Introduction
The European eel, Anguilla anguilla Linnaeus 1758, is one of the most important fish species for European fisheries and aquaculture. Traditional eel aquaculture in Europe started in the Mediterranean, notably in Italy, in the form of extensive pond cultivation (Heinsbroek, Reference Heinsbroek1991). First aquaculture production in Germany was established in the late 1980s in heated effluents and recirculation aquaculture systems (Heinsbroek, Reference Heinsbroek1991). However, all kind of aquaculture activities depended on wild caught and imported glass eels from France, Italy, UK, Portugal or Spain (Gousset, Reference Gousset1990; Dekker, Reference Dekker, Aida, Tsukamoto and Yamauchi2003). Therefore, in 1980, first glass eels of the Japanese eel, A. japonica Temminck & Schlegel, 1846 were imported into European aquaculture systems (Kirk, Reference Kirk2003).
In contrast to eel culture, eel fishing has a very long tradition all over Europe. First documented management and stocking activities in Germany date back to the late 1800s century, while landing numbers have been recorded since the early 1900s century (Dekker, Reference Dekker2019), with highest landings in the 1950th. Commercial eel landings in Europe dropped significantly from their peak in the late 1970s to the early 2000s (ICES, 2021). Referring to ICES North Sea recruitment index (ICES, 2021), the numbers of arriving glass eels are currently below 2% compared to the recruitment level before 1980. In a similar magnitude the recruitment dropped in other parts of Europe, where the current level equals less than 10% of the recruitment strength before 1980 (ICES, 2021). The European eel is considered critically endangered by the IUCN in 2008 (International Union for Conservation of Nature and Natural Resources) (Jacoby and Gollock, Reference Jacoby and Gollock2014). To counteract the drastic decline and critical stock situation, a broad discussion on possible reasons and preventive measures were initiated. For example, to cumulate the European eel conservation efforts, the European Union implemented an eel recovery plan in 2007 with new measures for stock recovery (Council regulation (EC) no. 1100/2007).
Anguillicola crassus, Niimi & Hagaki, 1974 is a common parasite of the Japanese eel, reaching regular prevalences of infection between 24.5–40.0% along the Japanese coast (Nagasawa et al., Reference Nagasawa, Kim and Hirose1994). According to Koops and Hartmann (Reference Koops and Hartmann1989), A. crassus was introduced to Europe in the early 1980s, most probably from Taiwan through stocking for the aquaculture industry. It was then reported for the first time in Northern Germany in 1982 (Neumann, Reference Neumann1985; Spangenberg and Reinhold, Reference Spangenberg and Reinhold1992), and rapidly became the predominant parasite of A. anguilla over most of Europe within 10 years (Moravec et al., Reference Moravec, Di Cave, Orecchia and Paggi1993; Kirk, Reference Kirk2003), reaching a prevalence of infection up to 97% in the Havel river in 1986 (Koops and Hartmann, Reference Koops and Hartmann1989). The eel becomes infected with A. crassus by ingesting the intermediate or paratenic hosts, which are copepods and ostracods and diverse fish species, molluscs, amphibians and insect larvae respectively (Emde et al., Reference Emde, Rueckert, Kochmann, Knopf, Sures and Klimpel2014).
Besides overfishing and environmental issues such as pollution, climatic and oceanic changes and barriers preventing up-stream migration in rivers (Kirk, Reference Kirk2003; Friedland et al., Reference Friedland, Miller and Knights2007), the occurrence of A. crassus is considered a further possible reason for the observed population decline. This parasite is assumed to have negative impact on the European eel population (Kirk, Reference Kirk2003) by causing the Anguillicolosis, impairing the swim bladder function and thus, inhibiting a successful spawning migration of mature silver eels (Palstra et al., Reference Palstra, Heppener, van Ginneken, Székely and van den Thillart2007; Barry et al., Reference Barry, Mcleish, Dodd, Turnbull, Boylan and Adams2014). During its spawning migration, the eel must overcome a distance of more than 5,000 km from Europe to the Sargasso Sea. A daily vertical migration from more shallow swimming depth at night, averaging 282 ± 138 m, to more deeper swimming depths during daytime averaging 564 ± 125 m, was observed (Aarestrup et al., Reference Aarestrup, Økland, Hansen, Righton, Gargan, Castonguay, Bernatchez, Howey, Sparholt, Pedersen and McKinley2009). This strenuous migration is potentially made impossible by a severely damaged, non-functioning swim bladder (e.g. Barry et al., Reference Barry, Mcleish, Dodd, Turnbull, Boylan and Adams2014; Dezfuli et al., Reference Dezfuli, Maestri, Lorenzoni, Carosi, Maynar and Bosi2021; Simon et al., Reference Simon, Ubl, Lewin and Dorow2023).
We present a long-term data set of the European eel population in the German Baltic Sea and various inland habitats of Mecklenburg-Western Pomerania (MWP), together with statistical analyses of A. crassus infection patterns since 1991. European eels were sampled for 30 years to detect the long-term changes. Possible reasons for the observed trends of both, the host and the parasite population developments, are discussed.
Materials and Methods
Sample collection and examination
The study is based on various eel related surveys in the north-eastern German state MWP. According to LALLF (https://www.lallf.de/fischerei/statistik/fangstatistik-kuestengewaesser/), annual commercial eel landings in MWP reached a mean of 41.6 t between 2011 and 2020 in coastal waters. Within the same period, the average annual commercial landings in inland waters of MWP were 48.3 t. In inland waters, eel stocking activities were conducted since more than 60 years (Dorow and Paetsch, Reference Dorow and Paetsch2017). In the first place, stocking should ensure the commercial eel fishing. Since the implementation of the European eel regulation, annual stocking is also a management measure to increase the silver eel escapement.
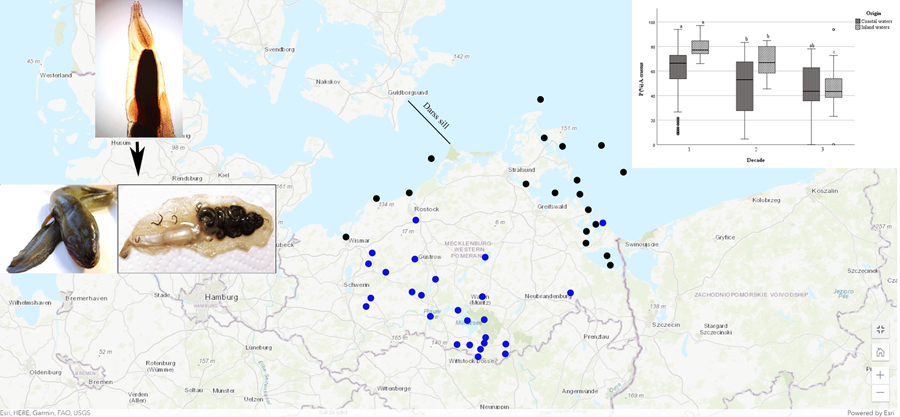
European eels integrated in this study originated from various scientific studies and samplings, such as eel monitoring programmes and commercial fisheries. Data were collected through the scientific staff of the Thuenen Institute of Baltic Sea Fisheries, Rostock (1991–2003) and the Mecklenburg-Vorpommern Research Centre for Agriculture and Fisheries, Rostock. Analysed data were discriminated by the origin of the caught eels and distinguished between inland habitats and coastal water samplings (Baltic Sea group). In inland waters, samples covered 32 different water bodies and streams. Baltic Sea eel were collected from more than 23 sampling locations, 6 of them from the western part of the German Baltic Sea (West of Darss sill) and 17 from the eastern part (East of the Darss sill, see Fig. 1). Details are given in Supplementary Table S1.

Figure 1. Map with marked sampling points of European eel in Mecklenburg-Western Pomerania. Blue dots show freshwater samples, black dots are sampling locat from the Baltic Sea. Darss sill is dividing the western and the eastern part of the German Baltic Sea. (Map by: ArcGis Online, ESRI)
Beside developmental stages (yellow eel, silver eel), the mean weight and length were recorded. The swim bladder damage status was assessed by defining 5 degrees of damage according to Hartmann (Reference Hartmann1994). Value 1 represents a normal swim bladder with no pathological sign, while 5 describes severe damaged swim bladders, with thickened wall and disfunction. Based on the rating of the individual samples, the mean overall damage degree per sampling was calculated as a rough measure for swim bladder damage for each water body:
where n1 is the number of eels with damage degree 1, n2 the number of eels with damage degree 2 etc. All eels were included (infected and uninfected).
Parasitological parameters
The swim bladder of 16 508 specimens of European eel were examined for A. crassus. Number of found specimens was counted and the prevalence (%), mean intensity (mI), intensity (I) and mean abundance (mA) were calculated. Parasitological terms follow Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997).
Statistical analyses
Statistical analyses were conducted using IBM, SPSS V.25 (Chicago, Illinois, USA) and were calculated based on the mean values and standard deviation of all eel samplings (n = 217). Sampling data were classified into 3 group sets of data, representing 3 decades (D) (D1 = 1991–2000; D2 = 2001–2010, D3 = 2011–2020). Initially, datasets of D1 – D3 were analysed for normality and homoscedasticity and accordingly, the following analyses were adjusted. One-way ANOVA was run in case of normally distributed data to compare the 3 decades in each habitat. In the case of not-normally distributed data, WELCH ANOVA was used. To compare the data by decades, the one-way analysis was followed by post-hoc test, while Tukey's HSD was used in the case of data and Dunnett T3 in case of heteroscedasticity. T-test was run to compare 2 groups, the coastal and inland fish in the same decades. Significance level was set at P = 0.05 in all cases. Significant differences between studied means are indicated by letters a, b or c, while the same letters stand for no significant differences between the 2 compared groups.
Results
Sampling set up
In total, 16 508 European eel from 217 samplings were investigated between the years 1991 and 2020 (see Table 1). Of these, 12 744 European eels from 147 samplings were caught along the coastline of MWP (Fig. 1). Each sampling included 86.7 fishes in average, with a total length of 48.2 cm in average and a mean weight of 270.8 g. In lakes and streams of MWP, 3,764 European eels were caught from 70 samplings with an average of 53.7 specimens per sample, each. Mean total length was 55.0 cm and mean weight 354.5 g.
Table 1. Number of samplings and examined European eel during 3 decades

Parasitological infection rates
During the entire examination period of 30 years, the mean prevalence of eel infected with A. crassus was 55.4% (±22.3 s.d.) in coastal and 60.8% (±20.1 s.d.) in inland waters. The highest average infection rates were recorded during the first sampled decade between 1991 and 2000 in both habitats. Inland caught eels were infected with a mean prevalence of 79.5% (±8.4 s.d.) and eels from the Baltic Sea coastal areas with 61.3% (±19.1 s.d.) (see Table 2). During the second decade, the observed prevalence decreased in coastal waters to 46.2% (±24.5 s.d.) and in inland waters to an average of 66.8% (±13.1 s.d.). During the last decade between 2011 and 2020, the prevalence in coastal waters remained at a mean value of 45.6% (±21.8 s.d.) while the prevalence of infection in eels from inland waters further decreased to 46.1% (±16.2 s.d.). For inland waters, the observed changes in prevalence of infection were significant between all three decades. In coastal waters, the prevalence of infection between decades 1 to 2 was significantly different (P < 0.05). Between D1 and D3 and D2 and D3 no significant differences were observed (Fig. 2). Overall mean prevalence of all studied eel samples from both habitats continuously decreased over time from 64.9% (D1) to 51.2% (D2) and 46.0% (D3).
Table 2. Results of Prevalence (P%), mean intensity (mI), intensity (I), mean abundance (mA) and mean Hartmann-class (mHC) (±s.d.) of infection with Anguillicola crassus in the swim bladder of European eel for inland and coastal areas in Mecklenburg-Western Pomerania given over time


Figure 2. Boxplot diagram of the mean prevalence (P (%)) of European eel infected with Anguillicola crassus from the Baltic Sea and coastal brackish waters and from inland freshwater habitats during 3 decades. Significant differences between mean prevalences are marked with a, b or c; while 2 groups with same letters do not vary significantly.
The mean number of nematodes per infected eel ( = mean intensity) was highest during the first decade. Altogether, 62 852 specimens of A. crassus were found, with 1 to 220 parasites per infected eel. Similar to the prevalence of infection, inland caught European eel showed the highest number of A. crassus per individual, with 8.8 (±4.3 s.d.) parasites compared with 6.3 (±2.8 s.d.) in coastal waters during D1. The values decreased during the studied periods of 3 decades and the lowest intensity was observed during D3 between 2011 and 2020 in inland waters, where only 3.8 (±1.7 s.d.) nematodes per infected eel were found. The observed decrease in intensity was highly significant for inland waters during all three decades (P ⩽ 0.001) (Fig. 3). In coastal waters, the intensity only slightly decreased to 5.6 ± 2.8 in D3. No significant differences were found here.

Figure 3. Boxplot diagram of mean intensity (mI) of European eel infected with Anguillicola crassus from the Baltic Sea and coastal brackish waters and from inland freshwater habitats during 3 decades. Significant differences between the mean intensity are marked with a, b or c; while 2 groups with same letters do not vary significantly.
By comparing the mean number of A. crassus for all sampled eel (mean abundance) including non-infected specimens, the observed trend of distribution of the mean intensity and prevalence could be confirmed. The highest mean abundance was found during the first decade (1991–2000) in the inland waters (7.2 vs 4.0), while between 2011 and 2020 the lowest mean abundance was observed (1.9) (Table 2). For eel from inland waters, all differences between the decades were significant, while those from the Baltic Sea only showed significant differences between D1 and D2 (P < 0.05) (Fig. 4).

Figure 4. Boxplot diagram of mean abundance (mA) of European eel infected with Anguillicola crassus from the Baltic Sea and coastal brackish waters and from inland freshwater habitats during 3 decades. Significant differences between the mean abundance are marked with a, b or c; while compared values with same letters do not vary significantly.
The mean Hartmann-class (mHC) followed the same trend as the other parasitological parameters. All values continuously decreased over time, with significant differences between all 3 decades in inland waters (Table 2). The highest average damage status was found during the first decade in eels caught in freshwater (2.6 ± 0.5) and brackish water (2.1 ± 0.6), respectively. Within the following decades, a strong and significant decrease of the mean damage status was especially observed in freshwater. For damage status of eel from the coastal waters of the Baltic Sea, only for D2 and D3 no significant differences were observed (P = 0.686) (Fig. 5), where the mean Hartmann-class was relatively low during both decades (1.4 and 1.5).

Figure 5. Boxplot diagram of mean Hartmann-class (mHC) of European eel infected with Anguillicola crassus from the Baltic Sea and coastal brackish waters and from inland freshwater habitats during 3 decades. Significant differences between the mHC are marked with a, b or c; while compared values with same letters do not vary significantly.
Discussion
The present study analysed a 30 years long data series of the infection rates, damage and thus parasite–host relationship of the neozoon parasite A. crassus (Nematoda) in its new host European eel in German (MWP) coastal and freshwater habitats. During the first decade, starting appr. 10 years after the first occurrence of this parasite in German waters, the parasite prevalence, mean intensity and abundance reached highest values. Because A. crassus was introduced to Germany in the 1980s, the first years after the introduction event to Europe represented a period of very high infection rates. The European eel, a naive definitive host, was very susceptible to the infection with the invading nematode A. crassus and seemed to have no or little defence mechanisms (Kirk, Reference Kirk2003). Studies by Koops and Hartmann (Reference Koops and Hartmann1989), Möller et al. (Reference Möller, Holst, Lüchtenberg and Petersen1991) or Sprengel and Lüchtenberg (Reference Sprengel and Lüchtenberg1991) indicated that the first infections with A. crassus in water bodies in Northern Germany occurred between 1985 and 1987, reaching a prevalence of 4% (in 1986, North Sea, near Helgoland) – 97% (in 1986, Havel River). The best studied region during this period was the lower Elbe river, where no infection was found in 1985, while 1 year later the rate already varied between 6 and 27% (Koops and Hartmann, Reference Koops and Hartmann1989). Between October 1987 and September 1988, the average infection with A. crassus reached 57.7% (Möller et al., Reference Möller, Holst, Lüchtenberg and Petersen1991), which is similar to our observed average prevalence rate of 61.3% in coastal waters within the first decade (1991 to 2000).
The mean overall prevalence of A. crassus in the eel during the first decade was 79.5% in inland waters. This demonstrates a generally higher infection rate in freshwater ecosystems compared with brackish water ones. Although the number of samples taken in inland and coastal waters is not the same, the statistical comparison is still significant because the number of eels in each decade is at least 500. In an earlier long-term study on A. crassus infections in European eel from Northern Germany between 1996 and 2011 by Wysujack et al. (Reference Wysujack, Dorow and Ubl2014) a continuously high prevalence of approximately 70–85% in several inland waters in Northern Germany (river and lakes) and in the Baltic Sea were reported. The authors observed no trend of decreasing infection rates over time. Studies in Belgium (river in Flanders) and France (in oligosaline Fumemorte Canal in Camargue) described the settlement of A. crassus as a 2-stage pattern, consisting of a rapid spread with increasing prevalences during the first years followed by ceiling levels at 60–70% (Audenaert et al., Reference Audenaert, Huyse, Goemans, Belpaire and Volckaert2003; Levebvre and Crivelli, Reference Lefebvre and Crivelli2004). Similarly, a stabilization of the A. crassus prevalence at levels of ~60% was reported by Schabuss et al. (Reference Schabuss, Kennedy, Konecny and Herzig2005) in the Neusiedler See (Austria) and in Lake Balaton (Hungary). A similar decreasing trend is herewith reported from inland waters during the last decade of the years 2011 to 2020. Compared to the second decade, a harsh reduction of the infection rate by more than 20% was observed. In coastal waters, the prevalence decreased slightly and stabilized at an average level of 45.6% (see Table 1). In total, a massive reduction of prevalence by around 33% in inland waters during this 30-years of long-term analysis was observed. In coastal waters, the reduction was less clear with ca. 16% over 30 years. Interestingly, all these prevalence of infection data seem to not correspond to another long-term data set from Portugal. Pereira et al. (Reference Pereira, Braga, Moura and Antunes2022) studied European eel from Minho River (Portugal) over a period of 26 years between 1995 and 2021. In general, the mean prevalence was lowest during the first decade (30.7%, 1995/96), increasing to 67.9% during the second decade (2009/11), subsequently followed by a slightly decrease to 63.1% during the third decade (2017/21). Because the authors stated that the first infection of A. crassus in Minho River was recorded in 1995, their study covered the immediate beginning and development of the infection. Thus, in case of the Minho River, the infection of A. crassus in the European eel started with an initial phase at low prevalences and remained on a similar high infection level as observed from other regions (see above) during the following years from 1995 to 2018. These results seem to contrast our data, concerning the overall peak of infection and the long-term development. However, as stated by Koops and Hartmann (Reference Koops and Hartmann1989), also the infection in German waters needed some years to reach its peak, similar to the first period identified by Pereira et al. (Reference Pereira, Braga, Moura and Antunes2022). On the other hand, the peak reached in Germany was much higher (up to 100%) compared with Minho River (67.9%). A possible reason for this difference might be seen the different population densities of the host in Minho River. A lower population size at the beginning of the parasite invasion might result in a lower prevalence of infection with A. crassus and subsequently followed by a lower adaptation pressure onto the host.
Distinct differences between the infection levels of the Baltic Sea and the inland freshwater environments were observed. The lower prevalence and minor infection intensity in the first and second decade resulted in an overall decreasing damage status of the swim bladders in the coastal waters of the study region. The infection in the coastal waters was clearly lower than in freshwater and thereby the results of former studies were confirmed (see Kennedy, Reference Kennedy2007; Székely et al., Reference Székely, Palstra, Molnár and van den Thillart2009; Wysujack et al., Reference Wysujack, Dorow and Ubl2014). Simon et al. (Reference Simon, Ubl, Lewin and Dorow2023) found the same trend in their study, indicating that eel from the open coastal waters have a higher fitness compared to species originating from nearby brackish lagoons. It has been shown that hatching rate, survival and infectivity of the second larval stage of A. crassus declines with increasing salinity (Kirk et al., Reference Kirk, Kennedy and Lewis2000). Even though adult parasites can survive in seawater for a certain time and may even produce eggs, the completion of the life cycle in saline waters appears to be limited due to physiological effects of salinity as well as to the fact that many of the marine copepods are of the insuitable size to serve as intermediate hosts (see Kennedy, Reference Kennedy2007). Several studies support the absence or rare abundance of A. crassus under high saline conditions (Jakob et al., Reference Jakob, Hanel, Klimpel and Zumholz2009; Giari et al., Reference Giari, Castaldelli, Gavioli, Lanzoni and Fano2021).
The mean intensity of infection was also highest during the first decade inside the freshwater habitats with 8.8 ± 4.3 specimens per fish. This mean intensity continuously decreased over time with minimum of 3.8 ± 1.7 in the third decade. The mean intensity in brackish waters was generally lower and showed stabile or only slightly decreasing trends over time. In other German inland waters, early studies like Spangenberg and Reinhold (Reference Spangenberg and Reinhold1992) reported 10–37 nematodes per swim bladder in rivers around Berlin and Potsdam, 5.0–5.3 nematodes per swim bladder in the river Rhine (Sures et al., Reference Sures, Knopf, Würtz and Hirt1999), and 4.2–5.9 A. crassus per eel in the river Weser (Reimer, Reference Reimer2000). Similar to our study, the values were highest in the early 1990s and decreased over time. A similar infection history was found in a long-term study at the upper Lake Constance in South Germany. Bernies et al. (Reference Bernies, Brinker and Daugschies2011) recorded a drastic initial increase of the infection intensity, which peaked at 16 nematodes per swim bladder 4 years after the first occurrence of the parasite in the lake in 1988. After this peak, the values started to drop rapidly within 2 years. This decrease then slowed down but continued consistently until the end of the study in 2009, when the mean infection intensity was only 3.3 ± 0.5 nematodes per infected eel. The same trend was also observed for the mean abundance.
The observed mHC was highest in the 1990s, indicating moderate impairment of the swim bladders. Within the following years, the damage status significantly decreased in both freshwater and Baltic Sea habitats to lower average values. Wysujack et al. (Reference Wysujack, Dorow and Ubl2014) found similar values of the Hartmann classes in Northern Germany, with the highest mean damage degree of 2.71 ± 0.32 in river Weser and the lowest value of 1.05 ± 0.04 in the coastal waters of MWP. Pietrock et al. (Reference Pietrock, Schreckenbach and Thürmer2002) investigated in total 406 European eel from 6 lakes in Brandenburg in 2001 and found a mHC of 2.6. Other authors used the Swim bladder Denerative Index (SDI) after Lefebvre, Contournet and Crivelli (Reference Lefebvre, Contournet and Crivelli2002) with values between 0 and 6. Pereira et al. (Reference Pereira, Braga, Moura and Antunes2022) calculated a mean SDI of 2.51 ± 1.12 for eel in Minho River, Portugal.
According to these available data sets and our new data, it seems that the eel population in the third decade was much more adapted to the parasite infection compared with the first and second decade, both with less inflamed swim bladder walls and less infection intensities. In comparison to Pereira et al. (Reference Pereira, Braga, Moura and Antunes2022), it must be considered that our data represent the decades 2–4 after first invasion, where a continuous host–parasite adaptation period following a peak infection level takes place. Several reasons can be argued to explain the observed shift from an infection peak towards decreasing infection levels:
1. The individual host acquires parasite immunity over time and is able to reduce the number of parasites. Knopf et al. (Reference Knopf, Madriles Helm, Lucius, Bleiss and Taraschewski2008) experimentally infected eel with L3-larvae of A. crassus and observed an increased inflammatory reaction in the surroundings of the nematodes in the swim bladder wall. The inflammatory infiltrate consisted of monocytes and macrophages, granulocytes and lymphocytes and was only observed in the surrounding of some of the present A. crassus larvae. Thus, an activation of the defence cells resulting in an increased migration activity in infected eels was documented (Knopf et al., Reference Knopf, Madriles Helm, Lucius, Bleiss and Taraschewski2008). Bracamonte et al. (Reference Bracamonte, Knopf and Monaghan2021) stated that the encapsulation of nematodes, which is commonly observed in native hosts, also takes place in the novel host A. anguilla. These authors described an increased frequency of encapsulation over time which was positively associated with the reduction in numbers of adult specimens of A. crassus inside the eels. The mean infection intensity of adult parasites was 5.1 ± 1.6 in non-capsulating eel compared to 2.0 ± 0.5 in the encapsulating eels.
2. Heavily infected eels might die after a certain time. Studies from the early 2000s showed that A. japonica has more effective defence mechanisms against A. crassus compared with the European eel (Knopf and Mahnke, Reference Knopf and Mahnke2004). Egusa (Reference Egusa1979) reported only negligible pathological effects of this parasite onto A. japonica but certain mortality among A. anguilla cultivated in Japan. In all above cases, a decreasing infection rate must be accompanied by a likewise decreasing infection intensity, as recorded in the present study. The first option was challenged by Denny et al. (Reference Denny, Denny and Paul2013), Neto et al. (Reference Neto, Costa, Costa and Domingos2010) and Pereira et al. (Reference Pereira, Braga, Moura and Antunes2022), who did not observe any negative effect of A. crassus infection on the eel body condition during their continental growth phase. The general body condition seemed to be not negatively influenced by the swim bladder damage caused by the nematode, which was also observed in all other studies, including the present one. Consequently, direct mortality might therefore not explain the observed changing prevalence and infection intensities.
3. Infected eels die on their way to the Sargasso Sea, only allowing better adapted or lower infected specimens to reproduce, driving towards a better adapted population. The functionality of the swim bladder still comes into focus when dealing with the long spawning migration of European eel (Palstra et al., Reference Palstra, Heppener, van Ginneken, Székely and van den Thillart2007; Newbold et al., Reference Newbold, Hockley, Williams and Kemp2015) and not during the continental growth phase. Thus, a higher mortality during the spawning migration is feasible caused by observed infection values. As infected eels have to utilize 20% additional energy reserves than they would normally need (Palstra et al., Reference Palstra, Heppener, van Ginneken, Székely and van den Thillart2007), it could prematurely deplete the energy needed to reach the spawning location in the Sargasso Sea. This would lead to the death before mating thus to a reduction of numbers of heavily infected eels taking part at the spawning (Pelster, Reference Pelster2015). Infection with A. crassus also impairs the mechanism of gas secretion by severely reducing the level of oxygen in the swim bladder through infection (Kirk, Reference Kirk2003). Metabolic processes that depend on the gas gland cells and thus on gas secretion could also be impaired (Pelster, Reference Pelster2015). In addition, the ability to regulate the eel's capacity to descend to depths in water may be inhibited (Palstra et al., Reference Palstra, Heppener, van Ginneken, Székely and van den Thillart2007). In this sense, it has been hypothesized that full maturation of the reproductive organs does not occur unless individuals swim to deeper sea water (Sébert et al., Reference Sébert, Amérand, Vettier and Dufour2007; Sjöberg et al., Reference Sjöberg, Petersson, Wickström and Hansson2009). Consequently, parasite infection will result in a loss of potential spawners for the eel population, with individuals dying either on the way to or inside the Sargasso Sea. Therefore, well-adapted eels are likely to be the main contributors to the maintenance of the overall spawning stock.
4. Parasite avoidance strategies of either the definitive or the intermediate hosts. Parasites are known to alter the host behaviour in order to have a better transmission to the next host (Palm et al., Reference Palm, Theisen, Pikalov, Kleinertz and Roitberg2018). Consequently, survival of heteroxenous parasites depends on successful strategies to either manipulate the host or prevent detection through the immune system. A neozoic parasite such as A. crassus cannot infect its host undetected, without the possibility of adopting avoidance strategies to elude the immune response. This kind of reactions has already been observed by Bracamonte et al. (Reference Bracamonte, Knopf and Monaghan2021) and Knopf et al. (Reference Knopf, Madriles Helm, Lucius, Bleiss and Taraschewski2008) and might also play a role resulting in the observed infection patterns. However, most A. crassus larvae occur in several species of cyclopoid copepods, which are the obligate first intermediate hosts. Additionally, about 50 paratenic hosts, like several amphibians or insects and at least 37 fish species are known as potential participants of its life cycle (Emde et al., Reference Emde, Rueckert, Kochmann, Knopf, Sures and Klimpel2014). With the very generalistic feeding regime or European eel (Bouchereau et al., Reference Bouchereau, Marques, Pereira, Guélorget and Vergne2009; Denis et al., Reference Denis, Rabhi, Le Loc'h, Ben Rais Lasram, Boutin, Kazour and Amara2022), such avoidance of intake of hosts seems impossible.
It is interesting to note that, in accordance to the observed decline in parasite intensity in eel during the third decade, studies by Dorow et al. (Reference Dorow, Lewin, Lill, Ubl and Frankowski2021, Reference Dorow, Kullmann, Buck and Frankowski2023) using different stock assessment methods indicate independently from each other an increased eel population in coastal waters of MWP since 2016. Thus, a higher number of definitive hosts in the region might support increasing infection levels with A. crassus in future, placing also more pressure onto the eel defence strategies. This hypothesis could compete with the stabilization of eel stocks through increased host–parasite adaptation. Accordingly, it is recommended to continue the established standardized assessment A. crassus to document to parasite infection development in the future. In the face of eel conservation efforts being implemented on a European scale, such data are needed to evaluate the impact of A. crassus on individual health status and reproduction potential.
Conclusions
In summary, our analyses using a 30-year long data set demonstrate that the infection of European eel with A. crassus in Mecklenburg-Western Pomeranian inland and coastal waters reached high infection rates during the first 10 years of investigation (D1). This period, however, must be considered already a second phase in the infection dynamics of A. crassus in German coastal and inland waters, most probably following a first phase of strongly increasing infection rates until reaching a peak level. Then, the prevalence, mean intensity, mean abundance and swim bladder damage status continuously decreased during subsequent years and in both habitats. Thus, the observed trend suggests that a steady state of infection has been reached in the eel population, with moderate levels of infection.
Of the 4 proposed explanations for the continuous decline in the infection rate, the most plausible one is the increase in the number of individuals with an immune response capacity against A. crassus, which was absent in the eel population that suffered a higher mortality during the initial infection period. Because eel from coastal waters tend to be generally less infected with A. crassus, we suggest that eel stocks in coastal waters may be of higher value for eel conservation efforts because they seem to be less impaired regarding the spawning migration compared to eels using nearby inland waters as growing habitats. Regarding the number and quality of spawners, the shown differences in the parasite intensity with A. crassus within different habitats during the continental life phase of the European eel should be considered for future management strategies.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182024000039
Data availability statement
The data that supports the findings of this study is available as supplementary materials.
Acknowledgements
We are very thankful to the colleagues from the Thünen-Institute, especially Eka Hahlbeck, and the Mecklenburg-Vorpommern Research Centre for Agriculture and Fisheries (LFA-MV) for sampling and archiving European eel data over 3 decades. We thank the anonymous reviewers for providing valuable and constructive comments on our manuscript.
Authors’ contributions
MD, JS and SM designed the study, JS and PU performed statistical analyses and interpreted the data; PU drafted the manuscript, MD, HP and SM revised the manuscript. All Authors edited and approved the final version of the manuscript.
Financial support
No direct financial support
Competing interests
None.
Ethical standards
All applicable institutional, national and international guidelines for the care and use of animals were followed.