Article contents
X-ray powder reference patterns of the Fe(Sb2+ x Te1− x ) skutterudites for thermoelectric applications
Published online by Cambridge University Press: 07 May 2014
Abstract
The crystal structure and powder X-ray diffraction (XRD) patterns for three skutterudite samples, Fe(Sb2+ x Te1− x ), x = 0.05, 0.10, 0.20, have been determined. These compounds crystallize in the cubic space group 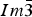 $Im\bar 3$ . Te was found to randomly substitute in the Sb site. Because of the fact the covalent radius of Sb is greater than that of Te, a trend of increasing lattice parameter has been observed as the x value in Fe(Sb2+ x Te1− x ) increases [cell parameters range from 9.10432(4) to 9.11120(3) Å for x = 0.0 to 0.2, respectively]. The Fe–Sb/Te bond distance also increases progressively [from 2.5358(4) to 2.5388(4) Å] as the Te content decreases. While average Sb/Te–Sb/Te distances in the four-membered rings are similar in these three compounds, the average Sb/Te–Sb/Te edge distances in the octahedral framework increase progressively from 3.5845(12) to 3.5900(13) Å. Reference XRD patterns of these three phases have been prepared to be included in the Powder Diffraction File (PDF).
$Im\bar 3$ . Te was found to randomly substitute in the Sb site. Because of the fact the covalent radius of Sb is greater than that of Te, a trend of increasing lattice parameter has been observed as the x value in Fe(Sb2+ x Te1− x ) increases [cell parameters range from 9.10432(4) to 9.11120(3) Å for x = 0.0 to 0.2, respectively]. The Fe–Sb/Te bond distance also increases progressively [from 2.5358(4) to 2.5388(4) Å] as the Te content decreases. While average Sb/Te–Sb/Te distances in the four-membered rings are similar in these three compounds, the average Sb/Te–Sb/Te edge distances in the octahedral framework increase progressively from 3.5845(12) to 3.5900(13) Å. Reference XRD patterns of these three phases have been prepared to be included in the Powder Diffraction File (PDF).
Keywords
Information
- Type
- Technical Articles
- Information
- Copyright
- Copyright © International Centre for Diffraction Data 2014
References
- 3
- Cited by

