Published online by Cambridge University Press: 11 March 2015
X-ray structural characterization and X-ray reference powder patterns have been determined for two series of iron- and cobalt-containing layered compounds (Bax Sr1− x )2Co2Fe12O22 (x = 0.2, 0.4, 0.6, 0.8) and (Bax Sr1− x )Co2Fe16O27 (x = 0.2, 0.4, 0.6, 0.8). The (Bax Sr1− x )2Co2Fe12O22 series of compounds crystallized in the space group R 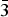 $\bar 3$ m (No. 166), with Z = 3. The structure is essentially that of the Y-type hexagonal ferrite, BaM 2+Fe6 3+O11. The lattice parameters range from a = 5.859 15(8) to 5.843 72(8) Å, and c = 43.4975(9) to 43.3516(9) Å for x = 0.2 to 0.8, respectively. The (Bax Sr1− x )Co2Fe16O27 series (W-type hexagonal ferrite) crystallized in the space group P63/mmc (No. 194) and Z = 2. The lattice parameters range from a = 5.902 05(12) to 5.8979(2) Å and c = 32.9002(10) to 32.8110(13) Å for x = 0.2 to 0.8. Results of measurements of the Seebeck coefficient and resistivity of these two sets of samples indicated that they are insulators. Powder X-ray diffraction patterns of these two series of compounds have been submitted to be included in the Powder Diffraction File.
$\bar 3$ m (No. 166), with Z = 3. The structure is essentially that of the Y-type hexagonal ferrite, BaM 2+Fe6 3+O11. The lattice parameters range from a = 5.859 15(8) to 5.843 72(8) Å, and c = 43.4975(9) to 43.3516(9) Å for x = 0.2 to 0.8, respectively. The (Bax Sr1− x )Co2Fe16O27 series (W-type hexagonal ferrite) crystallized in the space group P63/mmc (No. 194) and Z = 2. The lattice parameters range from a = 5.902 05(12) to 5.8979(2) Å and c = 32.9002(10) to 32.8110(13) Å for x = 0.2 to 0.8. Results of measurements of the Seebeck coefficient and resistivity of these two sets of samples indicated that they are insulators. Powder X-ray diffraction patterns of these two series of compounds have been submitted to be included in the Powder Diffraction File.