Article contents
Studies on intrategumental pH and its regulation in adult male Schistosoma mansoni
Published online by Cambridge University Press: 06 April 2009
Summary
The intrategumental pH in adult male Schistosoma mansoni as measured with pH-sensitive microelectrodes is between 7.0 and 7.2, a value about one pH unit more alkaline than expected for equilibrium. This value is maintained for at least 4 h after isolation in media buffered with Hepes or CO2/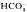 with or without serum present. CCCP (1 μM) and FCCP (10 μM) cause rapid acidification. DNP (1 mM) and Na-orthovanadate (1 mM) acidify but also cause significant depolarization of the tegument. NH
with or without serum present. CCCP (1 μM) and FCCP (10 μM) cause rapid acidification. DNP (1 mM) and Na-orthovanadate (1 mM) acidify but also cause significant depolarization of the tegument. NH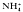 (20 mM) depolarizes and acidifies the tegument with no evidence of transient alkalinization. High K+ (25 mM) accelerates the acidification. Removal of the NH+4 causes a large transient further acidification with recovery requiring as long as 40 min. High K+ (25 mM) delays the early stage of the recovery. Presence of CO2/HCO
(20 mM) depolarizes and acidifies the tegument with no evidence of transient alkalinization. High K+ (25 mM) accelerates the acidification. Removal of the NH+4 causes a large transient further acidification with recovery requiring as long as 40 min. High K+ (25 mM) delays the early stage of the recovery. Presence of CO2/HCO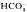 in the incubation medium does not accelerate the recovery rate nor does SITS (100 μM) inhibit the recovery. Intrategumental Na+ is elevated after an acid load and amiloride (3 mM) as well as low Na+ medium interfere with recovery from the acid load indicating that a Na+–H+ exchanger may be present in the tegumental membrane.
in the incubation medium does not accelerate the recovery rate nor does SITS (100 μM) inhibit the recovery. Intrategumental Na+ is elevated after an acid load and amiloride (3 mM) as well as low Na+ medium interfere with recovery from the acid load indicating that a Na+–H+ exchanger may be present in the tegumental membrane.
- Type
- Research Article
- Information
- Copyright
- Copyright © Cambridge University Press 1990
References
- 9
- Cited by


