No CrossRef data available.
Article contents
Crystal structure and X-ray powder diffraction data of barium copper iodate Ba2Cu(IO3)6
Published online by Cambridge University Press: 20 July 2023
Abstract
X-ray powder diffraction data, unit-cell parameters, and space group for the barium copper iodate, Ba2Cu(IO3)6, are reported [a = 7.48540(15) Å, b = 7.51753(19) Å, c = 7.64259(17) Å, α = 98.8823(7)°, β = 95.0749(7)°, γ = 97.6297(7)°, V = 418.528(9) Å3, Z = 1, and space group P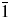 $\bar{1}$]. All measured lines are indexed and are consistent with the corresponding space group. The single-crystal diffraction data of Ba2Cu(IO3)6 are also reported [a = 7.493(3) Å, b = 7.521(6) Å, c = 7.644(5) Å, α = 98.855(18)°, β = 95.060(16)°, γ = 97.62(2)°, V = 419.3(5) Å3, Z = 1, and space group P
$\bar{1}$]. All measured lines are indexed and are consistent with the corresponding space group. The single-crystal diffraction data of Ba2Cu(IO3)6 are also reported [a = 7.493(3) Å, b = 7.521(6) Å, c = 7.644(5) Å, α = 98.855(18)°, β = 95.060(16)°, γ = 97.62(2)°, V = 419.3(5) Å3, Z = 1, and space group P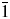 $\bar{1}$]. The crystal structure of Ba2Cu(IO3)6 features isolated [Cu(IO3)6]4− anionic clusters separated by Ba2+ cations. The experimental powder diffraction pattern matches well with the simulated pattern derived from the single crystal data.
$\bar{1}$]. The crystal structure of Ba2Cu(IO3)6 features isolated [Cu(IO3)6]4− anionic clusters separated by Ba2+ cations. The experimental powder diffraction pattern matches well with the simulated pattern derived from the single crystal data.
Information
- Type
- New Diffraction Data
- Information
- Copyright
- Copyright © The Author(s), 2023. Published by Cambridge University Press on behalf of International Centre for Diffraction Data


