Introduction
The coronavirus disease 2019 (COVID-19) pandemic has created challenges in maintaining the safety of Emergency Medical Services (EMS) providers while also allowing the provision of maximally aggressive respiratory therapies for patient care. Many patients transported by EMS require respiratory therapies, including nebulized medications, continuous positive airway pressure (CPAP) non-invasive ventilation, or heated high-flow nasal cannula oxygen therapy (HHFNC). These treatments are considered aerosol-generating procedures (AGPs) and thus have the potential to increase spread of infectious viral agents, such as COVID-19. Reference Pasnick, Carlos, Dela Cruz, Gross, Garrison and Jamil1,Reference Cournoyer, Grand’Maison and Lonergan2 Use of such therapies places EMS providers at particular risk given the small physical confines of an ambulance or helicopter and lack of ventilation systems designed to mitigate aerosolization of infectious agents. Consequently, many EMS agencies have limited the use of these AGPs despite the potential patient benefit.
Prior data indicate EMS providers can be actively infected during transport of COVID-19 patients. At the start of the pandemic in New York City (New York USA), there was a significant increase in 9-1-1 calls for respiratory complaints, cardiovascular complaints, and cardiopulmonary arrest. There was also an increase in high-acuity life-threatening calls resulting in increased exposure of EMS staff to aerosolizing procedures and augmented risk of contracting COVID-19. Reference Prezant, Lancet and Zeig-Owens3 Data from King County, Washington (USA) demonstrated that 16.3% (182 of 1,115) of encounters for COVID-19 had one or more aerosolizing procedures performed by EMS. Incidence of COVID-19 in EMS personnel during that period was 0.57 infections/10,000 person-days. Reference Brown, Schwarcz and Counts4 Out of 274 EMS encounters with COVID-19-confirmed patients, there were 151 person-exposures among 129 EMS providers, resulting in 981 quarantine days with a 0.4% positive test rate. Reference Murphy, Barnard and Drucker5 Additionally, early in the pandemic testing of 3,326 first responders in Arizona (USA), 1.5% tested positive for COVID-19. Reference Shukla, Lau and Towns6 Heinzerling, et al found that 67% of medical personnel who assisted COVID-19-infected patients undergoing nebulization developed infection themselves. The authors noted that since the virus has spread within the hospital environment, it should be assumed that chances of getting infected are significantly higher in the limited space of an ambulance. Reference Heinzerling, Stuckey and Scheuer7 The increasing exposure to respiratory complaints in the EMS field necessitates updated personal protective equipment (PPE) to minimize prehospital exposures, especially during AGPs. The risk of COVID-19 infection and/or mandatory quarantine following a significant exposure can be mitigated by improving prehospital PPE.
During the COVID-19 pandemic, the University of Michigan Department of Emergency Medicine (Ann Arbor, Michigan USA), Michigan Center for Integrative Research in Critical Care (M-CIRCC; Ann Arbor, Michigan USA), and the College of Engineering (Ann Arbor, Michigan USA) collaborated with a local manufacturing company to develop a device capable of mitigating these risks. The device (AerosolVE Helmet; Inspire Rx LLC; Ann Arbor, Michigan USA) consists of a helmet equipped with an air purifying filter, based on the common powered air purifying respirator (PAPR), but is uniquely re-engineered to reverse air flow. This design allows room air to be pulled into the helmet which is then passed through a high efficiency particulate air (HEPA) filter before being released back into the ambient environment (Figure 1). This creates a negative pressure environment within the helmet, thus infectious particles produced and aerosolized by the patient and/or any AGP are contained within the helmet and filtered before release into ambient air. The device is designed to be able to accommodate use of HHFNC, CPAP/BiPAP, and nebulized aerosol therapies. The secured face shield can be opened for emergency access to the patient’s face. The lower portion (bib) of the helmet is loosely fitted to allow large volumes of room air to be pulled through the helmet and to allow provider and patient access to the patient’s face by the patient or provider without having to open the face shield. The HEPA filter is integral to the motor (fan) and is designed to pull air through the helmet at 220 liters/minute. When compared to current recommendations for hospital negative pressure rooms of at least 12 air exchanges per hour, 8 the helmet produces 840 air exchanges per hour, or 70-times more air exchanges than current hospital room recommendations. The helmet, shroud, and hose are designed to be disposable. The motor and filter are designed to be reusable with filter life of 12 months or until the filter alarm activates. An alarm exists to detect when flow decreases to 170 liters/minute.
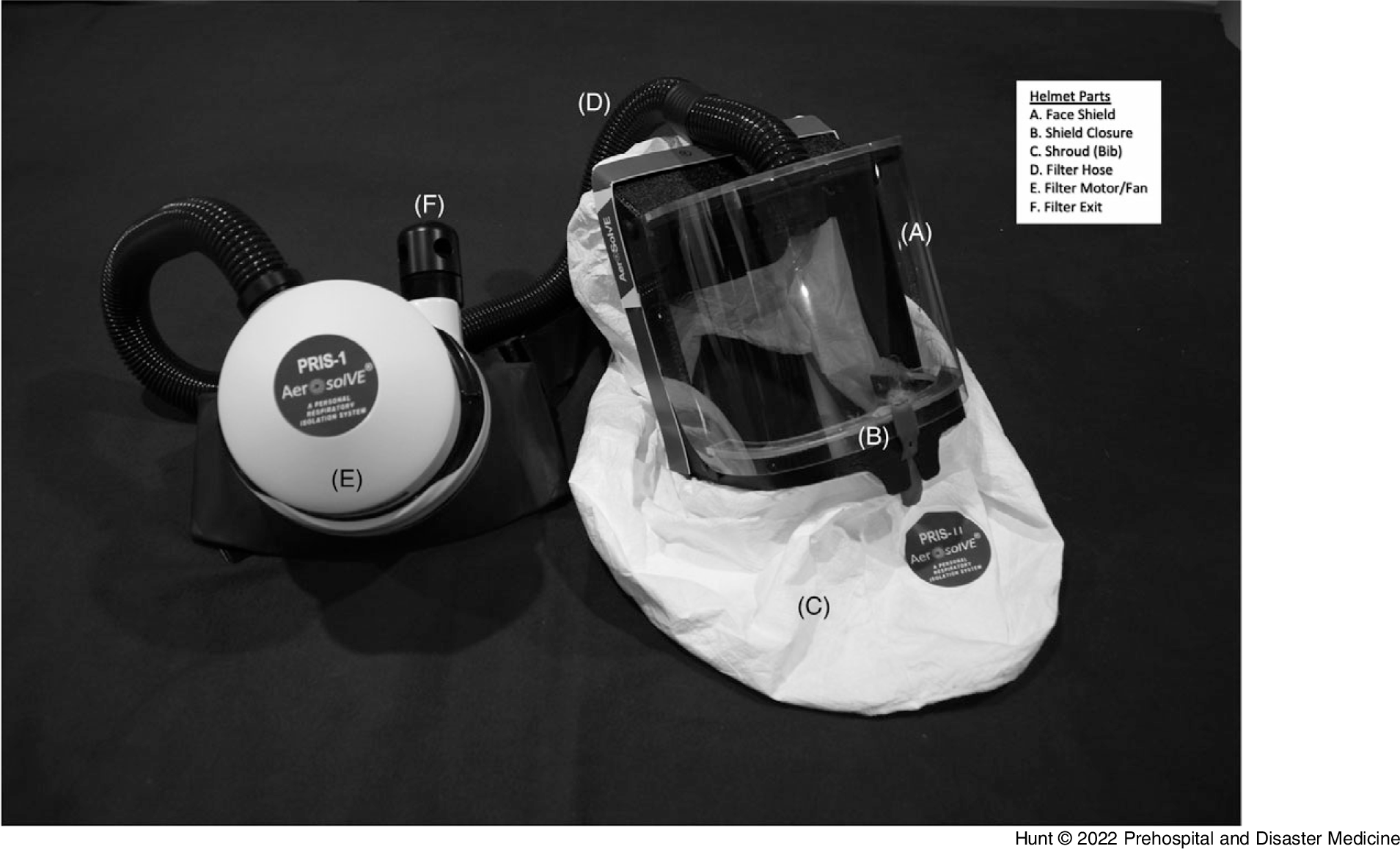
Figure 1. AerosolVE Helmet.
Note: The AerosolVE helmet is a negative pressure device modeled after a PAPR. The clear face shield (A) allows for good visibility and can be opened by the red tab (B) for immediate access to the patient’s face. The filter and motor (E) can be held or worn by a belt.
Abbreviation: PAPR, powered air purifying respirator.
The helmet and a similar negative pressure tent were initially developed for use in hospitals. Previous publications have demonstrated their efficacy in reducing air particle counts, likely improving the safety of providers caring for patients with communicable respiratory illnesses. Reference Haas, Bassin and Kota9–Reference Haas and Bassin12
The prehospital transport environment varies significantly from a hospital room, and environments between transport vehicle platforms (ie, jet versus helicopter versus ground ambulance) are also not equivocal. Thus, testing the device in each different transport platform is integral to ensure efficacy and safety.
The objective of this study was to test the effects of the negative pressure helmet device on air particle counts in healthy volunteers undergoing a variety of AGPs in simulated prehospital settings. It is hypothesized the AerosolVE helmet would prevent increases in air particle counts in the ambient cabin air.
Methods
This was an open-label study of the efficacy of the AerosolVE helmet and filtration system. Fifteen healthy volunteers were enrolled, twelve men and three women, and were distributed amongst three transport platforms: a LearJet 75 configured for medical transport, a Eurocopter EC155 medical helicopter, and a Ford E450 modular ambulance. While not a requirement, all volunteers had been fully vaccinated for COVID-19 prior to participation. Each participant was screened for signs and symptoms of COVID-19 or other respiratory illness prior to enrollment. Sodium chloride particles, generated by a TSI 8026 particle generator (TSI Inc; Shoreview, Minnesota USA), were emitted near the subjects’ mouths to simulate bioaerosol generation associated with viral respiratory infections and to maximally test the system. A TSI 3007 condensation particle counter (TSI Inc; Shoreview, Minnesota USA) was used to detect and quantify air particle counts at different locations, including particle leakage from the helmet and particle concentration inside the helmet. The device is capable of detecting and quantifying particles in the range of 0.01 to >1.0µm which would include the size of the COVID-19 virus (0.1µm). Reference Bar-On, Flamholz, Phillips and Milo13 This study was approved by the University of Michigan Institutional Review Board and required written consent (protocol # HUM00192223).
Baseline ambient air particle counts were obtained in the closed cabin of the selected vehicle without oxygen (O2) devices on and without active particle generation. This was to determine the ambient particle counts present related to dust and other environmental particles. The participant was then seated in the standard patient transport position and the helmet was placed on the participant. The helmet was in the standard configuration as provided from the manufacturer. The particle generator was inserted into the helmet from underneath the helmet’s lower bib and turned on. This was meant to simulate active expiration and aerosolization of infectious particles from a patient. Particle counts were obtained in numerous locations around the helmet and in various locations about the transport platform’s cabin (Figure 2A-C). Once counts had been obtained without use of an O2 device, the procedure was repeated with the participant wearing a non-rebreather mask (NRB) at 15L/minute O2 flow, a CPAP mask with pressure of 5cm of water, and a high-flow nasal cannula (HFNC) with 30L/minute of flow. These tests were performed in succession without any break in the cabin to ensure no inadvertent disruption of ambient particle levels (eg, dust in the outside air entering the cabin that would affect interpretation of subsequent particle counts). The participant had to briefly remove the helmet for each change of O2 delivery device. At each testing location, ten particle counts were obtained and the mean recorded. This was to account for respiratory variation and other environmental factors that may cause small shifts in particle counts at that location (eg, participants breathing in particles resulting in a momentary reduction in counts inside the helmet). Primary outcome was the difference in ambient particle counts and counts close to the helmet compared to counts inside the helmet with the filter motor on.
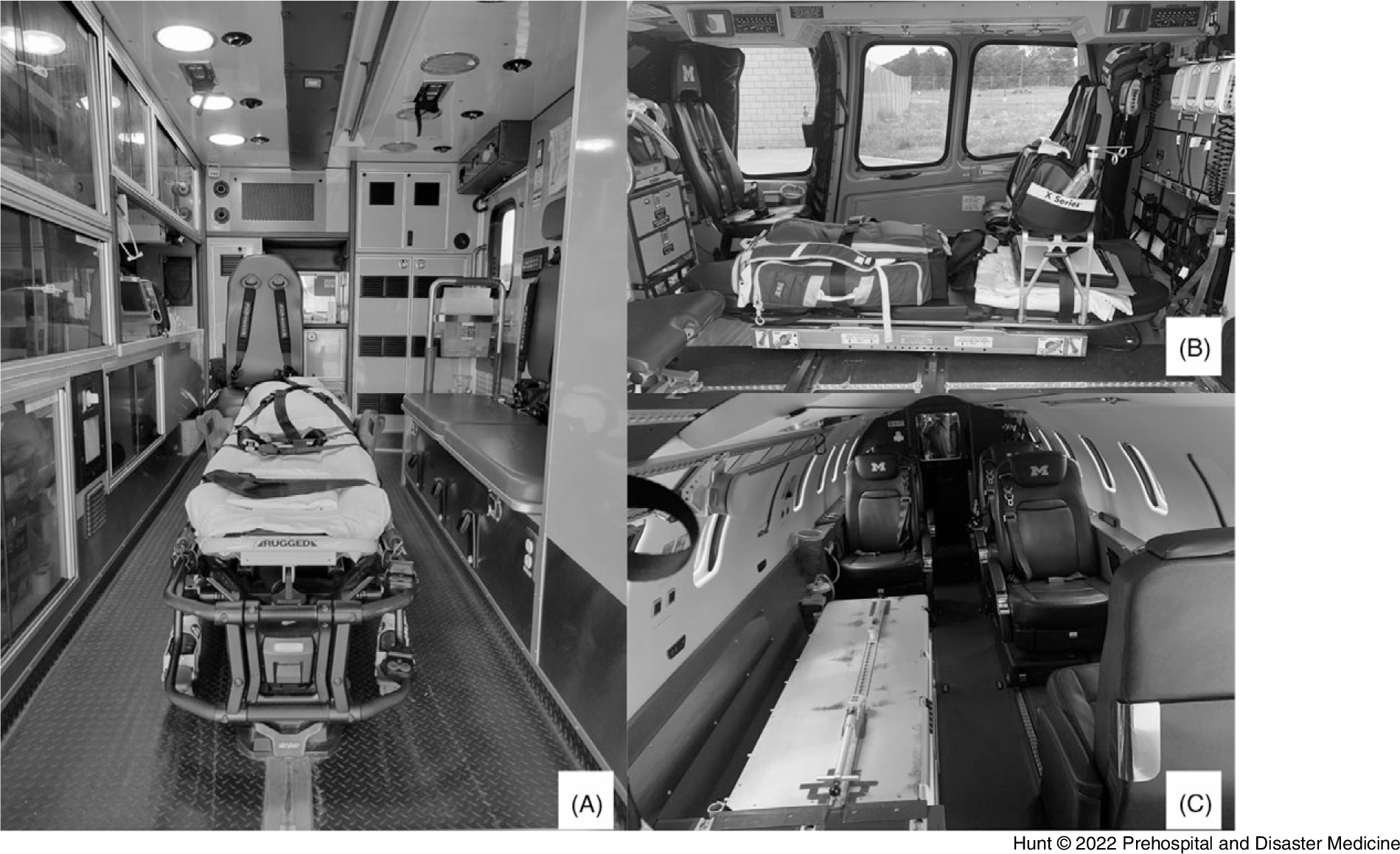
Figure 2. EMS Transport Platforms.
Note: Patient compartment view of each transport platform. Driver/pilot compartment not visible. A) Ground Ambulance. B) Medical Helicopter. C) Medical Jet.
Abbreviation: EMS, Emergency Medical Services.
Statistical Analysis
Data are presented as mean (standard deviation). One way ANOVA was used to compare the primary outcome (particle count inside helmet with filter on) to particle count in the ambient air and the environment close to the helmet. Statistical significance was considered as α=0.05. All data were analyzed using PRISM 9 (GraphPad Software; San Diego, California USA).
Results
Table 1 and Figure 3 present the mean air particle counts with and without the use of each O2 delivery device. Without the helmet fan on, the particle generator alone and all AGPs produced particle counts inside the helmet significantly higher than ambient particle counts. With the helmet fan on, particle counts near the helmet showed no significant elevation compared to baseline ambient particle counts during the use of the particle generator alone or with use of any of the AGPs. Additionally, particle counts were significantly lower in the helmet while the motor was on compared to when the motor was off for each O2 delivery device. Table 2 demonstrates that particle counts taken at the fan exit (post-HEPA filter) were reduced to nearly zero compared to counts inside the helmet. Interestingly, with the face shield up (which could be necessary for emergent access), there was no significant difference in the particle counts in the ambient air in the ambulance or the helicopter with all simulated AGPs. In the jet, likely because the ambient particle counts were so low at baseline, there was a significant difference in particle counts with the face shield up compared to ambient with the NRB and HFNC. However, cabin particle counts remained unchanged. No significant difference was seen in this platform with CPAP.
Table 1. Mean (SD) Particle Counts for Each Transport Platform and AGP
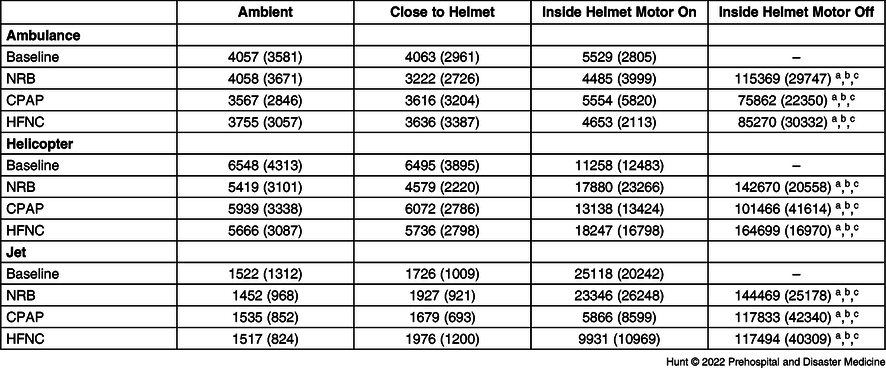
Note: Mean (standard deviation) particle counts for each transport platform and each AGP tested around the cabin, close to the helmet, inside the helmet with the motor on and off.
Abbreviations: AGP, aerosol-generating procedure; NRB, non-rebreather mask; CPAP, continuous positive airway pressure; HFNC, high-flow nasal cannula.
a Denotes significant difference between “Inside Helmet Motor off” and “Ambient” (P<.0001).
b Denotes significant difference between “Inside Helmet Motor off” and “Close to Helmet” (P<.0001).
c Denotes significant difference between “Inside Helmet Motor off” and “Inside Helmet Motor on” (P<.0001).

Figure 3. Mean Particle Counts by Platform and AGP.
Note: Mean particle counts by EMS transport platform and by AGP. Scale differs by platform.
Abbreviations: AGP, aerosol-generating procedure; EMS, Emergency Medical Services; NRB, non-rebreather mask; CPAP, continuous positive airway pressure; HFNC, high-flow nasal cannula.
Table 2. Mean Particle Counts at Filter Exit

Note: Mean particle counts (95% confidence interval) at the filter exit to the patient compartment with the filter motor and the particle generator on.
Abbreviations: O2, oxygen; NRB, non-rebreather mask; CPAP, continuous positive airway pressure; HFNC, high-flow nasal cannula.
Discussion
The results of this analysis demonstrated no significant increase in the ambient particle counts near the helmet nor in the cabin. This held true for each transport platform and for each O2 delivery device. The particle count in the helmet during the AGP without the motor on was used as a surrogate for a close exposure of an EMS provider to the patient in the absence of the helmet device.
By containing simulated infectious particles, the AerosolVE Helmet appears to reduce the risk of aerosolized particles being released into the patient compartment and thus may reduce risks to the patient attendant(s). Interestingly, a gradual decline in the ambient air particle counts was noticed during the serial tests within the cabins of the various transport vehicles. This likely is a result of the entraining of environmental air (and particles) into the helmet and subsequent filtration. Table 2 shows the mean particle counts at the filter exit with an average of 0.75 particles detected despite significantly higher particle levels in the helmet, indicating excellent filtration efficiency. Additionally, even with the face shield open, no significant change in ambient particle counts was observed in most settings, indicating that the powerful draw of air into the helmet prevents loss of particles into the patient compartment. These results show this device not only filters air but also effectively contains simulated infectious particles, preventing disbursement and potentially reducing risk to providers during AGPs.
The testing protocol specifically excluded the use of a nebulizer, a common respiratory care device used in the treatment of respiratory distress in the prehospital setting. Previous testing had shown similar generation of aerosolized particles from the particle generator and the nebulizer. Reference Haas, Bassin and Kota9 The authors chose to use only the generator for simplicity. They also chose not to test participants in various transport positions. Given the dynamics of the AerosolVE device (ie, pulling environmental air into the helmet), any increase in gaps under the shroud would allow more air flow and would likely improve the air exchange; thus, various positions would be unlikely to have a significant negative impact on results. Lastly, the authors elected not to modulate the air flow (ie, heat, ventilation, air conditioning [HVAC]) in the patient compartment of the ambulance as they wanted to specifically test the device in a “worst-case scenario” (no air exchange present). Any HVAC use would only serve to improve the ambient air quality. In addition, for the helicopter and jet, the authors would not have been able to test the device with HVAC running as this requires the aircraft to be running. The baseline ambient particle counts, as a result from dust or exhaust, were too high to measure any difference generated by the particle generator.
Despite the protracted nature of the current COVID-19 pandemic, there are no negative pressure or containment technologies approved for prehospital patient transport use. While a number of negative pressure/isolation “tents” have gained Emergency Use Authorization (EUA) for use within hospitals, none to the authors’ knowledge are approved for prehospital use. Furthermore, some of these rely on regular hospital wall suction to produce a negative pressure within the tent. The efficacy of this level of negative pressure and flow to reduce AGP increases in ambient particle counts back to baseline has not been reported.
Due to the risk of contamination, many EMS systems have reduced their transport of patients with known or highly suspected COVID-19 infections or modified protocols to reduce risk to providers (eg, using supraglottic airways rather than oral endotracheal intubation, metered-dose inhalers rather than nebulizer treatment). While the ability was tested of the AerosolVE Helmet to reduce AGP particle counts to baseline, the device can likely provide benefit during transport of any patient with unknown COVID-19 status, regardless of symptoms, as a means to reduce EMS provider exposure and allow for maximal patient therapy. It could also allow transport of multiple infected patients without increasing the risks of infection to EMS providers.
Similar challenges exist in the transport of military patients with COVID-19 as have been described in the civilian EMS sector, and the likelihood of a multiple-patient transport environment is higher in the military sector. Negative pressure transport conexes have been designed to transport multiple infected individuals. Reference Correll14 Use of devices like the helmet described in this report may offer additional options.
Limitations
This study was conducted on healthy volunteers who were breathing normally (not coughing) with simulated infectious droplets dispersed within the helmet. As this study was to determine efficacy of the device’s ability to prevent increases in cabin particle counts and particle counts near the subject in the EMS transport environment, it utilized a small convenience sample of volunteers rather than a large population. Given the efficiency of the device in its ability to filter particles, it is unlikely that additional test participants would have made a significant difference in the results obtained. Future studies will be required examining the device and particle counts with real patients being transported and undergoing AGPs.
As mentioned above, testing was not possible in the helicopter or jet with HVAC cabin air modulation due to high levels of environmental particles with the aircraft running. It is believed this creates a “worst-case scenario” in that no cabin air is exchanged, however, it is difficult to say with certainty how HVAC would have impacted results.
There were no reported or observed safety events during the use of the AerosolVE helmet. Although the study originally intended to deliver CPAP pressure at 10cm of water and HFNC flow at 60L/minute, due to participant discomfort, these were reduced to 5cm of water and 30L/minute, respectively.
Conclusion
Given the risk posed to prehospital medical providers by communicable respiratory diseases, development of novel devices to improve safety for these caretakers while still enabling use of respiratory therapies is of paramount importance. This holds true not only in the setting of a pandemic, but also during traditional “respiratory virus season” when a healthy workforce is critical to the function of the prehospital system. The AerosolVE Helmet demonstrated efficacy in creating a negative pressure environment around simulated patients and provided significant filtration of simulated respiratory droplets, thus making the confined space of various EMS transport vehicle types potentially safer for EMS personnel.
Conflicts of interest/funding
Kevin Ward has intellectual property regarding the AerosolVE Helmet through the University of Michigan which has been licensed to Inspire Rx LLC. Kevin Ward, Ben Bassin, and Nathan Haas have equity in Inspire Rx LLC. Ben Bassin is the Chief Medical Officer for Inspire Rx LLC. The remaining authors have no competing interests.








