Introduction
The Balearic Mallorca Island, belonging to Spain, is a popular holiday destination with more than 17 million tourists in 2023, of which about 5 million tourists are Germans. Touristic and public life has adapted to German speaking tourists (Germany, Austria, Switzerland), and nowadays, about 20 000 Germans are permanent residents with more than 60 000 Germans spending at least 3 months per year in Mallorca [Spanish Statistical Office (ine.es)].
Ixodid ticks are important obligate blood-feeding arthropod vectors of pathogens, and human parasitism by these ticks is a common event in the world (Sonenshine et al., Reference Sonenshine, Lane, Nicholson, Mullen and Mullen2002). In the family Ixodidae, there are currently 762 recognized species, divided into 2 groups, the Prostriata and the Metastriata, with 15 extant genera and 2 extinct genera (Guglielmone et al., Reference Guglielmone, Nava and Robbins2023). In Europe, ixodid tick species belong to 5 genera: Ixodes (Prostriata) as well as Dermacentor, Haemaphysalis, Hyalomma and Rhipicephalus (Metastriata) (Nowak-Chmura, Reference Nowak-Chmura2013). Ticks are hosting a large variety of microorganisms in their microbiome, among them pathogens, like rickettsiae and microorganisms, which form a crucial element in the various physiological processes as nutrition, development, reproduction, vector capacity and immunity (Stich et al., Reference Stich, Schaefer, Bremer, Needham and Jittapalapong2008; Bonnet et al., Reference Bonnet, Binetruy, Hernández-Jarguín and Duron2017). Certain microorganisms serve as endosymbionts, which may be essential or facultative for tick physiology (Francisella-like and Coxiella-like endosymbionts). Other microorganisms may be harmful pathogens for vertebrates, like Francisella tularensis and Coxiella burnetii, although they have not been identified to cause disease in their tick vectors. Different factors have influence on the tick microbiota composition, such as tick species, life stage and environment (Ponnusamy et al., Reference Ponnusamy, Gonzalez, Van Treuren, Weiss, Parobek, Juliano, Knight, Roe, Apperson and Meshnich2014; Van Treuren et al., Reference Van Treuren, Ponnusamy, Brinkerhoff, Gonzalez, Parobek, Juliano, Andreadis, Falco, Ziegler, Hathaway, Keeler, Emch, Bailey, Roe, Apperson, Knight and Meshnick2015; Aivelo et al., Reference Aivelo, Norberg and Tschirren2019).
While on the Spanish mainland 22 ixodid tick species are known (Guglielmone et al., Reference Guglielmone, Nava and Robbins2023), data on the tick fauna on the Spanish islands are much less known. Guglielmone et al. (Reference Guglielmone, Nava and Robbins2023) did not include Mallorca as a separate entity in the last list of ixodid ticks in the world. Recently, a study reported 12 tick species in Mallorca (Monerris Mascaro and del Mar Colom Noguera, Reference Monerris Mascaró and del Mar Colom Noguera2020). However, the recent study did not investigate the potential pathogens in Mallorcan ticks. Therefore, the aim of the current study was to investigate tick fauna and tick-borne pathogens in the ticks collected from dogs, a cat and humans in Mallorca.
Materials and methods
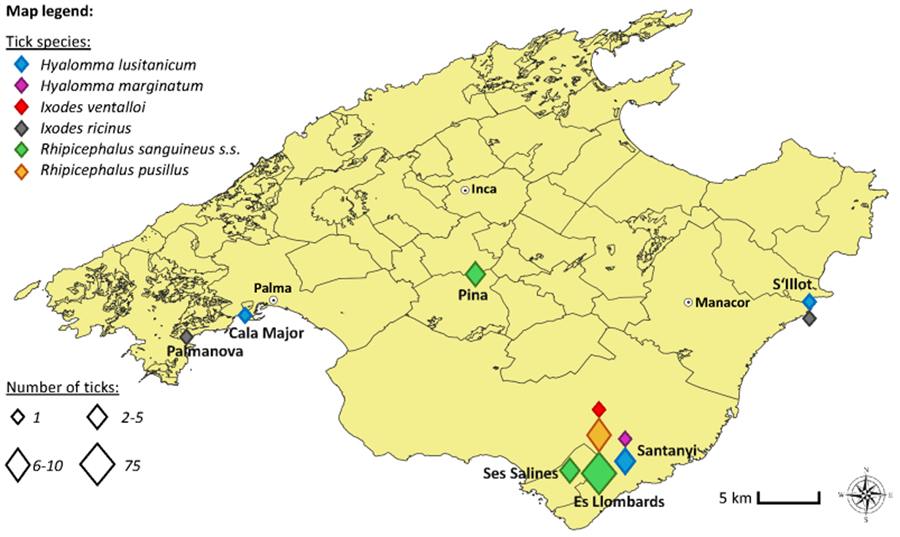
Ticks were collected based on a citizen science project. One of the authors (M.B.) released a call, based on an interview article published in a German speaking Mallorca journal (www.mallorcazeitung.es) in August 2021. Tourists and residents were asked to send in any ticks they could find or collect in Mallorca. Along with the ticks, citizens were asked to provide information on the date and location of collection, and the host. To enhance participant engagement, citizens received feedback and were informed about the tick species that they had submitted and which pathogens the respective ticks carried. Ticks were received at irregular intervals, from March to October 2023. All data on collected ticks and their respective information are summarized in Table 1 and shown on a map (Fig. 1).
Table 1. Primers and probes used for molecular investigation of tick species and their pathogens

a In brackets are number of positive samples.
b Rickettsia sp., as could not be sequence due to low amount of DNA.
c Rickettsia massilliae was identified after sequencing.

Figure 1. Map of the collection places created using QGis Version 3.34 Prizren, scale 1:220.000.
Ticks were identified based on morphological identification keys (Walker et al., Reference Walker, Keirans and Horak2000; Pérez-Eid, Reference Pérez-Eid2007; Nava et al., Reference Nava, Beati, Venzal, Labruna, Szabó, Petney, Saracho-Bottero, Tarragona, Dantas-Torres, Silva, Mangold, Guglielmone and Estrada-Peña2018), using a Keyence VHX-900F microscope (Itasca, IL, USA). DNA was extracted from individual ticks using the QIAamp Mini DNA extraction kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. The 16S rRNA gene of ticks was amplified according to Halos et al. (Reference Halos, Jamal, Vial, Maillard, Suau, Le Menach, Boulouis and Vayssier-Taussat2004), visualized in 1.5% agarose gel, purified using QIAquick® PCR Purification Kit (250) (Qiagen, Hilden, Germany), and subsequently bi-directionally sequenced, consensus sequences derived and submitted to GenBank. Additionally, DNA was analysed using pan-Rickettsia real-time PCR to amplify part of the gltA gene (Wölfel et al., Reference Wölfel, Essbauer and Dobler2008), followed by PCR amplification of 23S-5S intergenic spacer region (Chitimia-Dobler et al., Reference Chitimia-Dobler, Rieß, Kahl, Wölfel, Dobler, Nava and Estrada-Peña2018), ompB (Roux and Raoult, Reference Roux and Raoult2000), and gltA (Nilsson et al., Reference Nilsson, Lindquist and Pahlson1999), followed by Sanger sequencing to identify the Rickettsia species. Furthermore, the extracted tick DNA was analysed for the presence of Francisella spp. and Francisella-like endosymbionts (Gehringer et al., Reference Gehringer, Schacht, Maylaender, Zeman, Kaysser, Oehme, Pluta and Splettstoesser2013) using LightMix® F. tularensis 16S rRNA gene according to the manufacturer's instructions (TibMolBiol, Berlin, Germany). To detect genomic DNA from Coxiella burnetii and Coxiella-like endosymbionts the method described by Frangoulidis et al. (Reference Frangoulidis, Kahlhofer, Said, Osman, Chitimia-Dobler and Shuaib2021) was applied. The screening for Anaplasma phagocytophilum was performed with real-time PCR using a protocol by Courtney et al. (Reference Courtney, Kostelnik, Zeidner and Massung2004). A conventional PCR targeting a fragment (411–452 bp) of the 18S rRNA of piroplasms was performed using a protocol by Casati et al. (Reference Casati, Sager, Gern and Piffaretti2006). All PCRs included a positive control (Table 2) and purified water as negative control. Moreover, Francisella specific PCR includes an internal control to rule out PCR inhibition by sample ingredients. Table 2 summarizes the information on PCR methods.
Table 2. Overview of the collection dates and places of the different collected tick species and the number of Rickettia spp. positive specimens

For ticks, the 16S rDNA sequences were screened with BLASTn analysis (Altschul et al., Reference Altschul, Gish, Miller, Myers and Lipman1990) and representative related sequences downloaded from GenBank (https://www.ncbi.nlm.nih.gov/nucleotide). Sequences were aligned using the online version of MAFFT (Katoh and Standley, Reference Katoh and Standley2013) with default parameters and maximum likelihood analyses performed with IQ-Tree2 v1.6.12 (Minh et al., Reference Minh, Schmidt, Chernomor, Schrempf, Woodhams, von Haeseler and Lanfear2020). The optimal substitution model used was TIM2 + F + G4 and 10 000 bootstraps were performed to obtain nodal support values. The tree was rooted with Ixodes species.
The phylogenetical analysis of the Rickettsia-positive specimens subsequent to Sanger sequencing was conducted by an external contractor (Eurofins, Germany). Sequences were analysed using BioEdit Alignment Editor Version 7.1.1 (Hall, Reference Hall1999) and compared with sequences deposited in the GenBank database of the National Centre for Biotechnology Information (NCBI) using the Basic Local Alignment Search Tool (BLAST) (Altschul et al., Reference Altschul, Gish, Miller, Myers and Lipman1990). Maximum likelihood analysis was performed in MEGA v7.0.14 (Kumar et al., Reference Kumar, Stecher and Tamura2016) based on the Tamura-Nei model (Tamura and Nei, Reference Tamura and Nei1993) with 1000 bootstraps.
Results
A total of 91 ticks were received from German tourists and residents in Mallorca. Six tick species could be identified: Ixodes ricinus (n = 2), Ixodes ventalloi (n = 1), Hyalomma lusitanicum (n = 7), Hyalomma marginatum (n = 1), Rhipicephalus sanguineus s.l. (n = 71) and Rhipicephalus pusillus (n = 9). Detailed information on the studied tick specimen presented in Table 1 and Fig. 1.
Sixty-seven specimens of Rhipicephalus sanguineus s.l., a three-host tick, were collected from dogs and 4 female specimens from a cat. All Rhipicephalus-ticks were adults (32 males and 39 females). The 16S rDNA sequences identified the species as Rh. sanguineus s.s. (temperate lineage, (accession numbers: PP227945–PP228018) (Fig. 2). All Rh. pusillus adults (4 males and 5 females) were collected from dogs, together with Rh. sanguineus s.s. Seven out of the 9 Rh. pusillus were confirmed amplifying the 16S rRNA gene and the sequences were submitted to GenBank (accession numbers: PP478783–PP478790), Hyalomma lusitanicum was collected from 1 human, 1 dog and 5 specimens were collected from the ground, together with a H. marginatum male in the community of Santanyi. Both I. ricinus (three-host tick, female and nymph) were collected from humans. Ixodes ventalloi female was found on a dog, together with Rh. sanguineus s.s.

Figure 2. Phylogenetic analysis of the 16S rDNA sequences of ticks from Mallorca. The species name and accession numbers are indicated.
All ticks tested negative for Anaplasma phagocytophilum, Coxiella spp., Francisella spp., and piroplasms (Babesia, Theileria, Cytauxoon spp.).
In 32 of 71 (45%) specimens of Rh. sanguineus s.s., Rickettsia spp. could be detected. However, only 18/32 (56.2%) PCR-positive Rickettsia spp. contained sufficient amount of DNA to enable sequencing and subsequent sequence analysis (Table 1). In all sequenced specimens R. massiliae was identified. The phylogenetic analysis of R. massiliae 23S-5S intergenic spacer region is shown in Fig. 3. All sequences were submitted in GenBank as follow: 23S-5S intergenic spacer region (accession numbers: PP263040–PP263054), ompB (accession numbers: PP263035–P263036), and gltA (accession numbers: PP263037–PP263039). The I. ventalloi female and the both I. ricinus tested positive in the Rickettsia screening PCR, but the DNA sequencing failed, precluding the identification of the Rickettsia sp. In total, DNA sequencing was unsuccessful for 14/32 (43.7%) samples due to the low amount of specific DNA.

Figure 3. Phylogenetic analysis of the 23S-5S intergenic spacer region of Rickettsia massiliae in Mallorca.
Discussion
The Balearian Island of Mallorca is a main destiny of tourism. In 2023, a new record was observed with more than 12 million tourists visiting the island (http://www.mallorcamagazin.com/nachrichten/tourismus/2023/10/09/115575). The island in the Mediterranean Sea lies within the area of distribution of tick species with known major importance as vectors, e.g., Rh. sanguineus s.l., and pathogens of medical and veterinary importance, e.g., Mediterranean Spotted Fever and Crimean-Congo Haemorrhagic Fever. While several studies are available on ticks and tick-borne pathogens on the Spanish mainland, no data are available on the occurrence and prevalence of these pathogens in Mallorca. Also, since the discovery, that Rh. sanguineus s.l. as a complex of 3 species, no studies were conducted to clarify which of the 3 species might be present on the Balearian Islands and specifically on the Island of Mallorca. This knowledge about the tick fauna might be of importance for diagnostics and treatment of illnesses transmitted by ticks on the island.
Microclimatic conditions influence the tick species activity, abundance and survival (Bertrand and Wilson, Reference Bertrand and Wilson1996; Rynkiewicz and Clay, Reference Rynkiewicz and Clay2014). Ticks spend 90% of their life off-host in the environment where they quest for a suitable host and moult between life stages (Anderson, Reference Anderson2002), except for the one-host tick species which spends 90% on their host. During the last years, the increasing expansion of the distribution of ticks and tick-borne diseases due to climatic changes have been observed (Gray and Ogden, Reference Gray and Ogden2021; Semenza and Paz, Reference Semenza and Paz2021; Semenza et al., Reference Semenza, Rocklöv and Ebi2022). Studies as presented here are therefore also an important data basis for monitoring and surveillance of future developments in tick dispersal and expansion of distribution.
In this study, 91 ticks representing 6 species of hard ticks were collected by German tourists and residents living in Mallorca mainly from dogs, a cat, humans and from the ground. All hard tick species, except H. marginatum, found in this study had also been described by Monerris Mascaro and del Mar Colom Noguera (Reference Monerris Mascaró and del Mar Colom Noguera2020), who reported 12 tick species among more than 2000 ticks collected from sheep, wildlife and from vegetation by flagging in Mallorca. In this study on ticks in Mallorca, however, the presence of ticks on pet animals was not examined. Also, no exact differentiation of Rh. sanguineus s.l. was conducted. However, this differentiation becomes more and more important, as studies now have shown that the 3 accepted extant species of this tick species complex exhibit differing vector capacities for pathogens, e.g., rickettsiae (Chitimia-Dobler et al., Reference Chitimia-Dobler, Kurzrock, Molčányi, Rieß, Ute Mackenstedt and Nava2019a, Reference Chitimia-Dobler, Schaper, Rieß, Bitterwolf, Frangoulidis, Bestehorn, Springer, Oehme, Drehmann, Lindau, Mackenstedt, Strube and Dobler2019b).
In the study of Monerris Mascaro and del Mar Colom Noguera (Reference Monerris Mascaró and del Mar Colom Noguera2020), Rh. turanicus was reported in Mallorca. Another study found Rh. turanicus also in Menorca (Castella et al., Reference Castellà, Estrada-Peña, Almeria, Ferrer, Gutiérrez and Ortuño2001). More recent genetic studies from the Canary Islands and from mainland of Spain and Portugal are not in agreement with these former data, as only Rh sanguineus s.s. has been identified in these studies (Nava et al., Reference Nava, Beati, Venzal, Labruna, Szabó, Petney, Saracho-Bottero, Tarragona, Dantas-Torres, Silva, Mangold, Guglielmone and Estrada-Peña2018; Chitimia-Dobler et al., Reference Chitimia-Dobler, Kurzrock, Molčányi, Rieß, Ute Mackenstedt and Nava2019a, Reference Chitimia-Dobler, Schaper, Rieß, Bitterwolf, Frangoulidis, Bestehorn, Springer, Oehme, Drehmann, Lindau, Mackenstedt, Strube and Dobler2019b). There, Rh. turanicus could not be identified to confirm the old reports, but all investigated ticks were determined as Rh. sanguineus s.s. in analogy to Rh. sanguineus s.l. There is now a new classification of Rh. turanicus s.l., such as Rh. turanicus s.s., Rh. afranicus (Bakkes et al., Reference Bakkes, Chitimia-Dobler, Matloa, Oosthuysen, Mumcuoglu, Mans and Matthee2020), and the more recently described Rh. secundus (Mumcuoglu et al., Reference Mumcuoglu, Estrada-Peña, Tarragona, Sebastian, Guglielmone and Nava2022). 1The possible presence of Rh. turanicus in Mallorca should be reconsidered and confirmed by further investigations. In the current study only Rh. sanguineus s.s. was found in Mallorca. Rhipicephalus sanguineus s.s. has a large distribution in Europe (including Canary Islands), U.S.A., parts of South America (Nava et al., Reference Nava, Beati, Venzal, Labruna, Szabó, Petney, Saracho-Bottero, Tarragona, Dantas-Torres, Silva, Mangold, Guglielmone and Estrada-Peña2018; Chitimia-Dobler et al., Reference Chitimia-Dobler, Kurzrock, Molčányi, Rieß, Ute Mackenstedt and Nava2019a, Reference Chitimia-Dobler, Schaper, Rieß, Bitterwolf, Frangoulidis, Bestehorn, Springer, Oehme, Drehmann, Lindau, Mackenstedt, Strube and Dobler2019b), and in some regions in Algeria (Laatamna et al., Reference Laatamna, Oswald, Chitimia-Dobler and Bakkes2020). The Algerian study (Laatamna et al., Reference Laatamna, Oswald, Chitimia-Dobler and Bakkes2020) detected a large spectrum of pathogens in Rh. sanguineus s.s., such as Hepatozoon canis, Babesia vogeli. Anaplasma platys, Ehrlichia canis, R. massiliae and Rickettsia conorii conorii. In this study in Mallorca, only R. massiliae was detected. This might indicate, that R. massiliae predominantly circulates in Mallorca rather than R. conorii. One resident, who sent ticks collected from one of her cats, reported a history of a severe clinical rickettsiosis with long-term sequelae after a tick bite on her neck. Although R. conorii IgM was detected by laboratory diagnostics it cannot be ruled out that the causative agent was R. massiliae, as serological cross-reaction among SFG rickettsiae are common and a diagnostic differentiation between R. conorii and R. massiliae infections is difficult (Hechemy et al., Reference Hechemy, Raoult, Fox, Han, Elliott and Rawlings1989; Raoult and Paddock, Reference Raoult and Paddock2005). Rhipicephalus sanguineus s.l. feeds frequently on humans, especially in the adult stage (Guglielmone and Robbins, Reference Guglielmone and Robbins2018). None of the four Rh. sanguineus s.s. collected from the cat tested positive for R. massiliae.
Rhipicephalus pusillus ticks are commonly found in southern Europe (Portugal, Spain and France) and northern Africa (Tunisia and Morocco). It is presumed a 3-host tick and has European rabbit as primary host, but has been reported from other hosts (Walker et al., Reference Walker, Keirans and Horak2000). This tick species is also considered exclusively endophilic (Osácar, 1992 cited in Estrada-Peña et al., Reference Estrada-Peña, Venzal and Nava2018) rarely parasitizing humans (Guglielmone and Robbins, Reference Guglielmone and Robbins2018). Rickettsia massiliae was first isolated in 1992 from Rh. sanguineus ticks collected near Marseille, France (Beati and Raoult, Reference Beati and Raoult1993). Rickettsia massiliae has been identified in southern Spain (Marquez, Reference Marquez2008) and in the Canary Islands (Fernández de Mera et al., Reference Fernández de Mera, Zivkovic, Bolanños, Carranza, Pérez-Arellano, Gutiérrez and de la Fuente2009), but not in Mallorca, so far. Rhipicephalus pusillus is considered vector of R. massiliae and the primary hosts are rabbits and hares. In Europe, Lepus europaeus (European hare) and rabbits are reservoirs of Rickettsia conorii, Rickettsia slovaca, C. burnetiid and Francisella tularensis holarctica (Rehácek et al., Reference Rehácek, Urvölgyi, Brezina, Kazár and Kovácová1978; Pérez Castrillón et al., Reference Pérez-Castrillón, Bachiller-Luque, Martín-Luquero, Mena-Martín and Herreros2001; Fernández de Mera et al., Reference Fernández de Mera, Zivkovic, Bolanños, Carranza, Pérez-Arellano, Gutiérrez and de la Fuente2009; Eremeeva and Dasch, Reference Eremeeva and Dasch2015). All Rh. pusillus (n = 9) collected from dogs in Mallorca tested negative for Rickettsia spp. and Coxiella spp. Considering the result that almost 50% of the Rh. sanguineus s.s. ticks carried R. massiliae could be interpreted such that Rh. pusillus is not playing an important role for R. massiliae in Mallorca or at all.
Hyalomma lusitanicum is probably the most abundant exophilic tick species in the central and southern part of the Iberian Peninsula, but also in other European countries (France, Italy) and North Africa (Algeria and Morocco) (Válcarel et al., Reference Válcarel, González, González, Sánchez, Tercero, Elhachimi, Carbonell and Olmeda2020). Hornok et al. (Reference Hornok, Grima, Takács, Szekeres and Kontschán2020) reported this species from Malta, collected from rabbits and cats, which is supported by a personal report of a resident in Malta to one author (LCD), who collected many H. lusitanicum adults from the ground in his garden and sent them for identification and further analyses. Hyalomma lusitanicum is a 3-host tick species, immatures are endo- and exophilic, while adults are exophilic. Wild rabbits and hares are considered as the main hosts and many other wild and domestic animals as secondary hosts. It can sometimes also be found on humans, but humans are not the preferred host and thus, it is only a sporadic parasite of humans (Guglielmone and Robbins, Reference Guglielmone and Robbins2018; Válcarel et al., Reference Válcarel, González, González, Sánchez, Tercero, Elhachimi, Carbonell and Olmeda2020). On the other hand, it has been reported that attachments to humans have increased in recent years, and more frequent human infestation has been reported in Portugal (Valcárcel et al., Reference Válcarcel, Elhachini, Vilá, Tomassone, Sánchez, Selles, Kouidri, González, Martin-Hernández, Valcárcel, Fernández, Tercero, Sanchis, Bellido-Blasco, González-Coloma and Olmeda2023). One H. lusitanicum female was found attached to a human head, which is the first report of a human H. lusitanicum infestation in Mallorca. The patient did not develop any disease, but a local reaction to the bite on her head was visible for more than a week. In the present study, all H. lusitanicum ticks tested negative for any of the investigated pathogens. However, H. lusitanicum is a known vector for C. burnetii, Theileria equi and Theileria annulata. It may be involved in the cycle of other pathogens such as Crimean-Congo Haemorrhagic Fever (CCHF) virus, A. phagocytophilum, F. tularensis and R. aeschlimannii (Válcarel et al., Reference Válcarel, González, González, Sánchez, Tercero, Elhachimi, Carbonell and Olmeda2020).
Hyalomma marginatum is a 2-host tick species. It has a large distribution in North Africa, Asia and many European countries including Spain (Válcarel et al., Reference Válcarel, González, González, Sánchez, Tercero, Elhachimi, Carbonell and Olmeda2020). According to the ECDC map, H. marginatum has not been observed in Mallorca hitherto, but on the other neighbouring small islands (ECDC, 2023). In the present study, a male was collected from the ground together with 5 specimens of H. lusitanicum. It is important to know the geographical distribution and potential introduction of H. marginatum into new areas, concerning its vector competence for CCHF virus (Válcarel et al., Reference Válcarel, González, González, Sánchez, Tercero, Elhachimi, Carbonell and Olmeda2020) and R. aeschlimannii (Beati et al. (Reference Beati, Meskini, Thiers and Raoult1997). The found male tested negative for all investigated pathogens. Nevertheless, the occurrence of H. marginatum must be considered a risk for public and animal health and should be monitored closely. Migratory birds play an important role in the epizootiology and epidemiology of ticks and tick-borne pathogens and have received increased attention in recent years (Chitimia-Dobler et al., Reference Chitimia-Dobler, Kurzrock, Molčányi, Rieß, Ute Mackenstedt and Nava2019a, Reference Chitimia-Dobler, Schaper, Rieß, Bitterwolf, Frangoulidis, Bestehorn, Springer, Oehme, Drehmann, Lindau, Mackenstedt, Strube and Dobler2019b; Grandi et al., Reference Grandi, Chitimia-Dobler, Choklikitumnuey, Strube, Springer, Albihna, Jaenson and Omazic2020). One prominent example is the introduction of H. marginatum and H. rufipes into Germany and the fact that 50% of the specimens carried R. aeschlimannii (Chitimia-Dobler et al., Reference Chitimia-Dobler, Kurzrock, Molčányi, Rieß, Ute Mackenstedt and Nava2019a, Reference Chitimia-Dobler, Schaper, Rieß, Bitterwolf, Frangoulidis, Bestehorn, Springer, Oehme, Drehmann, Lindau, Mackenstedt, Strube and Dobler2019b).
Two I. ricinus (a female and a nymph) were removed from humans. This tick species is very common parasite of humans, despite not being specifically reported from humans in Mallorca (Guglielmone and Robbins, Reference Guglielmone and Robbins2018). Both I. ricinus tested positive for Rickettsia spp., however, the species identification was not successful. Ixodes ricinus is both vector and reservoir for 2 Rickettsia species from the Spotted Fever Group, Rickettsia helvetica and Rickettsia monacensis (Simser et al., Reference Simser, Palmer, Fingerle, Wilske, Kurtti and Munderloh2002; Parola et al., Reference Parola, Paddock, Socolovschi, Labruna, Mediannikov, Kernif, Abdad, Stenos, Bitam, Fournier and Raoult2013). Interestingly, the research of Maitre et al. (Reference Maitre, Wu-Chuang, Mateos-Hernández, Foucault-Simonin, Moutailler, Paoli, Falchi, Diaz-Sánchez, Banović, Obregón and Cabezas-Cruz2022) showed that a R. helvetica infection in I. ricinus reduces significantly the diversity of the microbiota and the connectivity of the co-occurrence network.
In this study we report for the first time I. ventalloi feeding on a dog. The I. ventalloi female was collected feeding at the same time from that respective dog together with 8 Rh. sanguineus s.s. (3 males and 5 females). Ixodes ventalloi has been already reported from Spain (including Mallorca), Portugal, southern part of France and Italy, Cyprus and North Africa. Lagomorphs, carnivores, and rodents are hosts for all life stages (Estrada-Peña et al., Reference Estrada-Peña, Venzal and Nava2018). In the study of Estrada-Peña et al. (Reference Estrada-Peña, Venzal and Nava2018) the carnivores from which this tick species was collected are listed in detail, but it was never found on dogs so far. Additionally, to the mentioned birds in the study of Estrada-Peña et al. (Reference Estrada-Peña, Venzal and Nava2018) I. ventalloi nymphs were found on European robin (Erithacus rubecula) and Black redstart (Phoenicurus ochruros) in Ponza, Italy (Rollins et al., Reference Rollins, Schaper, Kahlhofer, Frangoulidis, Strauß, Cardinale, Springer, Strube, Bakkes, Becker and Chitimia-Dobler2021). A summary of I. ventalloi collections from different hosts was done by Santos and Santos-Silva (Reference Santos and Santos-Silva2018), which also outlined the fact that this species can be collected by dragging over the grassy ground. In our study the Rickettsia screening PCR was positive, but subsequent identification via sequencing failed due to the low amount of DNA (CT 36.9). Ixodes ventalloi was collected from a dog, which was concomitantly infested with 8 Rh. sanguineus s.s. adults. Only I. ventalloi and 1 Rh. sanguineus female tested positive for Rickettsia spp., which could be identified as R. massiliae only in Rh. sanguineus s.s.. This finding could indicate that the dog was not the source of infection, but the ticks had already acquired the infection during the larva or nymph stages. Several pathogens, including C. burnetii, Rickettsia spp., Anaplasma spp. and Borrelia spp. or protozoa, were detected in I. ventalloi collected from different animals, humans or from vegetation (Santos and Santos-Silva, Reference Santos and Santos-Silva2018).
Conclusion
Mallorca Island is a main tourist destination in the Mediterranean. The presence of R. massiliae on the island constitutes a risk for human infection and should be considered in clinical diagnostics. Also, the detection of H. marginatum poses a potential public health risk and the occurrence and distribution as well as the carrier status for certain pathogens, especially CCHF virus should be monitored closely. The detection of unusual tick species, e.g., H. lusitanicum, infesting humans shows that under specific conditions rare tick species may infest humans and therefore, also may serve as vectors of unusual pathogens to humans. The results emphasize specific risks associated with ticks and tick-borne pathogens on the Island of Mallorca and appeal for more intensive surveillance, and also for intensified vector control on pets.
Data availability
All the sequences were submitted in GenBank and are available for further studies.
Acknowledgments
We thank Ralf Petzold, editorial office of the Mallorca Zeitung, for the article published in August 2021 about ticks and which was based on the interview with M.B. We thank the German tourists and residents on the island of Mallorca for sending us ticks. We like to thank Dana Rüster for her technical assistance in the laboratory.
Author contributions
LCD identified the tick species and wrote the manuscript; LCD, GD and SS tested the ticks for Rickettsia including species identification, MB organized tick collection and wrote the first draft of the manuscript. HB tested the ticks for Francisella spp; KM tested the ticks for Coxiella burnetii; AO tested the ticks for Babesia and Anaplasma species. LM made the map. SW did the Rickettsia phylogenetic analysis; BJM did the tick phylogenetic analysis and submitted the sequences in GenBank. All authors read and approved the final version of the manuscript.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors
Competing interests
The authors declare there are no conflicts of interest.
Ethical standards
Not applicable