Introduction
Schistosomiasis in the People's Republic of China (China), caused exclusively by infection with the species Schistosoma japonicum, is a zoonotic parasitic disease that goes back to antiquity with eggs identified in mummies from the Han dynasty (206 BC) (McManus et al., Reference McManus, Gray, Li, Feng, Williams, Stewart, Rey-Ladino and Ross2010; Reference McManus, Dunne, Sacko, Utzinger, Vennervald and Zhou2018; Yeh and Mitchell, Reference Yeh and Mitchell2016). Historically, schistosomiasis ranks as the most important helminth infection in China, with the main hotspots being the river bank areas of the five provinces along the middle and lower reaches of the Yangtze River (Hunan, Hubei, Jiangxi, Anhui and Jiangsu) and the mountainous areas of Sichuan and Yunnan (McManus et al., Reference McManus, Gray, Li, Feng, Williams, Stewart, Rey-Ladino and Ross2010; Li et al., Reference Li, Hou, Tan, Williams, Gray, Gordon, Kurscheid, Clements, Li and McManus2020). The major endemic foci are found in the marshlands of the Dongting and Poyang lakes, China's two largest inland lakes (Fig. 1). China is a global leader in schistosomiasis control with its strong political commitment and concerted long-term control efforts involving a variety of approaches developed and rigorously implemented over the past several decades. Control measures include snail control, removal of bovines from endemic areas, chemotherapy, improved sanitation, access to safe water and health education (Wang et al., Reference Wang, Chen, Guo, Zeng, Hong, Xiong, Wu, Wang, Wang, Xia, Hao, Chin and Zhou2009; Zhou et al., Reference Zhou, Liang, Chen, Rea, He, Zhang, Wei, Zhao and Jiang2011). In 2010, Chinese Vice-Premier Li Keqiang first championed the goal of upscaling this integrated strategy of blocking infection transmission to achieve schistosomiasis elimination (defined as reducing the locally acquired infection rate to zero) by 2020 (Utzinger et al., Reference Utzinger, Brattig, Leonardo, Zhou and Bergquist2015). In 2018, of 28 456 villages in 450 endemic counties (covering 260 million people, of which over 70 million were at risk of infection), 58.4% reached the criterion of elimination, 27.6% of transmission interruption and 14.0% of transmission control, due to implementation of these measures in the China National Schistosomiasis Control Programme (CNSCP) (Zhang et al., Reference Zhang, Xu, Guo, Dai, Dang, Lü, Xu, Li and Zhou2019). The elimination target was revised upwards from 2020 to 2025 (Zhou et al., Reference Zhou, Guo, Wu, Jiang, Zheng, Dang, Wang, Xu, Zhu, Wu, Li, Xu, Chen, Wang, Zhu, Qiu, Dong, Zhao, Zhang, Zhao, Xia, Wang, Zhang, Lin, Chen and Hao2004; Reference Zhou, Bergquist, Leonardo and Olveda2008a; Gray et al., Reference Gray, Williams, Li and McManus2008), and then 2030, as specified in the Healthy China 2030 strategic plan issued by the Chinese government (China, Reference China2016; Xu et al., Reference Xu, Li, Zhang, Bergquist, Dang, Wang, Lv, Wang, Lin, Liu, Ren, Yang, Liu, Dong, Zhang and Zhou2020).
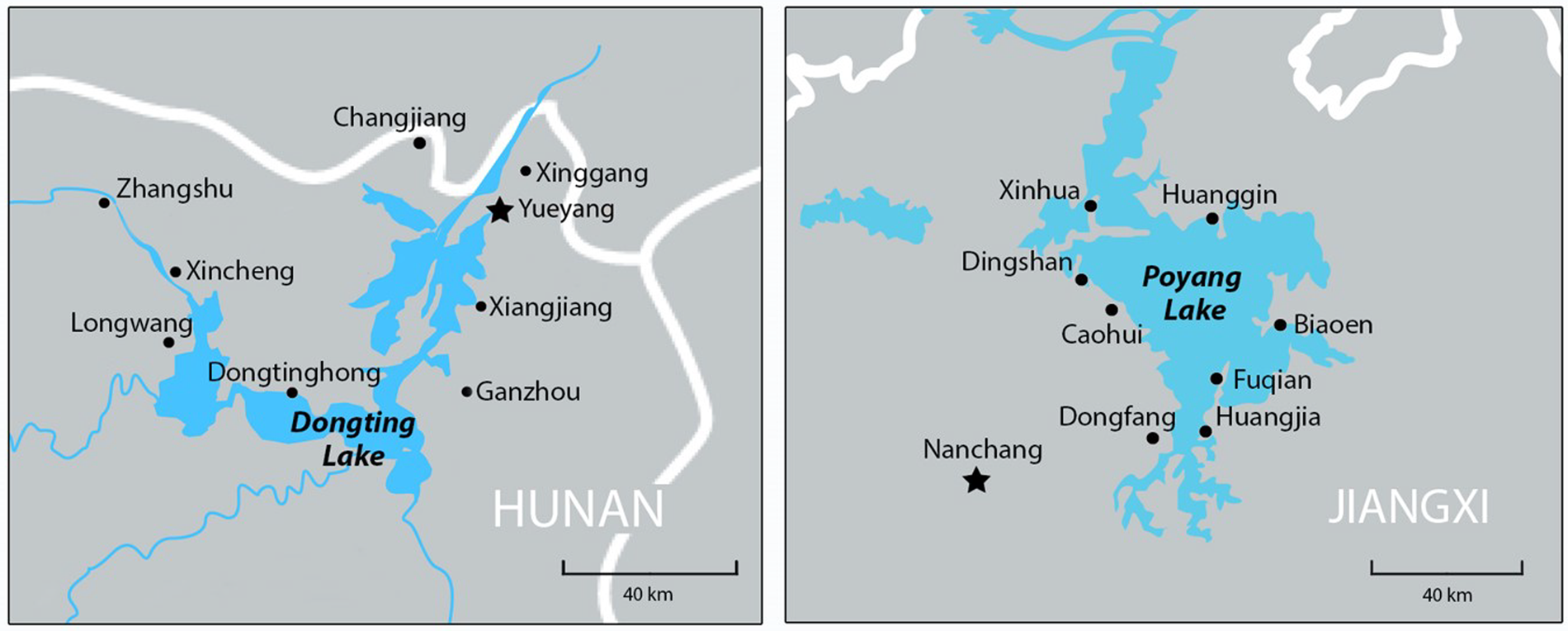
Fig. 1. The location of Hunan (1) and Jiangxi (2) provinces with the eight study villages in each province identified. Yueyang and Nanchang (provincial capital) cities, where the Hunan and Jiangxi Institutes of Parasitic Diseases are respectively located are also shown.
As schistosomiasis transmission decreases in China, accurate diagnosis will be essential for case detection and for determining true infection rates. The microscopy-based Kato-Katz (KK) thick smear technique (Katz et al., Reference Katz, Chaves and Pellegrino1972) recommended by the World Health Organization (WHO) to assess schistosome prevalence and intensity in humans is the standard tool used for stool examinations in the CNSCP (Chen et al., Reference Chen, Wang, Chen, Bergquist, Tanner, Utzinger and Zhou2012; Reference Chen, Guo, Fu, Liu, Lin, Xiao, Sun, Cong, Liu and Hong2021). However, it is laborious and its sensitivity is compromised in low-intensity infections and in areas of low prevalence (Weerakoon et al., Reference Weerakoon, Gobert, Cai and McManus2015). Similarly, conventional microscopy or the use of sentinel mice (Yang et al., Reference Yang, Sun, Liang, Wu, Li, Zhang, Huang, Hang, Liang, Bergquist and Zhou2013) are the current mainstays for detecting infected snails and have similar limitations. Furthermore, the numbers of pre-patent infected snails are likely to exceed those with mature cercariae and sporocysts, and germ balls are easily missed (Qin et al., Reference Qin, Xu, Feng, Lv, Qian, Zhang, Li, Lv, Bergquist, Li and Zhou2018). Innovative DNA-based assays [e.g. real-time polymerase chain reaction (qPCR) and, loop-mediated isothermal amplification (LAMP)] provide a far more sensitive and precise approach for detection of schistosome infections in humans and animals, and in snail intermediate hosts (Weerakoon et al., Reference Weerakoon, Gobert, Cai and McManus2015; Qin et al., Reference Qin, Xu, Feng, Lv, Qian, Zhang, Li, Lv, Bergquist, Li and Zhou2018). Indeed, without addressing the limitations in the currently used conventional diagnostic procedures and, with the marked ecological and demographic changes that China is facing, exacerbated by the possible repercussions of the COVID-19 pandemic on the CNSCP (Guo et al., Reference Guo, Zhang, Cao, Lü, Xu, Li and Zhou2020), the 2030 schistosomiasis elimination target set by the central government may yet prove challenging.
Here we present the results of the 2016 baseline survey of a longitudinal study on schistosomiasis prevalence in 16 villages in Hunan and Jiangxi provinces. We examined humans, animals and snails for infections using validated and highly sensitive molecular diagnostics procedures (Gordon et al., Reference Gordon, Acosta, Gray, Olveda, Jarilla, Gobert, Ross and McManus2012, Reference Gordon, Acosta, Gobert, Jiz, Olveda, Ross, Gray, Williams, Harn, Yuesheng and McManus2015a, Reference Gordon, Acosta, Gobert, Olveda, Ross, Williams, Gray, Harn, Li and McManus2015b; Tong et al., Reference Tong, Chen, Zhang, Yang, Kumagai, Furushima-Shimogawara, Lou, Yang, Wen, Lu, Ohta and Zhou2015; He et al., Reference He, Gordon, Williams, Yueshang, Wang, Hu, Gray, Ross, Harn and McManus2018), and compared the results of our survey with those from the CNSCP for the same villages.
Materials and methods
Ethics
Informed written consent was received from all individuals enrolled in the study, and from parents/guardians of minors. Ethical approval for human and animal work was provided by the Ethics Committees of QIMR Berghofer Medical Research Institute (Human Research Ethics Committee reference number P524; Animal Ethics Committee reference number A1003-601), Hunan Institute of Parasitic Diseases (HIPD), Jiangxi Institute of Parasitic Diseases (JIPD) and the National Institute of Parasitic Diseases (NIPD), Shanghai. Written informed consent was received from all individuals enrolled in the study, and from parents/guardians of minors.
All human subjects found to be positive for S. japonicum during the course of the study were referred to the local schistosomiasis control station for treatment with praziquantel (PZQ) at the WHO recommended dose of 40 mg kg−1. Written informed consent was received from all animal owners in the study sites, and the study was performed in accordance with the recommendations of the 2013 Australian code of practice for the care and use of animals for scientific purposes.
Study area
We identified 16 villages participating in the CNSCP around the Dongting and Poyang lakes. The villages included eight in each of Hunan and Jiangxi provinces that were selected based on published prevalence data (Gray et al., Reference Gray, Li, Williams, Zhao, Harn, Li, Ren, Feng, Guo, Guo, Zhou, Dong, Li, Ross and McManus2014; Dang et al., Reference Dang, Jin, Xu, Li, Zhou, Sun, Li and Lu2017; Reference Dang, Li, Guo, Xu, Li and Lu2021). Specifically, villages were selected using the following process: a sampling frame of 24 villages in schistosomiasis-endemic areas in each of Jiangxi and Hunan provinces was constructed. These were stratified according to human prevalence using cut-off points of 1 and 1.5% and placed in random order within the province and stratum. The cut-off of 1% was chosen as the tipping point for elimination, and 1.5% came from the mean prevalence of the villages as determined by the KK procedure. Cut-offs were initially selected based on power calculations for the study where a sample size of 3600 would provide a power of 79.8 and 95% assuming the KK would diagnose 1 and 1.5% of positively infected individuals, respectively.
Accordingly, eight villages were selected in each province in consecutive order on the randomized list. The villages selected in Hunan were: Changjiang, Dongtinghong, Ganzhou, Longwang, Xiangjiang, Xincheng, Xinggang, and Zhangshu; while in Jiangxi the selected villages were Biaoen, Caohui, Dingshan, Dongfang, Fuqian, Huanggin, Huangjia, and Xinhua (Fig. 1).
Study design and procedures
The baseline of a longitudinal cohort study was carried out across the 16 villages identified in Hunan and Jiangxi provinces. A demographics questionnaire, including details of medical history and water contact (Supplementary form 1; individual survey), was administered to all village residents and used to select individual cohort participants who had to meet the following criteria: (i) age 5–70 years; (ii) resident in the village for >12 months; (iii) not intending to migrate in the next 5 years; (iv) continuously resident in the village over the study period; (v) indicated having had water contact; and (vi) provided written informed consent (minors had the written informed consent of their parent or guardian). Using these selection criteria, a fixed cohort (N = 500–600) of villagers (with equal numbers of males and females) was recruited for the baseline (2016) and follow-up (2017–2019) surveys from each village. Approximately 10–20% loss to follow-up was expected based on our previous experiences in China. The primary end points of the study were human infection rates with secondary end points including animal infection rates, snail prevalence and density of infected snails.
Cohort members were each provided with a stool collection container labelled with the individual participant identification (PID) number. Subjects were directed to deposit a roughly chicken egg-sized amount of stool into the container. Containers were collected the following day and processed for storage and transportation (see the section on ‘Stool sample collection and storage’). A household questionnaire (Supplementary form 2) was administered to the households of all cohort participants, with one senior adult member from each household providing responses.
An animal survey was performed at the same time as the human stool collection. Households that indicated ownership of bovines or goats were provided with a stool container labelled with the animal's identification (AID) number and directed to place a minimum chicken egg-sized stool sample into the container. The stool samples were returned to central collection points to be collected by study personnel. Animal age and sex were captured in the household questionnaire (Supplementary form 2).
Stool sample collection and storage
On the day of stool collection, approximately ~1 g of human faeces was lightly homogenized using an applicator stick, placed into a labelled 15 mL tube and 80% (v/v) ethanol was added to completely cover the sample. Tubes were then stored at 4°C until transported to the National Institute of Parasitic Diseases (NIPD), Chinese Center for Disease Control and Prevention, Shanghai for DNA extraction. Bovine (5–10 g) and goat (approximately 2 g) stool samples were lightly homogenized with an applicator stick, placed into a labelled 50 mL tube, completely covered with 80% (v/v) ethanol, stored at 4°C, and transported to QIMR Berghofer (Brisbane, Australia) for DNA extraction and analysis.
Snail survey and collection
Snail hot spots were identified in the study area and a snail survey was performed in April–May 2015. GPS coordinates and photos of the areas where snails were collected were taken to record hot spot locations. Snail sampling was conducted using the standard Chinese random quadrant method (Ministry of Health Health, 2000). In a snail area, the potential hotspot location was divided into five sections. Sampling frames (0.11 m2) were labelled with a site identification code (SID), and the frame ID. Numbered frames were placed at 2 m intervals along the width and length of the potential snail hot spot location. All snails within each frame were collected and placed into a bag labelled with the SID, frame ID and the date of snail sampling. Snails were counted as they were placed into the bag. GPS coordinates were taken for each snail sampling location. Snails were transferred to HIPD in Hunan province and JIPD in Jiangxi province for microscopic examination to identify schistosome-positive snails by observing cercarial emergence, which were then pooled for LAMP. Snails were either processed immediately for DNA extraction (see below), or stored at −20°C until they could be processed.
Molecular detection of S. japonicum in human and animal stool samples
DNA extraction
Total DNA was extracted from human stool samples at NIPD in Shanghai, using QIAamp DNA Mini Kits (Qiagen; Hilden, Germany) following the manufacturer's protocol with some minor modifications. Two hundred milligrams of stool were placed into a 2 mL Eppendorf tube with 1 mL of MilliQ water, the sample was vortexed thoroughly and then centrifuged in a microfuge at maximum speed for 1 min to wash any residual ethanol out of the sample. The supernatant was discarded and the manufacturer's protocol was followed for the remaining steps.
Total DNA was extracted from animal stool samples at QIMR Berghofer, using a Maxwell 16 LEV Plant DNA kit (Promega Corporation; Alexandria, Australia) following the manufacturer's protocol with some minor modifications before adding the sample to the Maxwell cartridges. Two hundred milligrams of stool were placed into a 2 mL screw top tube with 1 mL of lysis buffer and 0.5 g of 0.5 mm zirconia/silica beads (Daintree Scientific; St Helens, Australia). The sample was homogenized in a Precelly's tissue homogenizer for 45 s at 2500 rpm. The sample was then centrifuged for 3 min at 800 g. After centrifugation 200 μL of the supernatant was added to the first well of the Maxwell 16 cartridge along with 200 μL of MilliQ water. Elution tubes contained 50 μL of elution buffer. The protocol for the Plant DNA extraction programme was selected and run on the Maxwell 16 robot. After the programme was completed any magnetic beads transferred into the elution tube were removed using a magnetic rack, and the eluted DNA was transferred to a new tube.
The recovered DNAs from the human and animal stool samples were checked for quantity and quality using a NanoDrop™ 2000 spectrophotometer and aliquots of diluted DNA (1:5) were used as the template for the qPCR and ddPCR assays targeting the mitochondrial subunit I of the NADH dehydrogenase (nad1) gene of S. japonicum (Lier et al., Reference Lier, Simonsen, Haaheim, Hjelmevoll, Vennervald and Johansen2006; Gordon et al., Reference Gordon, Acosta, Gray, Olveda, Jarilla, Gobert, Ross and McManus2012; Reference Gordon, Acosta, Gobert, Jiz, Olveda, Ross, Gray, Williams, Harn, Yuesheng and McManus2015a, Reference Gordon, Acosta, Gobert, Olveda, Ross, Williams, Gray, Harn, Li and McManus2015b).
Real-time PCR (qPCR)
The total reaction volume of 17 μL contained 10 μL SYBR Green (Invitrogen), 200 nm of each primer, 0.1 μg μL−1 of BSA, 3 μL of H2O and 2 μL of template DNA (50 ng μl−1). The PCR was performed as previously described (Lier et al., Reference Lier, Simonsen, Haaheim, Hjelmevoll, Vennervald and Johansen2006; Gordon et al., Reference Gordon, Acosta, Gray, Olveda, Jarilla, Gobert, Ross and McManus2012, Reference Gordon, Acosta, Gobert, Jiz, Olveda, Ross, Gray, Williams, Harn, Yuesheng and McManus2015a, Reference Gordon, Acosta, Gobert, Olveda, Ross, Williams, Gray, Harn, Li and McManus2015b). Results were quantified as eggs per gram of faeces (EPG) in the range 1–100 EPG using qPCR cycle threshold (Ct) scores based on egg seeding experiments as described previously (Gordon et al., Reference Gordon, Acosta, Gray, Olveda, Jarilla, Gobert, Ross and McManus2012, Reference Gordon, Acosta, Gobert, Jiz, Olveda, Ross, Gray, Williams, Harn, Yuesheng and McManus2015a, Reference Gordon, Acosta, Gobert, Olveda, Ross, Williams, Gray, Harn, Li and McManus2015b). The EPG as determined by qPCR is referred to as qEPG from here on.
The qPCR assays on human stool samples were performed at the Chinese National Human Genome Center (CNHGC) in Shanghai, on a 7300 Plus real-time PCR system (Applied Biosystems; California, USA) thermocycler, while the qPCR assays on animal stools were performed at QIMR Berghofer, on a Corbett Rotorgene 6000 (Qiagen) thermocycler.
Digital droplet PCR (ddPCR)
The ddPCR primers were the same as used for the qPCR (Lier et al., Reference Lier, Simonsen, Haaheim, Hjelmevoll, Vennervald and Johansen2006; Gordon et al., Reference Gordon, Acosta, Gray, Olveda, Jarilla, Gobert, Ross and McManus2012, Reference Gordon, Acosta, Gobert, Jiz, Olveda, Ross, Gray, Williams, Harn, Yuesheng and McManus2015a, Reference Gordon, Acosta, Gobert, Olveda, Ross, Williams, Gray, Harn, Li and McManus2015b; Weerakoon et al., Reference Weerakoon, Gordon, Gobert, Cai and McManus2016, Reference Weerakoon, Gordon, Cai, Gobert, Duke, Williams and McManus2017a, Reference Weerakoon, Gordon, Williams, Cai, Gobert, Olveda, Ross, Olveda and McManus2017b). ddPCR assays were performed as previously described (Weerakoon et al., Reference Weerakoon, Gordon, Williams, Cai, Gobert, Olveda, Ross, Olveda and McManus2017b) Negative (no DNA template) controls were used in all assays, as were positive controls containing S. japonicum egg DNA. Following PCR amplification, the 96-Well twin.tec™ PCR plate (Eppendorf; Hamburg, Germany) was placed in a QX200 Droplet Reader (Bio-Rad) for droplet reading and analysis. Fluorescence intensity of the no DNA template negative control was used to define the threshold for discrimination between droplets that contained target (positives) and those that did not (negatives), determined using QuantaSoft software version-1.3.2.0 (Bio-Rad) (Weerakoon et al., Reference Weerakoon, Gordon, Gobert, Cai and McManus2016, Reference Weerakoon, Gordon, Cai, Gobert, Duke, Williams and McManus2017a, Reference Weerakoon, Gordon, Williams, Cai, Gobert, Olveda, Ross, Olveda and McManus2017b). Total droplet numbers were used to assess the success of a sample in a run. Samples that had less than 10,000 total droplets were repeated, as were any that evidenced droplet shearing when viewing the 1D amplitude plots on the QuantaSoft software. The ddPCR analysis was performed at QIMR Berghofer.
Target gene copy number index (CNI)
The target gene copy number index (CNI) is defined as the number of target gene copies per 100 ng of template DNA (Weerakoon et al., Reference Weerakoon, Gordon, Williams, Cai, Gobert, Olveda, Ross, Olveda and McManus2017b). Target gene copy number per one mL of a 20 μL reaction mix was obtained using the QuantaSoft software (Weerakoon et al., Reference Weerakoon, Gordon, Williams, Cai, Gobert, Olveda, Ross, Olveda and McManus2017b). This was converted to CNI using the following formula

The higher the CNI, the higher the intensity of infection.
Molecular identification of S. japonicum in Oncomelania hupensis snails
DNA extraction
Snails were pooled by the collection site with a maximum of 50 snails per pool with additional pools made up of any remaining snails. Snails were crushed between two thick glass slides and large shell debris was removed. The snail soft tissues were pooled in clean 2.0 mL tubes (not exceeding 50 snail bodies in each), DNA lysis buffer (400–1000 μL) (Qiagen; Valencia, CA, USA) was added to each tube and the tissues were lightly homogenized. Then, 20–50 μL of Proteinase K (Qiagen) was added and the tubes were maintained for 2–3 h at 56°C in a water bath, after which the snail homogenate supernatants were passed through a DNA binding column (Qiagen). The DNA binding column was then washed with washing buffer (Qiagen) and genomic DNA was recovered from the column by elution with nuclease-free water. The recovered DNA was checked for quantity and quality using a NanoDrop™ 2000 spectrophotometer (Thermofisher Scientific) and subjected to testing by a field-verified LAMP assay targeting the 28S ribosomal S. japonicum gene (SJ28S) (GenBank accession no. Z46504) (Kumagai et al., Reference Kumagai, Furushima-Shimogawara, Ohmae, Wang, Lu, Chen, Wen and Ohta2010; Qin et al., Reference Qin, Xu, Feng, Lv, Qian, Zhang, Li, Lv, Bergquist, Li and Zhou2018).
LAMP assay
The SJ28S LAMP assay master mix (total volume 23 μl) was prepared containing 7.5 μL of MilliQ water, 12.5 μL of 2 × reaction buffer, 1 μL of primer mixture (Kumagai et al., Reference Kumagai, Furushima-Shimogawara, Ohmae, Wang, Lu, Chen, Wen and Ohta2010) [(F3 (GCTTTGTCCTTCGGGCATTA), B3 (GGTTTCGTAACGCCCAATGA), FIP (ACGCAACTGCCAACGTGACATACTGGTCGGCTTGTTACTAGC), BIP (TGGTAGACGATCCACCTGACCCCTCGCGCACATGTTAAACTC)], 1 μL of Bst enzyme (a Bacillus stearothermophilus DNA polymerase homologue used for DNA strand displacement), and 1 μL of calcein. To this 2 μL of the extracted pooled snail DNA was added and mixed well. Negative controls contained nuclease-free water instead of DNA, and positive controls contained S. japonicum genomic DNA isolated from adult worms. Reaction tubes were incubated at 65°C for 60–90 min followed by 85°C for 5 min to stop the reaction. The tubes were observed directly by the unaided eye to detect a colour change from brown (negative) to green (positive).
Wealth index
Poor health may be a consequence of poverty due to an inability to afford adequate nutrition, housing, education, and healthcare. Poverty as an indicator for schistosome infection is likely to be due to inadequate hygiene infrastructure such as flushable toilets and access to clean, safe water. A household wealth index was calculated and included in the analysis as an independent variable to adjust for potential socioeconomic confounders in the longitudinal cohort study. The household survey (Supplementary form 2) included a number of household characteristics and asset variables that can be used to estimate the household wealth index using a principle component analysis (Filmer and Pritchett, Reference Filmer and Pritchett2001; Vyas and Kumaranayake, Reference Vyas and Kumaranayake2006; Balen et al., Reference Balen, McManus, Li, Zhao, Yuan, Utzinger, Williams, Li, Ren, Liu, Zhou and Raso2010). The household characteristics used to estimate the wealth index were non-productive assets (motorbike, car, tractor, fridge, washing machine, rice cooker, microwave, TV, telephone and computer) and productive assets (land ownership, number of cattle, number of buffalo and number of goats); household utilities (type of source of drinking water, access to a sanitation facility, type of toilet and access to the internet) were also included in the wealth index estimation. Household wealth inequality is measured by dividing the wealth index into quintiles, with the lowest quintile representing the poorest 20% and the highest quintile representing the wealthiest 20% of households in the study area.
Data management
All data entry was completed using the REDCap web application (Harris et al., Reference Harris, Taylor, Thielke, Payne, Gonzalez and Conde2009, Reference Harris, Taylor, Minor, Elliott, Fernandez, O'neal, Mcleod, Delacqua, Delacqua, Kirby and Duda2019). All data were double entered by different study personnel. Data error checking was performed and differences between the two datasets were compared; any differences between the records were corrected based on the source document. Regular quality checks were made to find anomalous values which were then rectified. Data were stored centrally on a secured server within the University of Queensland under the auspices of authors GMW and SF. Once data entry and error checking were completed, the data were exported and translated into SAS version 9.4 (SAS Institute; Cary, NC, USA) for data analysis.
Statistical analysis
SAS software was used for most data analysis. Ninety-five per cent confidence intervals (95% CI) were calculated using standard formulae based on the binomial distribution (prevalence) and the lognormal distribution (infection intensity). To account for clustering effects within villages generalized estimating equations (GEE) were used to calculate P values with villages as a cluster effect. P < 0.05 was considered significant.
A stool sample was considered positive if a positive Ct score was obtained by qPCR (a Ct score equal or greater than 35 was considered negative; less than 34.99 was considered positive), or positive droplets over the threshold fluorescence for the ddPCR (Weerakoon et al., Reference Weerakoon, Gordon, Williams, Cai, Gobert, Olveda, Ross, Olveda and McManus2017b).
Egg counts from the qPCR assays were transformed to eggs per gram of faeces (qEPG) and geometric EPG (GMEPG) in infected stool samples calculated using the log-transformed egg counts. Confidence limits were calculated using standard formulae based on the binomial distribution (prevalence) and the lognormal distribution (infection intensity). P values for occupation prevalence were based on the ‘occupation 2’ variable where occupation groups were combined as there were too many variables with very low numbers in each for the calculation.
JMP (version 16.0.0) was used for a generalized regression using a beta-binomial maximum likelihood model to examine the relationship between the presence of schistosome-positive animals in a village and human prevalence.
Chinese National Schistosomiasis Control Programme (CNSCP) protocols
The CNSCP data were sought for 2015 and 2016 for all study villages to directly compare with the survey results described here. Under the CNSCP, cluster-randomized sampling of households from villages in endemic areas is performed to select households for inclusion in mass drug administration (MDA) and the monitoring of villagers for S. japonicum infections; blood samples (200 μL) are taken from individuals in the selected households and then tested serologically using the indirect haemagglutination assay (IHA) (Glinz et al., Reference Glinz, Silué, Knopp, Lohouringnon, Yao, Steinmann, Rinaldi, Cringoli, N'goran and Utzinger2010). Those who are IHA-positive are then asked to submit a single stool sample for KK examination (three slides; 41.7 template) (Katz et al., Reference Katz, Chaves and Pellegrino1972). MDA is also performed in endemic areas based on water contact data. Those who are considered to be at high to moderate risk of schistosomiasis based on water contact are treated with PZQ. MDA on humans is carried out by local health stations with MDA coverage rates of 30–60% of the population. There is no animal treatment as part of the CNSCP.
Snail surveys are also performed as part of the CNSCP by systematic and environmental sampling. Snails are pooled (50 snails per pool), DNA extracted, and LAMP performed to diagnose snails infected with S. japonicum (Tong et al., Reference Tong, Chen, Zhang, Yang, Kumagai, Furushima-Shimogawara, Lou, Yang, Wen, Lu, Ohta and Zhou2015).
Results
A flow diagram of the human and animal study participant recruitment and cohort study sample selection is shown in Fig. 2. In total, 9961 people were initially enrolled into the study at baseline, and 9794 completed the questionnaire and provided a stool sample (167 did not provide a stool sample). From each village, a random selection of study participants was taken and DNA was extracted from 5077 stool samples and qPCR was successfully performed on 4414. Of those with complete qPCR data, 490 were unable to be matched in the database and were excluded from the analysis. The total number of people from whom data were analysed in the study at baseline was 3924.
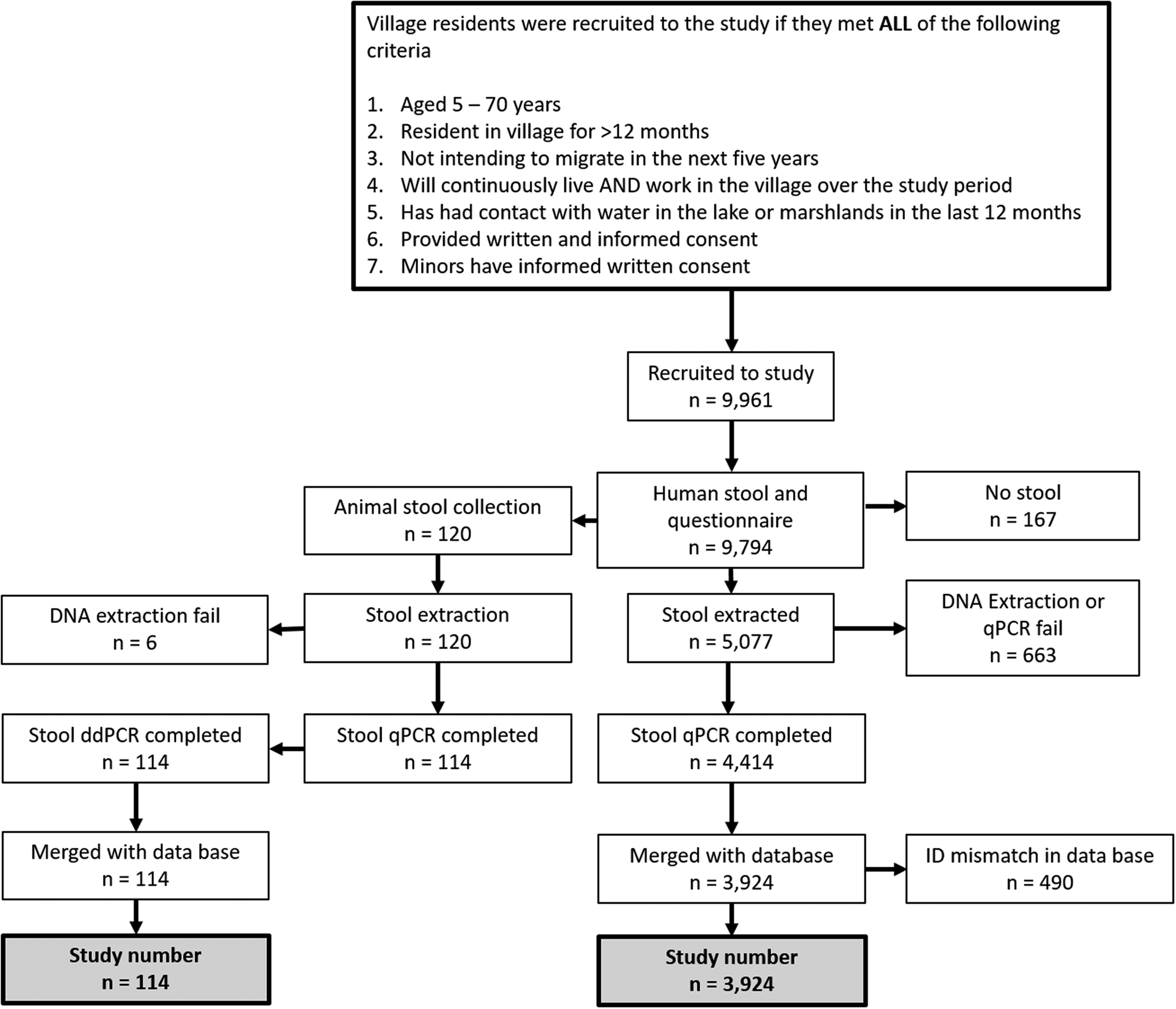
Fig. 2. Flow diagram of human and animal study participant recruitment and study sample selection.
A total of 120 animal (55 cattle, 30 buffalo, 35 goats) stool samples were analysed by qPCR and ddPCR for the presence of S. japonicum. Age and sex of bovines were ascertained by use of the household questionnaire given to the animal owners prior to faecal collections (Fig. 2). Animals surveyed in this study came from three villages in Hunan province, Longwang, Ganzhou, and Xinggang. All other villages (no animals were present in the selected Jiangxi villages) had no bovines or goats present at the time of the survey.
Study demographics
Of 3924 participants who had completed questionnaires and matched qPCR data, 51.5% of subjects were from Jiangxi, and 52.2% were male (Table 1, Supplementary Table 1). Ages ranged from 4 to 79 years, with a median age of 48 years.
Table 1. Prevalence and intensity of infection of S. japonicum in humans from 16 sentinel villages in Hunan and Jiangxi provinces determined by qPCR
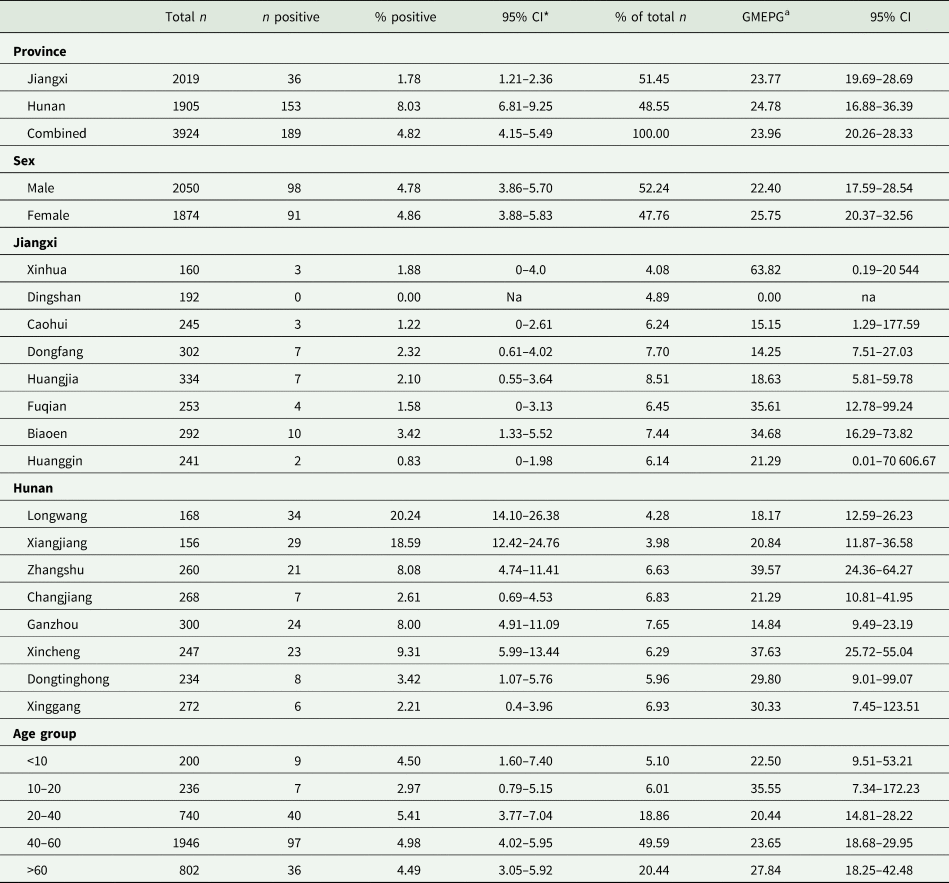
* 95% CI.
a Geometric mean eggs per gram of faeces.
Human S. japonicum prevalence
Hunan and Jiangxi cohorts combined
The total combined baseline prevalence in Hunan and Jiangxi provinces for S. japonicum, determined by qPCR was 4.8% (95% CI 4.2–5.5%); Hunan had a significantly (P < 0.0001) higher prevalence (8.0%; 95% CI 6.8–9.3%) than Jiangxi (1.8%; 95% CI 1.2–2.4%) (Table 1, Supplementary Table 1).
The village with the highest human prevalence in Jiangxi province was Biaoen (3.4%; 95% CI 1.33–5.5%), with the lowest recorded prevalence being Caohui (1.2%; 95% CI 0.0–2.6%); Dingshan recorded zero positives (Table 1, Fig. 3). There were no significant differences in prevalence between villages from Jiangxi where S. japonicum infections were recorded (Supplementary Table 2).
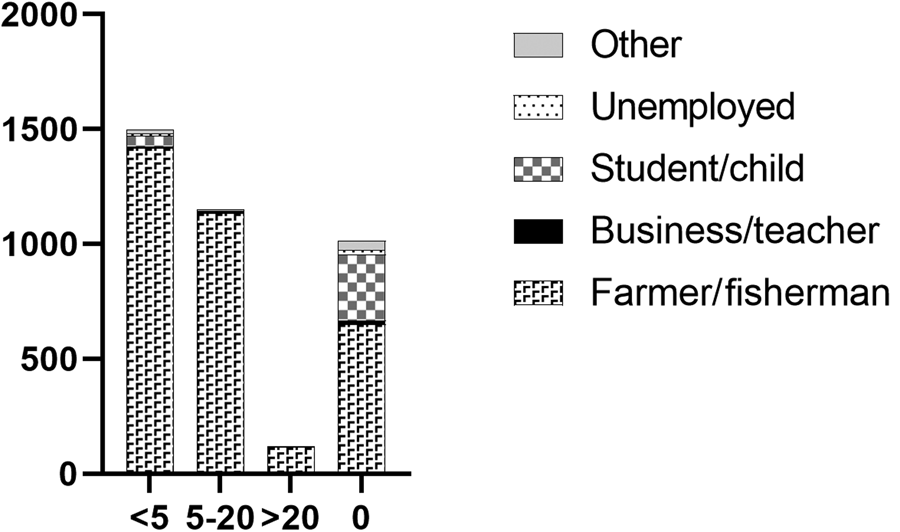
Fig. 3. Total number of individuals with previous praziquantel treatments shown with occupation.
In Hunan province, all villages recorded positively infected individuals; the highest prevalence occurred in Longwang (20.2%; 95% CI 14.1–26.4%) and Xiangjiang (18.6%; 95% CI 12.4–24.7%), with Xinggang (2.2%; 95% CI 0.5–4.0%) and Changjiang (2.6%; 95% CI 0.7–4.5%) having the lowest (Table 1). Longwang and Xiangjiang had a significantly higher prevalence than Zhangshu (P = 0.0004; P = 0.0019), Changjiang (P < 0.0001; P < 0.0001), Dongtinghong (P < 0.0001; P < 0.0001) and Xinggang (P < 0.0001; P < 0.0001); Xincheng had a significantly higher prevalence than Dongtinghong (P = 0.0008) and Xinggang (P = 0.0008) and Zhangshu had a significantly higher prevalence than Dongtinghong (P = 0.0327) and Xinggang (P = 0.0039) (Supplementary Table 2).
Approximately half of the study participants in both provinces were aged between 40 and 60 years (49.6%) and the highest number of positive infections (n = 97; 4.9%; 95% CI 4.0–6.0%) were recorded in this age group (Table 1). There were no significant differences in prevalence between any of the age groups or their education level, even when those below 20 years of age and those above 20 years of age were considered separately (Supplementary Table 2). The majority of village participants in the study were farmers and/or fishermen (85.6%), and these contributed the highest number, albeit not significantly, of positive cases (5.1%; 95% CI 4.4–5.9%), although the highest prevalence was in businessmen/teachers (5.4%; 95% CI 0.0–13.1); however, this was the smallest group by occupation (n = 37). (Supplementary Tables 1 and 2).
Most participants (98.8%) used either home or public latrines as their primary mode of defecation, although 5.5% admitted to defecating in the marshlands or lake as their secondary mode of defecation; of these only two individuals tested positive for S. japonicum. There was a high number (over 30% being farmers and/or fishermen) who provided no responses for secondary defecation locations. The majority of people did not report diarrhoea (91.3%), weakness (90.1%) or fever (95.1%) in the 2 weeks prior to commencement of the survey. Of those that did record these symptoms, the highest prevalence of S. japonicum was in those who reported more than 5 episodes in the fortnight; this was significantly higher for diarrhoea and weakness in infected vs uninfected individuals, but not for fever (Supplementary Tables 1 and 2).
Self-reported historical S. japonicum infection was high in the combined Hunan and Jiangxi province cohorts, with 68.1% reporting having had a previous infection, of which 5.2% (95% CI 4.4–6.1%) had a current infection determined by the qPCR analysis (Supplementary Table 1). Just over 70% of the study participants had previously received PZQ treatment for schistosomiasis at least once prior to study commencement, and 41.2% had been treated within the previous 2 years (Supplementary Table 1). The majority of those infected had not been treated in the previous 2 years, although this was not significantly associated with S. japonicum infection (P = 0.3745). Of the infected individuals, 22.7% (n = 43) had received no prior treatment for schistosomiasis. Very few cohort subjects reported having previously had advanced (0.7%) or acute (1.7%) schistosomiasis. The largest occupational groups with previous schistosome infection were farmers and/or fishermen (66.4%) with only 19.0% having no prior history of schistosomiasis, although 5.1% (95% CI 4.4–5.8) of individuals in this group were positive for S. japonicum (Supplementary Table 1). Very few of the occupational groups had previously been treated more than 20 times for schistosomiasis, but there was a clear trend for more schistosomiasis treatments in farmers and/or fishermen (Fig. 3, Supplementary Table 1).
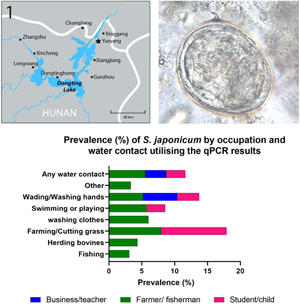
Water contact was more commonly reported in the summer months compared with spring and autumn. The most common water contact activities in all seasons involved fishing, farming, wading in water or washing hands. When combining responses to water contact for all seasons, farming or cutting grass (7.2%; 95% CI 5.7–8.7) and washing clothes (5.9%; 95% CI 3.1–8.7) had the highest cohort subject prevalence for S. japonicum (Supplementary Table 1). By occupation, farmers and fishermen had by far the highest recorded water contact (78.9%) in the 12 months prior to the beginning of the study followed by students and pre-school children (6.1%) (Fig. 4, Supplementary Table 1).
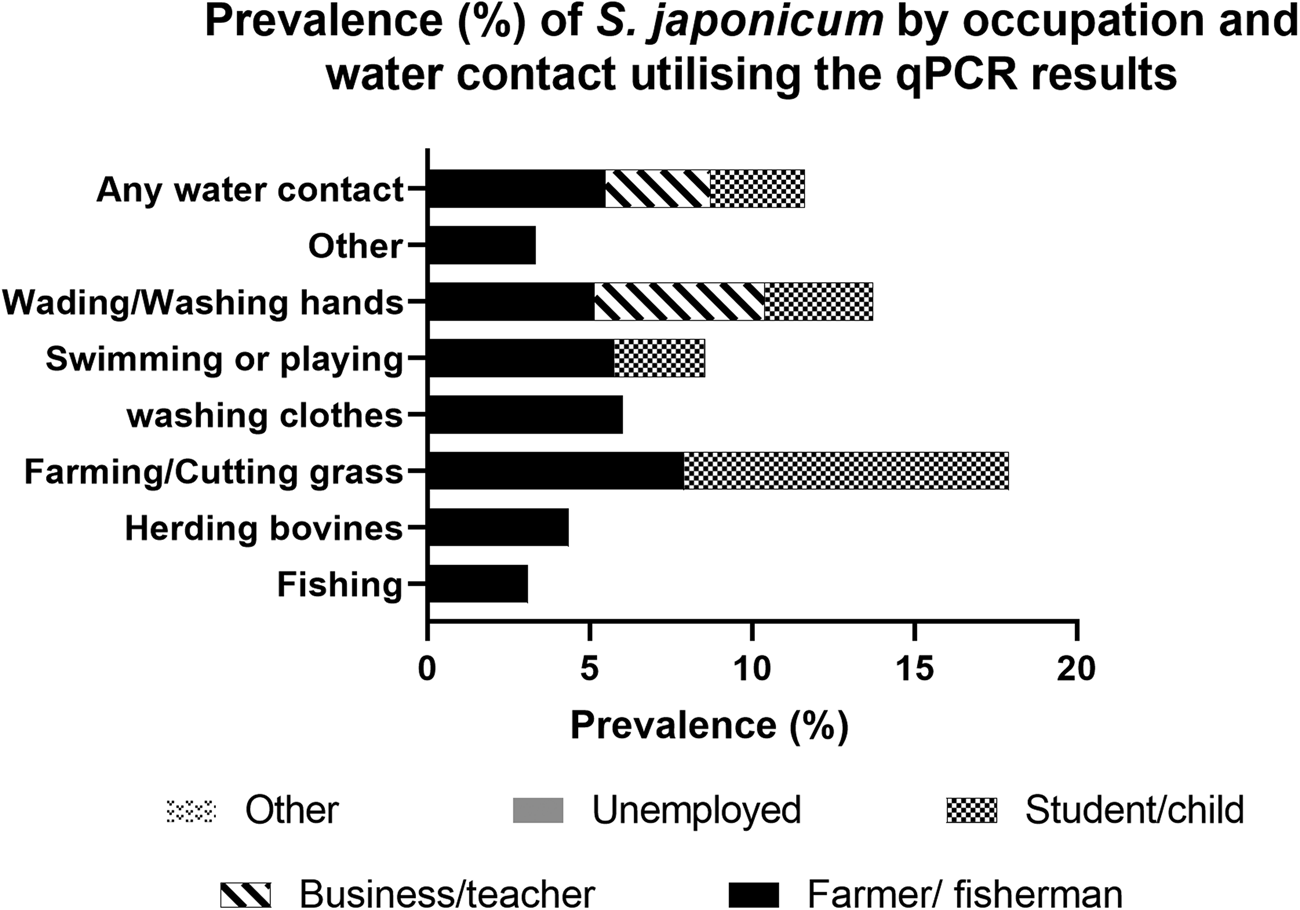
Fig. 4. Prevalence (%) of S. japonicum by occupation and water contact utilizing the qPCR results.
Results for Hunan only villages were also considered and the data are presented in Supplementary Tables 3 and 4.
Human S. japonicum infection intensity
Hunan and Jiangxi cohorts combined
There were some significant differences in infection intensity (GMEPG) between villages but no significant differences in GMEPGs between age, sex or province (Supplementary Tables 1 and 5). Infection intensity varied widely between villages in Jiangxi province compared with Hunan. The highest GMEPGs were evident in Xinhua, Jiangxi province (63.8; 95% CI 0.2–29 544) and Zhangshu, Hunan province (39.6; 95% CI 24.4–63.3) (Table 1). Subjects who received primary school (P = 0.0492) and college-level (P ≤ 00 001) education had significantly higher GMEPG than those who were illiterate, and those who had received college-level education had a significantly higher infection intensity than those at the middle school level (P ≤ 00001). Businessmen had the highest GMEPG, although only two individuals in this group were recorded as being infected with S. japonicum. There were no significant differences in GMEPG between the other occupational groups (Supplementary Tables 1 and 5).
When water contact for the summer, spring and autumn seasons was combined, swimming or playing was associated with a significantly higher GMEPG compared with other modes of water contact (57.7; P ≤ 0.0001) (Supplementary Tables 1 and 5).
The results for Hunan only villages were also considered and the data are presented in Supplementary Tables 3 and 4.
S. japonicum prevalence in animals
Animals were recorded as present in only three villages, all in Hunan province (Longwang, Ganzhou, Xinggang) (Table 2). Xinggang had the highest number of animals (n = 71) with cattle (n = 27), water buffalo (n = 9) and goats (n = 35) present. Longwang had goats only (n = 17), while Ganzhou had both cattle (n = 28) and water buffalo (n = 4).
Table 2. Results of animal ddPCR for S. japonicum infection stratified by village, sex, species, and age
* 95% CI.
a Target gene copy number index.
b Water buffalo or cattle, species unknown.
No animals were positive for S. japonicum infection by qPCR but 20% (95% CI 11.6–26.6) were positive by ddPCR (Table 2). Animal species data were collected via the household questionnaire but some of the bovine samples were unable to be matched to the questionnaire database and we were, therefore, unable to determine their gender, or if they were cattle or water buffalo. Accordingly, these samples were combined and referred to as bovines, with gender unknown. Where the animal species was known, it was reported accordingly. Ten samples had low quality and/or quantity of DNA and were not analysed by qPCR or ddPCR.
No goats were positive either by qPCR or ddPCR. The majority of animals positive for S. japonicum were from Xinggang (18/61) (Table 2). There were no significant differences associated with sex, age group, or village for prevalence or CNI and the average CNI was quite low for all variables (Table 2).
Snail survey and LAMP
In total, eight sites were sampled for oncomelanid snails in Hunan province, one per village, and 22 sites in Jiangxi with multiple sites in some villages, and multiple sub-sites per village (Supplementary Table 7). Multiple sites were sampled in some villages due to low snail numbers. The environment type (marshland) was the same for all villages. Vegetation type (grass) was the same for all Jiangxi villages but varied between grass, forest and reed type in the Hunan villages (Supplementary Table 7).
In Jiangxi, no snails were found in Biaoen, and only 12 specimens were collected in Caohui. In Hunan, no snails were found in Ganzhou; 115 oncomelanids were collected from Xinggang and 171 from Longwang. Snail numbers in the other villages from both provinces were >300 (Supplementary Table 7). No S. japonicum positive snails were identified by LAMP (Supplementary Table 7).
Chinese National Schistosomiasis Control Programme (CNSCP) data
All the study villages from Hunan and Jiangxi provinces recorded individuals IHA-positive for S. japonicum in the CNSCP in 2016 (Table 3). The highest prevalence was evident in Jiangxi province with four villages recording a prevalence greater than 10%; only one village in Hunan recorded a prevalence exceeding 10%. The lowest prevalence (0.98%) was recorded in Xiangjiang, Hunan province. Prevalence by KK was very low, with only one village in Jiangxi and three villages in Hunan recording positive infections of S. japonicum (Table 3). Whereas animals had all been removed from the Jiangxi study villages by 2016, some were still present in Biaoen village, Jiangxi province in 2015, with two animals (species not stated) (n = 109) KK-positive for schistosomiasis (Supplementary Table 8).
Table 3. Results of the Chinese National Schistosomiasis Control Programme in 2016, with qPCR results from the current study included for comparison

IHA, Indirect haemagglutination assay; KK, Kato-Katz; 95% CI, 95% confidence interval; qPCR, real-time PCR.
Wealth Index
Using a binary output of low-high, the prevalence of infection was higher in low wealth index households in the cohort villages than in higher wealth index households (AOR: 1.73; 95% CI 1.19–2.50) (Table 4).
Table 4. Demographic characteristics of the study participants and wealth variable calculation

* 95% Confidence interval.
Discussion
Human qPCR and comparison with the CNSCP data
In the CNSCP, subjects in at-risk households in endemic villages are screened by the IHA and those seropositive are requested to provide a faecal sample which is then examined by the KK microscopy technique for the presence of eggs. As shown in Table 3, very few IHA-positive individuals in our study cohort were subsequently shown positive by KK, highlighting the insensitivity of this microscopy-based procedure. The low specificity of the IHA can lead to a false-positive diagnosis as evidenced by the comparison of the qPCR results obtained in the current study with the IHA data reported for the CNSCP.
An earlier study published in 2015 compared results of IHA, KK, and circulating anodic antigen (CAA) on both urine and serum in individuals from Hunan province (van Dam et al., Reference Van Dam, Xu, Bergquist, De Dood, Utzinger, Qin, Guan, Feng, Yu, Zhou, Zheng, Zhou and Corstjens2015). The IHA in that study was also shown to generate a high number of false positives when using a composite reference standard of the KK and the CAA assays which supports the qPCR results we report here.
We found that the overall human prevalence of schistosomiasis determined by qPCR was significantly higher in Hunan province than in Jiangxi province (Table 1), although this was not reflected in the CNSCP data reported for the same villages in 2016 (Table 3). According to the CNSCP data, Jiangxi had a much higher S. japonicum prevalence by IHA than Hunan province, although the values were substantially lower for both provinces where prevalence was determined by the KK procedure; there were only three positives by KK in Jiangxi, and six in Hunan. As indicated, the KK is known to lack sensitivity, particularly in low-intensity infections (Glinz et al., Reference Glinz, Silué, Knopp, Lohouringnon, Yao, Steinmann, Rinaldi, Cringoli, N'goran and Utzinger2010; Habtamu et al., Reference Habtamu, Degarege, Ye-Ebiyo and Erko2011; Gordon et al., Reference Gordon, Acosta, Gray, Olveda, Jarilla, Gobert, Ross and McManus2012, Reference Gordon, Acosta, Gobert, Olveda, Ross, Williams, Gray, Harn, Li and McManus2015b). Sensitivity of the KK can be increased by examining multiple stools on different days and increasing the numbers of slides observed under the microscope; furthermore, the aggregation of eggs in faeces (resulting in a very uneven distribution), as well as day to day variation in egg output, severely impacts on the results of the KK, particularly if only a single stool sample is examined (Barreto et al., Reference Barreto, Smith and Sleigh1990; de Vlas et al., Reference De Vlas, Gryseels, Van Oortmarssen, Polderman and Habbema1992, Reference De Vlas, Engels, Rabello, Oostburg, Van Lieshout, Polderman, Van Oortmarssen, Habbema and Gryseels1997; Engels et al., Reference Engels, Nahimana and Gryseels1996a, Reference Engels, Sinzinkayo and Gryseels1996b; Utzinger et al., Reference Utzinger, Booth, N'goran, Müller, Tanner and Lengeler2001; Berhe et al., Reference Berhe, Medhin, Erko, Smith, Gedamu, Bereded, Moore, Habte, Redda, Gebre-Michael and Gundersen2004; Yu et al., Reference Yu, de Vlas, Jiang and Gryseels2007). Previous reports comparing the IHA and KK procedures in China found the IHA to have a high level of sensitivity but poor specificity (Yu et al., Reference Yu, de Vlas, Jiang and Gryseels2007; Zhou et al., Reference Zhou, Yang, Tao, Jiang, Zhao, Wei and Jiang2008b). Indeed, circulating anti-schistosome antibodies have been shown to drop but they can persist for months to years after successful treatment with PZQ and parasite clearance, resulting in false positives during serology (Feldmeier et al., Reference Feldmeier, Nogueira-Queiroz, Peixoto-Queiroz, Doehring, Dessaint, De Alencar, Dafalla and Capron1986). The higher prevalence observed in Jiangxi province by the CNSCP using the IHA may indicate that schistosomiasis prevalence in the selected Jiangxi villages had previously been higher than the selected Hunan villages where the prevalence by IHA was overall lower.
It is therefore likely that the IHA overestimates schistosomiasis prevalence but the procedure works well as an initial population screening method. In contrast, the KK procedure used in the CNSCP, performed on a single human stool sample with three slides subsequently examined microscopically, likely results in an underestimation of prevalence. The protocol is far from ideal, particularly with the current low intensity S. japonicum infections prevalent in China following the decades of control efforts and the yearly MDA programme in targeted communities. Accordingly, the true schistosomiasis prevalence probably lies somewhere in between the values derived from the CNSCP IHA and KK methods. The KK has long been preferred over more sensitive diagnostics in many schistosomisis-endemic countries due to its low cost and the relative ease of performing the procedure (Gray et al., Reference Gray, McManus, Li, Williams, Bergquist and Ross2010; Aula et al., Reference Aula, McManus, Mason, Botella and Gordon2021). In future, it would be profitable for the CNSCP to consider incorporating more accurate and easy to use diagnostic procedures, but the incurred increased costs will need to be carefully considered.
Real-time PCR is highly sensitive even in areas with low-intensity schistosome infections (Lier et al., Reference Lier, Simonsen, Wang, Lu, Haukland, Vennervald, Hegstad and Johansen2009; Gordon et al., Reference Gordon, Gray, Gobert and McManus2011; He et al., Reference He, Gordon, Williams, Yueshang, Wang, Hu, Gray, Ross, Harn and McManus2018) and we previously used this diagnostic procedure in China for the detection of S. japonicum infections in humans and animals (Van Dorssen et al., Reference Van Dorssen, Gordon, Li, Williams, Wang, Luo, Gobert, You, McManus and Gray2017; He et al., Reference He, Gordon, Williams, Yueshang, Wang, Hu, Gray, Ross, Harn and McManus2018). It is evident that while the results of the qPCR presented here do not match well with the CNSCP data, we believe that this DNA-based detection procedure provides a more accurate picture of the real status of schistosomiasis in Hunan and Jiangxi provinces – higher than the KK from the CNSCP, but lower than the IHA results (Tables 1 and 3).
Prevalence and intensity of S. japonicum infection in humans determined by qPCR
The cohort study population in both Hunan and Jiangxi provinces was demographically quite homogeneous, with the majority of participants from both provinces being farmers and/or fishermen, who owned their own land and house, worked in the village >5 years, and nearly 100% of whom lived and worked in their resident villages for more than 6 months a year (Supplementary Table 1). While there were some significant differences in prevalence between demographic variables, there were very few meaningful specific examples. In terms of occupation, as indicated, >85% of the study population were farmers and/or fishermen with nearly 69% classed as farmers only. Prevalence was highest in farmers/fishermen and businessmen/teachers, although this was not significantly different to other groups due to the low numbers of individuals categorized as having occupations other than farming and/or fishing; the businessmen/teacher occupation group had the lowest number of individuals with only two individuals positive for schistosomiasis. The intensity of infection in GMEPG was very high in a few study individuals which skewed the results in some instances, particularly where there were very few positive subjects in a particular classification (Supplementary Table 1).
An earlier study that we conducted in 2013 in four Chinese provinces (Hunan, Anhui, Hubei and Jiangxi) utilizing qPCR determined human S. japonicum prevalence values of 27.0% for Hunan and 10.3% for Jiangxi (He et al., Reference He, Gordon, Williams, Yueshang, Wang, Hu, Gray, Ross, Harn and McManus2018). In contrast, the results presented here for 2016 were 8.0% and 1.8% in Hunan and Jiangxi respectively. These substantially lower values were likely due to the impact of increased control efforts including the additional three annual rounds of MDA undertaken as part of the CNSCP. However, the 2013 survey examined far fewer individuals, with only 158 villagers from Jiangxi and 63 from Hunan, compared with, respectively, 2019 and 1905 subjects in the current study. By contrast, the infection intensity of S. japonicum determined by qPCR in the 2013 study was significantly lower than we recorded in 2016, with the average GMEPG in Hunan being 4.9 compared with 24.8 in the current study, and 3.5–7.0 in Jiangxi compared with 23.77 recorded here. No information on prior treatment was available for the 2013 study and the recorded infection intensity differences in the two surveys may have been due to different villages being sampled and that considerably more individuals were sampled in the current study. Only one village (Biaoen in Jiangxi), was sampled in both studies, with a much higher infection intensity (GMEPG; 3.48 vs 34.68) recorded in the current survey compared with the previous one, although the prevalence was higher in 2013 (13.00% vs 3.42%). Bovines, recognized major reservoirs of S. japonicum infection (Gray et al., Reference Gray, Williams, Li, Chen, Forsyth, Li, Barnett, Guo, Ross, Feng and McManus2009a; Reference Gray, Williams, Li, Chen, Forsyth, Li, Barnett, Guo, Ross, Feng and McManus2009b; McManus et al., Reference McManus, Dunne, Sacko, Utzinger, Vennervald and Zhou2018), were still present in Biaoen in 2015 (Supplementary Table 8) which likely contributed to parasite transmission there with the prevalence being the highest recorded for any of the sampled Jiangxi villages in the current study (Table 1).
Over the period 2004 to 2008 the infection prevalence of S. japonicum, determined by the insensitive KK method, was shown to be reduced from 11.27 to 3.12% in humans, and from 4.90 to 2.15% in bovines in the Dongting lake region of Hunan province (McManus et al., Reference McManus, Gray, Ross, Williams, He and Li2011), emphasising the general downward trend over time in schistosomiasis prevalence in this setting.
Prevalence and intensity of infection of S. japonicum in animals by qPCR and ddPCR
Three types of large ungulates (water buffalo, cattle, and goats) were examined in the current study by qPCR and ddPCR. These animals were present in only three villages in Hunan province in 2016 and there was no significant (P = 0.0913) relationship between human infection prevalence and their presence or absence in the study villages. In the current study all bovines had been removed from all study villages in Jiangxi province, although just three years prior in 2013, Biaoen (the village with the highest human prevalence recorded in the current survey) still maintained bovines, recording, by qPCR, a S. japonicum prevalence of 17.7% in 51 animals sampled (13% human prevalence was recorded; n = 100) (Table 2), and a prevalence of 1.3% (n = 109) in 2015 as part of the CNSCP (Supplementary Table 8).
Previously we demonstrated S. japonicum infections in goats and bovines from Hunan province using a range of diagnostic procedures (Van Dorssen et al., Reference Van Dorssen, Gordon, Li, Williams, Wang, Luo, Gobert, You, McManus and Gray2017). Goat stool appears to have a considerable number of inhibitors present that prevents amplification of the S. japonicum nad1 gene by qPCR, even in those samples parasite-positive by KK and MHT; however, these inhibitors are sufficiently diluted in the ddPCR assay, resulting in positive amplification and a higher prevalence than determined by the KK and MHT (Van Dorssen et al., Reference Van Dorssen, Gordon, Li, Williams, Wang, Luo, Gobert, You, McManus and Gray2017). We did not identify any positive goats by qPCR or ddPCR in the current survey, but we did identify S. japonicum-positive bovines utilizing the ddPCR. As we have previously used qPCR without issue on bovine stools to diagnose S. japonicum, we expected it to perform at least as well as the ddPCR in this study. The intensity of infection was relatively low as shown by the CNIs calculated from the ddPCR results (Table 2), and it may be that the detection level is lower for ddPCR than qPCR, with ddPCR proving the more sensitive diagnostic procedure (Weerakoon et al., Reference Weerakoon, Gordon, Gobert, Cai and McManus2016, Reference Weerakoon, Gordon, Cai, Gobert, Duke, Williams and McManus2017a, Reference Weerakoon, Gordon, Williams, Cai, Gobert, Olveda, Ross, Olveda and McManus2017b). In comparison, in our earlier 2013 study in Hunan province, cattle were positive by KK, MHT and qPCR with a GMEPG of 24.20 for KK and 480.96 by qPCR. These prevalence values were considerably higher than we recorded for water buffalo where none were positive by KK, only one was positive by MHT, and nine (n = 10) were positive by qPCR with a GMEPG of 132.57 (He et al., Reference He, Gordon, Williams, Yueshang, Wang, Hu, Gray, Ross, Harn and McManus2018). Cattle are considered to be more susceptible to S. japonicum infection than water buffalo (Yang et al., Reference Yang, Feng, Fu, Yuan, Hong, Shi, Zhang, Liu, Li, Lu and Lin2012a, Reference Yang, Fu, Feng, Shi, Yuan, Liu, Hong, Li, Lu and Lin2012b; He et al., Reference He, Gordon, Williams, Yueshang, Wang, Hu, Gray, Ross, Harn and McManus2018) given the latter are native animals whilst cattle are a relatively new introduction. The GMEPG calculated from the KK and qPCR assays in the earlier study was substantially higher than the CNIs presented here, confirming the lower intensity of infection in these animals in the current study. Older water buffalo excrete fewer viable eggs than animals <18 months old, a feature that may be due to self-cure, decreased egg viability, or decreased egg production by female worms (McManus et al., Reference McManus, Gray, Li, Feng, Williams, Stewart, Rey-Ladino and Ross2010; Li et al., Reference Li, McManus, Lin, Williams, Harn, Ross, Feng and Gray2014). In the current study, all water buffalo were under 20 months of age; thus the lower infection intensity we report likely reflects the overall reduction in transmission in the study area.
The Chinese government has, for some time, been removing cattle and water buffalo from schistosomiasis-endemic areas and replacing them with motorized tractors, or has fenced off these bovines to limit environmental contamination of the area with S. japonicum eggs (McManus et al., Reference McManus, Gray, Ross, Williams, He and Li2011; Li et al., Reference Li, Hou, Tan, Williams, Gray, Gordon, Kurscheid, Clements, Li and McManus2020). As indicated, bovines are recognized as major reservoir hosts of S. japonicum in China and contribute substantially to human transmission due to the large numbers of schistosome eggs they excrete daily (Guo et al., Reference Guo, Ross, Lin, Williams, Chen, Li, Davis, Feng, McManus and Sleigh2001; Gray et al., Reference Gray, Williams, Li, Chen, Forsyth, Li, Barnett, Guo, Ross, Feng and McManus2009b; He et al., Reference He, Gordon, Williams, Yueshang, Wang, Hu, Gray, Ross, Harn and McManus2018).
Previous schistosomiasis status
The majority of participants at baseline reported previously having had schistosomiasis (68.1%), although very few had advanced (0.7%; n = 29) or acute disease (1.7%; n = 66) (Supplementary Table 1). Schistosomiasis is a long-lived infection that can result in chronic disease with liver and spleen granulomas leading to hepatosplenomegaly (McManus et al., Reference McManus, Li, Gray and Ross2009, Reference McManus, Dunne, Sacko, Utzinger, Vennervald and Zhou2018; Gordon et al., Reference Gordon, Kurscheid, Williams, Clements, Li, Zhou, Utzinger, McManus and Gray2019). However, the regular MDA programme of the CNSCP has reduced the number of people developing the chronic disease through the regular treatment of those at risk of infection. The relatively high number of individuals positive in our cohort study that had never previously received treatment for schistosomiasis indicates a gap in the CNSCP that needs to be addressed by improving the accuracy of the diagnostics procedures used, and by increasing the precision of the targeted MDA programme to include treating all farmers/fishermen as they have the highest risk of infection.
Snail survey and LAMP
No S. japonicum-positive snails were identified in any of the 16 villages in this study and none were recorded in the CNSCP. More snails were collected in Jiangxi than Hunan although no snails were recorded in two villages, one each from Jiangxi (Biaoen) and Hunan (Ganzhou). This is likely due to the same snail survey sites being visited in the current study, and by the CNSCP field teams in some villages, which would lead to an overall lower number of snails being collected in those locations. As both villages where no snails were recorded did have positive human infections it is likely that snail habitats were missed as the presence of oncomelanids are pre-requisites for schistosomiasis transmission. That no positive snails were identified may be due also to the low overall prevalence and intensity of S. japonicum infection in both provinces. As indicated above, bovines are major reservoirs of S. japonicum infection and with their removal or a decrease in the numbers of these animals in an endemic area, there is a considerable reduction in the number of schistosome eggs being excreted into the environment. None-the-less, humans were clearly getting infected in both provinces and other animal species may have participated as transmission reservoirs; our previous survey failed to identify any patent infections in rodents in the Dongting lake area (Van Dorssen et al., Reference Van Dorssen, Gordon, Li, Williams, Wang, Luo, Gobert, You, McManus and Gray2017), so a human-snail-human route is a likely transmission pathway.
In both the CNSCP and in this study, Oncomelania snails were pooled in groups of 50 prior to DNA being extracted from each pool and subjected to LAMP. As the S. japonicum prevalence in animals and humans is reduced, the number of infected snails will also drop. Pooling is a cost-effective and fast way to survey a large number of snails, but is subject to the dilution effect (Hung and Swallow, Reference Hung and Swallow1999). In high-intensity transmission areas, the number of positive snails in a pool will be quite high and, therefore, readily identified. As the number of positive snails drops due to successful control efforts, the number of positive snails in a pool will also be reduced. The signal from one positive snail in a pool of 50 may be diluted to such an extent that it results in a false negative LAMP result. However, a previous study showed that a single infected snail in a pool of 100 was still detectable using the LAMP method (Kumagai et al., Reference Kumagai, Furushima-Shimogawara, Ohmae, Wang, Lu, Chen, Wen and Ohta2010). It is more likely that positive snails were simply missed during collection due to the low prevalence of S. japonicum in the study areas. Indeed, the total population of snails and the prevalence of infected snails have been shown to be falling in the Dongting lake area of Hunan with no infected snails found in surveys performed in 2006–2008 (McManus et al., Reference McManus, Gray, Ross, Williams, He and Li2011).
In future, qPCR or ddPCR could be used as an alternative to LAMP to assess snail prevalence as they are more sensitive assays (Weerakoon et al., Reference Weerakoon, Gordon and McManus2018).
Water contact
Reported water contact was high in the cohort study villages with 88% of villagers stating they had had water contact in the previous 12 months and 20% having water contact more than once a week (Supplementary Table 1). Water contact was at its highest for all villager activities in the summer, and at its lowest in autumn. There are two main S. japonicum transmission seasons in China. In the Dongting lake area, the first commences with the rainy season in April, at which time snail populations expand due to the warmer weather and increased water levels in the Dongting lake; the second peak in the transmission is from October to November (McManus et al., Reference McManus, Gray, Ross, Williams, He and Li2011). The Poyang lake is fed by inland rivers between April and June, raising the water level and accounting for the first transmission season, and is then fed by the Yangtze river between July and September – accounting for the second transmission season (Xue et al., Reference Xue, Wang, Zhang, Hao, Chen, Lin, Xu, Xia and Li2021).
During the colder months, the Oncomelania snails bury themselves underground to survive and transmission is therefore low to zero in winter. Thus, the highest numbers of snails, and thus transmission, and the peak of human water contact activities occur in the summer.
Farming/cutting grass was significantly associated with S. japonicum infection. A previous study in Hunan province, which used water contact diaries, also identified cutting grass as a significant risk factor for schistosomiasis, along with fishing, crossing water, washing and playing (Ross et al., Reference Ross, Yuesheng, Sleigh, Williams, Hartel, Forsyth, Yi and McManus1998). Night soil has traditionally been used as fertilizer in China and may contribute to the transmission of schistosomiasis, particularly among farmers (Carlton et al., Reference Carlton, Liu, Zhong, Hubbard and Spear2015).
Defecation modes
Open defecation is an important risk factor for schistosomiasis transmission, particularly when it occurs in snail habitat areas. In our study, very few individuals were defecating in the marshlands or lakes, and while only two of these individuals tested positive for schistosomiasis, human defecation may still be important for ongoing transmission in the area. It should also be considered that the questionnaire we employed may not have captured information on casual open defecation practices as it asked for two primary modes of defecation and not all modes of defecation.
Wealth index
As indicated earlier, poor health may be as a consequence of poverty due to the inability to afford adequate nutrition, housing, education, and healthcare. Poverty as an indicator for schistosome infection is likely to be due to inadequate hygiene infrastructure such as flushable toilets and access to clean, safe water. In general, economic growth in China has led to decreases in the numbers of individuals living in poverty and increases in overall community health (Ross et al., Reference Ross, Sleigh, Li, Davis, Williams, Jiang, Feng and McManus2001; Balen et al., Reference Balen, McManus, Li, Zhao, Yuan, Utzinger, Williams, Li, Ren, Liu, Zhou and Raso2010). However, there are still income and wealth gaps apparent, particularly between rural and urban locations (Balen et al., Reference Balen, McManus, Li, Zhao, Yuan, Utzinger, Williams, Li, Ren, Liu, Zhou and Raso2010). Overall, in the current study, there was a higher prevalence of S. japonicum infection in households with a lower wealth index than those with a higher wealth index (Table 4).
An earlier study examining different variables that may help assess household wealth also found that rural houses were less likely to have adequate access to good hygiene infrastructure, in the form of access to tap water and flushable toilets, than those in a peri-urban setting (Balen et al., Reference Balen, McManus, Li, Zhao, Yuan, Utzinger, Williams, Li, Ren, Liu, Zhou and Raso2010). This can obviously have a huge impact on the potential transmission of schistosomiasis in addition to giving insight into household wealth. In the current study, the majority of households had flushable toilets (78.4%) while just over half (54.8%) had access to tap water, the remaining households accessing water via a hand pump (15.8%) or well (27.8%) and used either dry latrines (3.79%) or wet latrines (17.58%) (Supplementary Table 9). Prevalence of schistosomiasis was similar between those with flushable toilets (7.7%; 95% CI 6.4–8.9) and those who used wet latrines (7.8%; 95% CI 5.2–10.3) (Supplementary Table 9).
Treatment options other than praziquantel
MDA with PZQ is a cornerstone of MDA for schistosomiasis control worldwide. Praziquantel is cheap, with the drug often provided free of charge due to arrangements with non-government organizations and pharmaceutical companies. MDA is also inexpensive to implement as it does not require case finding before administration, although a lack of case finding has been linked with low compliance in some endemic countries (Inobaya et al., Reference Inobaya, Chau, Ng, Macdougall, Olveda, Tallo, Landicho, Malacad, Aligato, Guevarra and Ross2018). Treatment with PZQ also does not prevent re-infection, and nor is it effective against migrating schistosomula, and thus infection and transmission continue. In China, PZQ is listed as a prescription medicine and outside of the control programme, people at risk of infection can only receive PZQ after receiving a positive diagnosis.
The antimalarial artemether, has shown efficacy against schistosomula, and although less effective against adults, it could be used as a phrophylactic control measure individually or in combination with PZQ (Xiao et al., Reference Xiao, Booth and Tanner2000; Reference Xiao, Tanner, N'goran, Utzinger, Chollet, Bergquist, Chen and Zheng2002; Li et al., Reference Li, Chen, He, Hou, Ellis and McManus2005). However, its use would be restricted to areas non-endemic for malaria due to the potential for drug-resistant Plasmodium parasites developing. A field trial in four endemic provinces in China found a much lower prevalence of S. japonicum in villages where artemether was given to local at-risk residents four times at intervals of 15 days compared to control villages (Xiao et al., Reference Xiao, Booth and Tanner2000).
More recently, a number of compounds derived from Paecilomyces sp., a soil fungus, have been investigated for anti-schistosomal properties with particular emphasis on migrating schistosomula. A number of these compounds have been tested in vitro with five compounds exhibiting death rates against skin schistosomula above 90% in the first 24 h. One of these, radicicol (RAD), has been tested in in vivo and is highly effective against early-stage skin schistosomula, reducing the worm burden by 91.2% (Xu et al., Reference Xu, Liu, Zeng, Wu, Yu, Gu, Xue and Wei2021).
It is clear that new anti-schistosomal drugs are being sought by a number of research groups in China (Xiong et al., Reference Xiong, Xu and Zheng2021), but they have yet to be shown as effective as PZQ or incorporated into the CNSCP. Areas in China where previously control had been withdrawn due to low prevalence in humans saw a resurgence with prevalence levels returning to high levels within a few years of schistosomiasis control cessation (Liang et al., Reference Liang, Yang, Zhong and Qiu2006; Li et al., Reference Li, Hou, Tan, Williams, Gray, Gordon, Kurscheid, Clements, Li and McManus2020). In order to achieve elimination and prevent rebound infections, both more sensitive diagnostics and treatment strategies need to be employed in the future.
Conclusion
The CNSCP has been very successful, reducing schistosomiasis prevalence from 12 million in 1949 to less than 200 000 in 2013 with a key element being the shift in focus from determining morbidity outcomes to detecting infections and measuring transmission (WHO, 2013; Lei et al., Reference Lei, Zheng, Zhang, Zhu, Xu, Xu, Fu, Wang, Li and Zhou2014; Yang et al., Reference Yang, Liu, Zhu, Griffiths, Tanner, Bergquist, Utzinger and Zhou2014). China now seeks to achieve the goal of schistosomiasis elimination by 2030, a revised date from earlier time scale pronouncements. This later date to achieve the goal of elimination indicates that while the overall prevalence is low, schistosomiasis is still persisting, a feature likely due in part to the diagnostics procedures currently employed whereby some individuals with schistosomiasis are misdiagnosed as being uninfected. The persistence of the disease may also be due to the reliance on PZQ for treatment, a drug with poor efficacy against schistosomula. Continued research into other drugs, including artemether, which do target juvenile stages and could be used in combination with PZQ should be encouraged. Continued challenges as China moves towards schistosomiasis elimination include the wide host range of S. japonicum with more than 40 species of mammalian host reservoir identified (He et al., Reference He, Salafsky and Ramaswamy2001), the large areas of snail habitats in endemic areas, and the unavoidable water contact for some occupational groups. The fact there is no currently available anti-schistosomiasis vaccine is also a limiting factor for control (Molehin et al., Reference Molehin, Rojo, Siddiqui, Gray, Carter and Siddiqui2016).
The lack of S. japonicum-positive snails evident in our study villages and recorded by CNSCP for the same locations may indicate decreased transmission in this setting; however further monitoring of additional snail habitats in the study villages would likely have resulted in some positive snails being identified. As we observed here, the use of targeted chemotherapy may miss treating some individuals infected with S. japonicum; a number of individuals, who had never received previous treatment for schistosomiasis, tested positive by qPCR, highlighting potential issues in disease surveillance. Similarly, a re-appraisal of the diagnostics procedures currently employed in the CNSCP appears warranted if the goal of schistosomiasis elimination by 2030 is to be realized.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182021001724.
Data
All data are presented either in the manuscript or as supplementary files. Raw data files will be made available upon request.
Acknowledgements
The authors sincerely acknowledge the assistance and work of members of the Hunan Institute of Parasitic Diseases (HIPD), Jiangxi Institute of Parasitic Diseases (JIPD) and the National Institute of Parasitic Diseases (NIPD), as well as regional staff and volunteers from the local village areas.
We also acknowledge Gunter Hartel from QIMR Berghofer for statistics support, and Madeleine Flynn also from QIMR Berghofer for graphical illustrations.
Author contribution
Conceived study: DPM, GMW, DJG, ACAC, XNZ, YL, JU. Project development: DPM, GMW, DJG, ACAC, XNZ, YL, JU, JK, SF, JX, CAG. Laboratory work: ZL, SL, XY, GL, DL, ZL, FH, JG, SX, JC, TS, CL, HZ, CAG. Field work and training: CAG, GMW, DJG, XNZ, YL, JK, SF, JX, HZ, ACAC. Project stage manager: JZ, ZL, DL, JG. Data entry: GL, SL, HZ. Data capture and cleaning: GMW, JK, SF. Data analysis: JK, CAG, KAA, GMW. Manuscript first draft: CAG. Manuscript editing: CAG, DPM, JK, ACAC, KAA, YL, DJG. Funding acquisition: DPM, DJG, JU, RB, XNZ, GMW.
Financial support
This work received financial support from a Project Grant (APP1098244; DPM, GMW, DJG, ACAC, XNZ, YL, JU), a Programme Grant (APP1132975; DPM), a Research Fellowship (APP1102926; DPM) and an Investigator Grant (APP1194462; DPM) from the National Health and Medical Research Council of Australia.
Conflicts of interest
The authors declare there are no conflicts of interest.
Ethical standards
Informed written consent was received from all individuals enrolled in the study, and from parents/guardians of minors. Ethical approval for human and animal work was provided by the Ethics Committees of QIMR Berghofer Medical Research Institute (Human Research Ethics Committee Reference Number P524; Animal Ethics Committee Reference Number A1003–601), Hunan Institute of Parasitic Diseases (HIPD), Jiangxi Institute of Parasitic Diseases (JIPD) and the National Institute of Parasitic Diseases (NIPD) in Shanghai.
All human subjects found to be positive for S. japonicum during the course of the study were referred to the local schistosomiasis control station for treatment with praziquantel (PZQ) at the WHO recommended dose of 40 mg kg−1.
Informed written consent was received from all animal owners in the study sites and the study was performed in accordance with the recommendations of the 2013 Australian code of practice for the care and use of animals for scientific purposes.