarez Rojas, Kronenberg, Miranda and Deplazes2020; Barlaam et al., Reference Barlaam, Temesgen, Tysnes, Rinaldi, Ferrari, Sannella, Normanno, Cacciò, Robertson and Giangaspero2021; Umhang et al., Reference Umhang, Bastien, Cartet, Ahmad, van der Ark, Berg, Bonelli, Davidson, Deplazes, Deksne, João Gargate, van der Giessen, Jamil, Jokelainen, Karamon, M'rad, Maksimov, Oudni-M'rad, Muchaamba, Oksanen, Pepe, Poulle, Rinaldi, Samorek-Pieróg, Santolamazza, Santoro, Santucciu, Saarma, Schnyder, Villena, Wassermann, Casulli and Boué2024). For other types of vegetables (mainly chards, parsley and spinach), a similar global value of 1.8% was found in the multicentre study of the OHEJP Meme project (Umhang et al., Reference Umhang, Bastien, Cartet, Ahmad, van der Ark, Berg, Bonelli, Davidson, Deplazes, Deksne, João Gargate, van der Giessen, Jamil, Jokelainen, Karamon, M'rad, Maksimov, Oudni-M'rad, Muchaamba, Oksanen, Pepe, Poulle, Rinaldi, Samorek-Pieróg, Santolamazza, Santoro, Santucciu, Saarma, Schnyder, Villena, Wassermann, Casulli and Boué2024). However, these values are much lower than the value of 30.7% obtained for various vegetables, excluding lettuces, in a highly endemic area in Poland (Lass et al., Reference Lass, Szostakowska, Myjak and Korzeniewski2015). This latter study revealed the first detection of Echinococcus spp. in food. The high levels of contamination by E. multilocularis found in various types of samples have been questioned and debated in several papers (Lass et al., Reference Lass, Szostakowska, Myjak and Korzeniewski2016; Robertson et al., Reference Robertson, Troell, Woolsey and Kapel2016; Torgerson, Reference Torgerson2016). The authors had suggested that choosing a sampling site in a hyper-endemic zone could help explain the high contamination levels, which would probably not be the same as those observed more globally in a given region. While the contamination rate for vegetables other than salad vegetables is very different from that obtained subsequently, it is not currently possible to compare mushrooms (36%) as this item was not targeted in other studies. However, it is possible to compare berries. Lass et al. (Reference Lass, Szostakowska, Myjak and Korzeniewski2015) reported contamination rates of 9.4% for forest fruits (raspberries, cranberries, blueberries, cowberries) collected in the forest and 20% for cultivated raspberries. No contamination by E. multilocularis has been reported on berries in some studies (Malkamäki et al., Reference Malkamäki, Näreaho, Lavikainen, Oksanen and Sukura2019; Temesgen et al., Reference Temesgen, Stigum and Robertson2022) and especially on berries (n = 30) or salad vegetables (n = 74) from areas with a high and low fox prevalence in Poland (Umhang et al., Reference Umhang, Bastien, Cartet, Ahmad, van der Ark, Berg, Bonelli, Davidson, Deplazes, Deksne, João Gargate, van der Giessen, Jamil, Jokelainen, Karamon, M'rad, Maksimov, Oudni-M'rad, Muchaamba, Oksanen, Pepe, Poulle, Rinaldi, Samorek-Pieróg, Santolamazza, Santoro, Santucciu, Saarma, Schnyder, Villena, Wassermann, Casulli and Boué2024). However, during this same multicentre study (Umhang et al., Reference Umhang, Bastien, Cartet, Ahmad, van der Ark, Berg, Bonelli, Davidson, Deplazes, Deksne, João Gargate, van der Giessen, Jamil, Jokelainen, Karamon, M'rad, Maksimov, Oudni-M'rad, Muchaamba, Oksanen, Pepe, Poulle, Rinaldi, Samorek-Pieróg, Santolamazza, Santoro, Santucciu, Saarma, Schnyder, Villena, Wassermann, Casulli and Boué2024), an overall contamination rate by E. multilocularis of 5.4% for strawberries and 7.3% for blueberries was obtained for the 7 endemic European countries concerned. The highest proportions were found in the 2 Balkan countries sampled, with 13.3% on strawberries (n = 30) and 13.3% on blueberries (n = 30) in Latvia, and 16.7% on strawberries (n = 30) in Estonia. When few strawberries were collected (n = 21) during this study, the only positive batch was identified among the 4 from Switzerland. Consequently, recent data acquisitions appear to confirm a high rate of E. multilocularis contamination among berries in some areas (Umhang et al., Reference Umhang, Bastien, Cartet, Ahmad, van der Ark, Berg, Bonelli, Davidson, Deplazes, Deksne, João Gargate, van der Giessen, Jamil, Jokelainen, Karamon, M'rad, Maksimov, Oudni-M'rad, Muchaamba, Oksanen, Pepe, Poulle, Rinaldi, Samorek-Pieróg, Santolamazza, Santoro, Santucciu, Saarma, Schnyder, Villena, Wassermann, Casulli and Boué2024), as initially observed in Poland (Lass et al., Reference Lass, Szostakowska, Myjak and Korzeniewski2015). However, this does not seem to be the case for vegetables other than salad vegetables, contrary to the high values obtained from the initial study in Poland. The observation of a significant difference in the rate of contamination between salad vegetables and berries is also supported by data from Pakistan, where E. multilocularis contamination affected 2% of salad vegetables (n = 100) and 56% of blueberry batches (n = 25) mainly from the same region (Umhang et al., Reference Umhang, Bastien, Cartet, Ahmad, van der Ark, Berg, Bonelli, Davidson, Deplazes, Deksne, João Gargate, van der Giessen, Jamil, Jokelainen, Karamon, M'rad, Maksimov, Oudni-M'rad, Muchaamba, Oksanen, Pepe, Poulle, Rinaldi, Samorek-Pieróg, Santolamazza, Santoro, Santucciu, Saarma, Schnyder, Villena, Wassermann, Casulli and Boué2024).
Table 5. Data from studies concerning detection of Echinococcus species in food
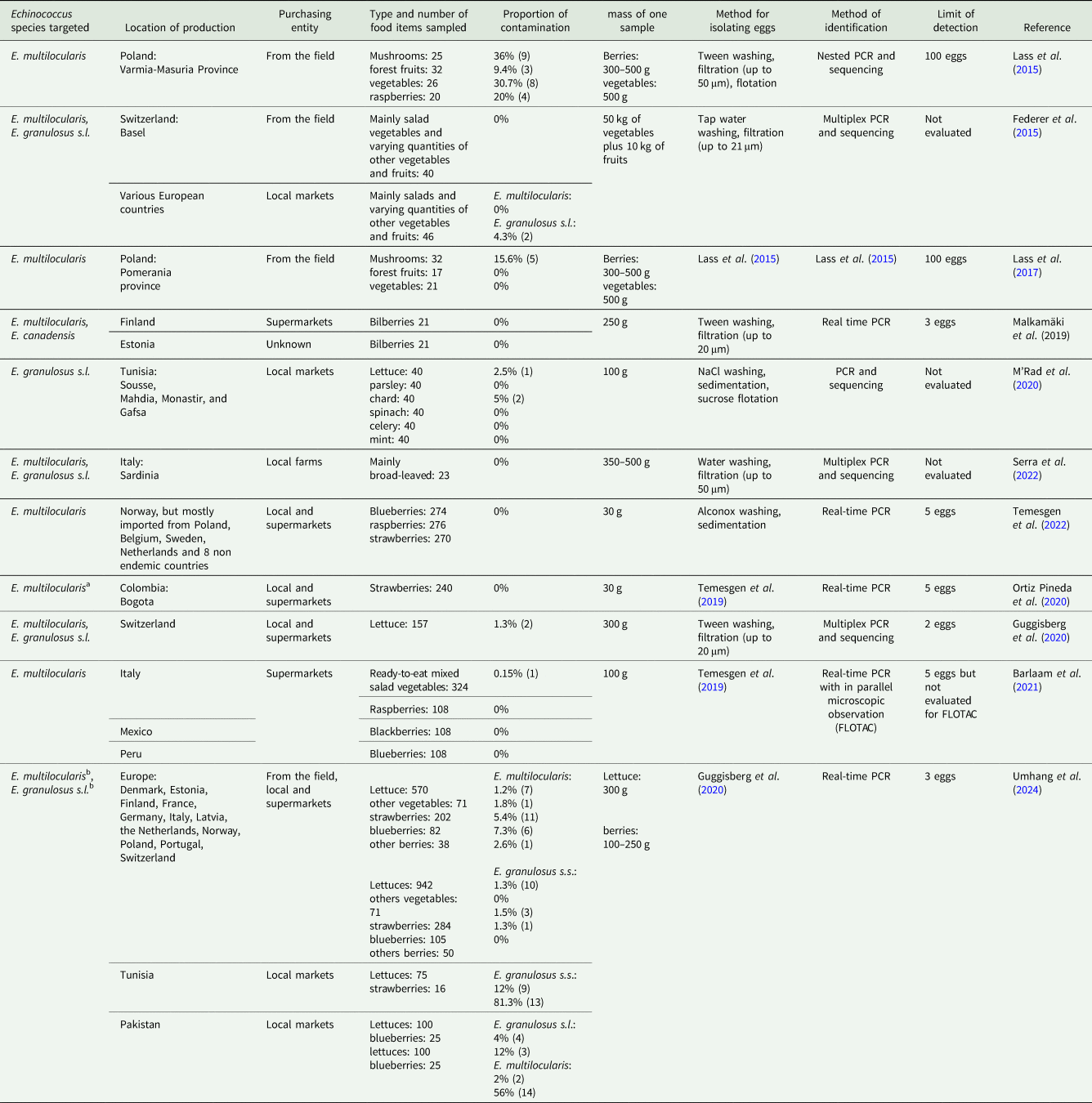
a Studies that assumed the non-endemic status for E. multilocularis in the country but detection conducted in the context of a muliplex PCR including other parasite species.
b Proportions were calculated considering only those European countries for which areas endemic for E. multilocularis have been sampled (Denmark, Estonia, France, Germany, Latvia, the Netherlands, Poland and Switzerland) and European countries where a domestic lifecycle is established for E. granulosus (Estonia, France, Italy, Latvia, Norway, Poland, Portugal).
The presence of a citation for the egg isolation and identification methods means that it is the same method used in the study cited.
From these same samples from Pakistan, a similar significant trend in favour of the higher contamination of berries than lettuces was observed for E. granulosus s.l. There was a 4% contamination rate for salad vegetables (n = 100) and a 12% contamination rate for blueberries (n = 25, all E. granulosus s.s., except for one case of E. canadensis). A significant difference in contamination between salad vegetables and berries was also described in Tunisia, a hyper-endemic area for E. granulosus s.s., with 12% of salad vegetables (n = 75) contaminated and 81.3% (n = 16) of strawberries (Umhang et al., Reference Umhang, Bastien, Cartet, Ahmad, van der Ark, Berg, Bonelli, Davidson, Deplazes, Deksne, João Gargate, van der Giessen, Jamil, Jokelainen, Karamon, M'rad, Maksimov, Oudni-M'rad, Muchaamba, Oksanen, Pepe, Poulle, Rinaldi, Samorek-Pieróg, Santolamazza, Santoro, Santucciu, Saarma, Schnyder, Villena, Wassermann, Casulli and Boué2024). However, in this specific situation, it is important to note that the strawberries all came from the region of Cap Bon in the northeast of the country, whereas the lettuces came from other parts of Tunisia that had a similar rate of contamination. Previously, E. granulosus s.s. eggs were only observed in 2% of the salad vegetables (n = 40) and 5% of the chards (n = 40) out of the various vegetables tested from local markets in 4 Tunisian cities (M'rad et al., Reference M'rad, Chaabane-Banaoues, Lahmar, Oumaima, Mezhoud, Babba and Oudni-M'rad2020). These lower proportions may be explained by the use of microscopic egg screening prior to molecular identification, a method whose performance was not evaluated in the study. In Europe, the average proportion of E. granulosus s.l. contamination of salad vegetables can be estimated at 1%, with similar values for strawberries (1.1%) and blueberries (1%) according to the 5 studies concerned (Federer et al., Reference Federer, Armua-Fernandez, Hoby, Wenker and Deplazes2015; Malkamäki et al., Reference Malkamäki, Näreaho, Lavikainen, Oksanen and Sukura2019; Guggisberg et al., Reference Guggisberg, Alvarez Rojas, Kronenberg, Miranda and Deplazes2020; Serra et al., Reference Serra, Masu, Chisu, Cappai, Masala, Loi and Piseddu2022; Umhang et al., Reference Umhang, Bastien, Cartet, Ahmad, van der Ark, Berg, Bonelli, Davidson, Deplazes, Deksne, João Gargate, van der Giessen, Jamil, Jokelainen, Karamon, M'rad, Maksimov, Oudni-M'rad, Muchaamba, Oksanen, Pepe, Poulle, Rinaldi, Samorek-Pieróg, Santolamazza, Santoro, Santucciu, Saarma, Schnyder, Villena, Wassermann, Casulli and Boué2024). The cases reported concern only identified endemic countries in the south (Italy and Portugal) and east of Europe (Latvia, Estonia and Poland), although uncertainty remains about the exact origin within Europe of the cases detected by Federer et al. (Reference Federer, Armua-Fernandez, Gori, Hoby, Wenker and Deplazes2016) in lettuces bought in Switzerland. While several European countries with low or very low endemic levels were involved without resulting in detection, the proportion reaches higher values for the countries from highly endemic areas, with an average proportion of 1.9% (n = 469). For instance, in Italy a total proportion of 3.2% of contaminated salad vegetables (n = 232) was reported, with detections ranging from 1.3% in Abruzzo to 3.8% in Sardinia and 6.5% in Campania (Umhang et al., Reference Umhang, Bastien, Cartet, Ahmad, van der Ark, Berg, Bonelli, Davidson, Deplazes, Deksne, João Gargate, van der Giessen, Jamil, Jokelainen, Karamon, M'rad, Maksimov, Oudni-M'rad, Muchaamba, Oksanen, Pepe, Poulle, Rinaldi, Samorek-Pieróg, Santolamazza, Santoro, Santucciu, Saarma, Schnyder, Villena, Wassermann, Casulli and Boué2024).
To sum up, it is only in the past decade that most data on the contamination of food by Echinococcus spp. have been produced. The detection rate of E. multilocularis and E. granulosus s.l. DNA in food mainly ranges from 0 to 5%, but can occasionally reach higher values, especially in berries. The large variation in the few available data reflects the heterogeneity of the studies carried out in terms of the variety of matrices, epidemiological context, number of samples tested per type of matrix and methods used. The food samples tested include various types of berries and a wide range of leafy vegetables, mainly lettuces. Four studies focused on one or two types of food items, which generally allows a high number of samples to be collected, resulting in an accurate estimation of the presence or absence of contamination, as opposed to sampling several different types of vegetables, often in small quantities for each. For now, it may be more appropriate to prioritize the production of robust data for specific food items rather than testing a large panel but with very few samples of each, as it would be difficult to draw relevant conclusions. The quantities of vegetable and berry matrices tested varied mostly between 30 g and 500 g, which can greatly impact the result depending on the method used. In this way, out of the 11 available studies, only six different methods were employed with detection limits ranging from 3 to 100 eggs, although this limit was not estimated for half of the methods used. However, based on current data, the contamination rate by E. multilocularis and E. granulosus s.s. appears to be significantly higher on berries than on salad vegetables and probably on other vegetables. Further studies conducted in various epidemiological situations will be necessary to clarify this preliminary trend.
Current gaps and future prospects
The need to use efficient detection methods
Various studies conducted over the past decade have provided much-needed data on the contamination of the environment and food by Echinococcus eggs. Despite the heterogeneity of the acquired data, it is possible to obtain a first overview of the contamination level for the different matrices tested. Overall, the contamination of soil, where eggs are initially deposited via feces, appears to be generally greater than in water or food following their dispersion. However, there is a need to consolidate these data to obtain more accurate estimates of contamination in different matrices. The multiplication of detection methods to detect taeniid eggs in the same matrix complicates the comparison of results (Saelens et al., Reference Saelens, Robertson and Gabriël2022). To achieve the goal of using standardised methods validated by an inter-laboratory ring trial (Hazards (BIOHAZ) et al., Reference Koutsoumanis, Allende, Alvarez-Ordóñez, Bolton, Bover-Cid, Chemaly, Davies, De Cesare, Herman, Hilbert, Lindqvist, Nauta, Peixe, Ru, Simmons, Skandamis, Suffredini, Cacciò, Chalmers, Deplazes, Devleesschauwer, Innes, Romig, van der Giessen, Hempen, Van der Stede and Robertson2018) it is currently crucial to employ only performance-evaluated methods for each matrix type taking great care to prevent contamination of samples during molecular analyses. The limit of detection may vary for the same method, for example, between leafy vegetables and berries, or between different types of water or soil. A limit of detection that is too high will result in an underestimation of the level of contamination. Currently, there are limited data available on the number of eggs detected in environmental or food samples, which is typically in the range of several dozen eggs per sample (Craig et al., Reference Craig, Macpherson, Watson-Jones and Nelson1988; M'rad et al., Reference M'rad, Chaabane-Banaoues, Lahmar, Oumaima, Mezhoud, Babba and Oudni-M'rad2020; Temesgen et al., Reference Temesgen, Stigum and Robertson2022). Although smaller quantities (<10 eggs) have also been observed (Shaikenov et al., Reference Shaikenov, Torgerson, Mathis, Deplazes, Rysmukhambetova and Massenov2004; Barlaam et al., Reference Barlaam, Temesgen, Tysnes, Rinaldi, Ferrari, Sannella, Normanno, Cacciò, Robertson and Giangaspero2021), the lower sensitivity of microscopic observation compared with molecular methods suggests that these high quantities of eggs observed do not correspond to the majority of contaminations, but only to the few most significant ones. There are currently no data on the minimum number of eggs that could lead to human infection (Alvarez Rojas et al., Reference Alvarez Rojas, Mathis and Deplazes2018). It may be difficult to systematically detect a single egg, as evidenced by the most effective methods currently requiring 2–5 eggs from food matrices even though fewer eggs may be detected but with a lower frequency. The precise quantification of the number of eggs present in soil, water and food samples is currently unknown but necessary to define relevant detection limits for methods. The amount of material tested for each sample is also important. Large quantities lead to methodological constraints in handling large volumes and generally result in a loss of sensitivity. This drawback can be compensated for by increasing the number of samples, probably giving a more relevant result but leading to a longer analysis time and higher cost.
Pursuing the acquisition of data on environmental and food contamination
In the coming years, the use of efficient methods will lead to more information being gathered on the contamination of soil, water, vegetables and berries. Conducting studies simultaneously seeking Echinococcus spp. and taeniid eggs in different matrices should shed light on the relative contamination of soil, water and food, as previously undertaken in some studies (Craig et al., Reference Craig, Macpherson, Watson-Jones and Nelson1988; Awosanya et al., Reference Awosanya, Olagbaju, Peruzzu, Masu, Masala and Bonelli2022; Serra et al., Reference Serra, Masu, Chisu, Cappai, Masala, Loi and Piseddu2022). It will also be necessary to investigate more specifically the differences in contamination between various types of leafy vegetables and berries. For instance, a difference in the attachment of Taenia laticollis eggs has been described between 2 types of berries (bilberries and cowberries) in the boreal forests of Finland (Malkamäki et al., Reference Malkamäki, Oksanen, Näreaho and Sukura2022). Such studies could potentially identify foods that are more at risk. Furthermore, understanding these differences in interactions between Echinococcus spp. eggs and the surfaces of different foods could lead to an adaptation of domestic and industrial washing methods. Currently, the effectiveness of washing methods has never been evaluated, despite washing being one of the main recommendations for preventing Echinococcus food contamination. In the current context of global trading, there is also the question of the risk to humans of contamination due to the importation of vegetables or berries from hyper-endemic areas. E. granulosus has been detected on salad vegetables purchased in Switzerland but originating from other European countries (Federer et al., Reference Federer, Armua-Fernandez, Gori, Hoby, Wenker and Deplazes2016). Similarly, contamination by protozoa from imported berries has also been described in Italy and Norway (Barlaam et al., Reference Barlaam, Temesgen, Tysnes, Rinaldi, Ferrari, Sannella, Normanno, Cacciò, Robertson and Giangaspero2021; Temesgen et al., Reference Temesgen, Stigum and Robertson2022). In addition, the favourable storage conditions of these exported foods promote the survival of eggs by maintaining a high level of humidity.
Furthermore, most of the articles have extended the identification of parasites by molecular biology beyond Echinococcus spp. to include other species of the Taeniidae family (genus Taenia, Hydatigera and Versteria) as well as protozoa (such as Toxoplasma, Cyclospora or Cryptosporidium). Only 2 studies conducted by the same group focus exclusively on one species (i.e. E. multilocularis) (Lass et al., Reference Lass, Szostakowska, Myjak and Korzeniewski2015, Reference Lass, Szostakowska, Myjak and Korzeniewski2017). Although the chosen washing methods can isolate a wide range of nematodes, cestodes and protozoa, it is important to note that the following steps of flotation and/or filtration and especially the molecular tests usually focus on only a few priority species due to their zoonotic potential. However, conducting a more comprehensive screening of parasite species can provide valuable insights into the contamination of food by parasite eggs, oocysts and cysts. To address the limitations and challenges of food sampling, a multi-species approach that encompasses more than one species or parasitic group would be more appropriate. This approach is already used by some groups notably using multiplex PCR to identify species in the Taeniidae family (Trachsel et al., Reference Trachsel, Deplazes and Mathis2007) or to identify a larger spectrum, covering Cyclospora sp., T. gondii and E. multilocularis (Temesgen et al., Reference Temesgen, Robertson and Tysnes2019). In regards to Echinococcus, detecting E. granulosus s.l. may always appear relevant due to its global distribution. While not systematically testing for E. multilocularis may be justified on non-endemic continents (i.e. Africa and South America), it would appear to be of primary interest elsewhere, even in areas not previously known to be endemic, as it may involve imported food or incidental detection, as in the case of locally produced salad vegetables in southern Italy (Barlaam et al., Reference Barlaam, Temesgen, Tysnes, Rinaldi, Ferrari, Sannella, Normanno, Cacciò, Robertson and Giangaspero2021).
Studying seasonal variations in environmental contamination rates would provide a better understanding of the dynamics of the presence of eggs in the environment. For instance, in Europe, the prevalence of E. multilocularis in foxes is generally higher in autumn and winter than in spring and summer, indirectly reflecting the proportion of rodents in the fox's diet (Burlet et al., Reference Burlet, Deplazes and Hegglin2011; Otero-Abad et al., Reference Otero-Abad, Armua-Fernandez, Deplazes, Torgerson and Hartnack2017). Does this supposed increase in environmental contamination still contribute to the risk of food contamination, which is mainly associated with warmer periods corresponding to food production? While egg dispersion can lead to their presence in cultivated areas, it is important to know whether eggs are still infectious after several months in the environment. Although available data demonstrate that this is possible, it is by no means systematic, as evidenced by the success rates of vole infestations ranging from 0 to 54% after infection by E. multilocularis eggs that had spent several months outdoors in experiments conducted by Veit et al. (Reference Veit, Bilger, Schad, Schäfer, Frank and Lucius1995). To more accurately assess environmental contamination, it appears necessary to estimate the proportion of eggs that are still viable rather than simply detecting their presence.
Essential need to assess the viability of Echinococcus eggs
Currently, data on environmental and food contamination by Echinococcus spp. are based on detecting parasitic DNA from eggs. However, it has not been possible to assess the viability of these eggs, which is a major issue in evaluating the risk of human contamination. Out of all the available studies, only one attempted – unsuccessfully – to evaluate the viability of eggs detected on lettuces (Guggisberg et al., Reference Guggisberg, Alvarez Rojas, Kronenberg, Miranda and Deplazes2020). This currently represents a significant methodological barrier, whether using in vitro or in vivo methods. Regarding the in vitro approach, hatching and activation of taeniid eggs in artificial gastric fluid may overestimate infectivity as eggs first lose their infective ability when they age (Coman and Rickard, Reference Coman and Rickard1977; Hazards (BIOHAZ) et al., Reference Koutsoumanis, Allende, Alvarez-Ordóñez, Bolton, Bover-Cid, Chemaly, Davies, De Cesare, Herman, Hilbert, Lindqvist, Nauta, Peixe, Ru, Simmons, Skandamis, Suffredini, Cacciò, Chalmers, Deplazes, Devleesschauwer, Innes, Romig, van der Giessen, Hempen, Van der Stede and Robertson2018). On the other hand, the comparison between oral, intraperitoneal and subcutaneous infection of mice showed that the latter route was the most sensitive, allowing for systematic parasite development (4/4) of up to 20 E. multilocularis eggs (Federer et al., Reference Federer, Armua-Fernandez, Hoby, Wenker and Deplazes2015). However, this detection threshold still appears too high when it comes to environmental or food contamination, bearing in mind that this threshold has not yet been established for in vitro methods. In addition to ethical considerations regarding animal welfare, replacing in vivo by in vitro methods would be appropriate, especially as some limitations can be successfully managed in this case (Alvarez Rojas et al., Reference Alvarez Rojas, Mathis and Deplazes2018). A similar in vitro method already exists for protozoa such as T. gondii and Cryptosporidium parvum, using CC-qPCR based on the development of oocysts in cell culture followed by real-time PCR detection to indirectly demonstrate parasitic proliferation (Rousseau et al., Reference Rousseau, Escotte-Binet, La Carbona, Dumètre, Chagneau, Favennec, Kubina, Dubey, Majou, Bigot-Clivot, Villena and Aubert2019; Géba et al., Reference Géba, Rousseau, Le Guernic, Escotte-Binet, Favennec, La Carbona, Gargala, Dubey, Villena, Betoulle, Aubert and Bigot-Clivot2021; Kubina et al., Reference Kubina, Costa, Favennec, Gargala, Rousseau, Villena, La Carbona and Razakandrainibe2021). The availability of a suitable method to assess the viability of Echinococcus spp. eggs is essential to be able to fully exploit the detection data from environmental and food matrices so as to more accurately evaluate the risk of human contamination.
Conclusion/future directions
During the last decade, there has been a renewed interest in studying environmental and food contamination by Echinococcus spp. eggs, which has provided an overview of the level of contamination in different matrices such as water, soil, berries and raw vegetables. Given the heterogeneity of the available data, it is necessary to continue acquiring information from different sources and in different epidemiological situations. However, it is essential to use methods whose performance has been evaluated and is relevant to the number of contaminated eggs and the type of sample involved. The quantitative data on egg contamination per sample is currently unknown, and must be estimated. Additionally, the development of standardised methods, ideally not limited to the Echinococcus genus, would facilitate the comparison of results on parasitic contamination. In the meantime, it is necessary to have accessible and efficient methods for producing data in hyper-endemic areas where economic resources are often limited. However, even in the presence of sensitive and robust methods for environmental or food detection, surveillance/monitoring of Echinococcus spp. via feces, where eggs are concentrated in large quantities, seems far more relevant (Alvarez Rojas et al., Reference Alvarez Rojas, Mathis and Deplazes2018). A better understanding of environmental contamination would also require additional data on the mechanisms involved in the dispersion of eggs, and especially the ability to estimate their viability on different matrices with a view to assessing the risk of human echinococcosis infection. If data are more and more available about the understanding and level of environmental and food contamination by Echinococcus eggs, there is still a long road to obtain an accurate estimation of the relative contribution of the different transmission pathway to human infection.
Data availability statement
Data will be made available on request.
Author contributions
G. U. and R. B. conceived and designed the study. G. U. and R. B. conducted data gathering. G. U. and R. B. wrote the article.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Competing interests
None.
Ethical standards
Not applicable.








