Article contents
Powder diffraction study of Pd2HgSe3
Published online by Cambridge University Press: 06 November 2017
Abstract
The Pd2HgSe3 phase was synthetized from individual elements by the silica glass tube technique and its crystal structure has been refined by the Rietveld method. The Pd2HgSe3 phase crystalizes in P 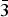 $\bar 3$ m1 space group with the unit-cell parameters a = 7.3096(2) Å, c = 5.2829(1) Å, V = 244.45(1) Å3, D c = 8.84 g/cm3, and Z = 2. In its layered crystal structure, the [PdSe6] octahedra share opposing Se–Se edges with adjacent [PdSe4] squares forming layers parallel with the (001) plane. The layers show AA type stacking along the c-axis. Hg atoms occupy the anti-cubooctahedral voids between two consecutive layers. Pd2HgSe3 is isostructural with Pt2HgSe3 and Pt4Tl2 X 6 (X = S, Se, or Te) phases. The structure can be viewed as a 2a.2a.c superstructure of PtSe2.
$\bar 3$ m1 space group with the unit-cell parameters a = 7.3096(2) Å, c = 5.2829(1) Å, V = 244.45(1) Å3, D c = 8.84 g/cm3, and Z = 2. In its layered crystal structure, the [PdSe6] octahedra share opposing Se–Se edges with adjacent [PdSe4] squares forming layers parallel with the (001) plane. The layers show AA type stacking along the c-axis. Hg atoms occupy the anti-cubooctahedral voids between two consecutive layers. Pd2HgSe3 is isostructural with Pt2HgSe3 and Pt4Tl2 X 6 (X = S, Se, or Te) phases. The structure can be viewed as a 2a.2a.c superstructure of PtSe2.
Information
- Type
- Technical Articles
- Information
- Copyright
- Copyright © International Centre for Diffraction Data 2017
References
- 7
- Cited by


