Introduction
Globally, the push for universal primary health care (PHC) has been slowly advancing with the Alma-Ata Conference identifying in 1978 the need for all governments to develop, implement and sustain PHC as part of their responsibility for the health of their populations (WHO, 1978). Thirty years later, stark inequalities persisted in access, quality and outcomes of health care leading to a renewed interest in the advancement of PHC (WHO, 2008). PHC became the approach of choice for reaching universal health coverage and addressing the social determinants of health. The Sustainable Development Goals (SDGs) continued this push specifically through the third SDG (UN, 2015). To meet these goals, countries have reexamined the processes used for providing access, including restructuring PHC services, and the utilization of community health workers (CHWs) has appeared as a viable strategy (Cosgrove et al., Reference Cosgrove, Monroy, Jenkins, Castillo, Williams, Parris, Tran, Rivera and Brownstein2014; Perry et al., Reference Perry, Zulliger and Rogers2014). Therefore, the Brazilian family health model is an important reference, with successes and difficulties experienced providing lessons that can be applied in multiple settings, although emphasizing that services must be planned according to local needs (Johnson et al., Reference Johnson, Noyes, Haines, Thomas, Stockport, Ribas and Harris2013).
The Brazilian Federal Constitution of 1988 guaranteed health as a right and the obligation of the State to establish policies for protecting this right, thus preparing the foundation for the creation of the largest universal public health system: the Brazilian Unified Health System [Sistema Único de Saúde (SUS)] (Bastos et al., Reference Bastos, Menzies, Hone, Dehghani and Trajman2017). SUS incorporates both public and private organizations and follows the organizational principle of decentralization. The Family Health Strategy (FHS) is the preferred method of providing PHC, aiming to reorient health services to focus on people instead of diseases and contributing the progress toward universal access. This strategy is a community-based approach where health teams are responsible for the health of residents of defined territories. The basic family health (FH) teams consist of a physician (general practitioner or FH specialist), nurse, nurse technician and CHWs and are responsible for a maximum of 4000 residents, with each CHW responsible for up to 750 people (Brazil, 2012). Perry et al. (Reference Perry, Zulliger and Rogers2014) highlight the utilization and roles of CHW in low-, middle- and high-income countries. In this strategy, CHWs are paid members of the team who support PHC principally through home visits for establishing rapport with the community, registering users and accompanying and promoting the health of covered families (Brazil, 2012).
As part of SUS’ founding principles of equity and universal coverage, areas of greater vulnerability are given priority when establishing FH clinics and coverage areas, although this strategy should eventually cover all Brazilians. Due to the profiles of the territories, in addition to health promotion, FH teams are responsible for the prevention of both chronic and infectious diseases in the covered areas. This strategy currently covers 63.1% of the Brazilian population, 44.5% of the inhabitants of the capital city of Rio de Janeiro, Rio de Janeiro State (RJ) and 100% of the residents of the Complexo de Manguinhos (CM), a dynamic agglomeration of favelas, or slums, in the Northern Zone of Rio de Janeiro, RJ (Brazil, 2015; Teais-Escola Manguinhos, n.d.). The expansion of the FHS both territorially and in services offered has been a result of the identification of qualitative and quantitative improvements through the implementation of this strategy, such as increased user satisfaction, the reduction of infant mortality, decreased hospitalization rates and improvements of other health indicators (Bastos et al., Reference Bastos, Menzies, Hone, Dehghani and Trajman2017; Macinko and Harris, Reference Macinko and Harris2015).
In the Global South, health needs are complex and community profiles present morbidity from a combination of external injuries and chronic and communicable diseases. Tremendous inequities persist and neglected diseases still perpetuate a cycle of poverty and disease in vulnerable communities (Araújo-Jorge, Reference Araújo-Jorge2011). Although the etiologic agents, means of transmission and low-cost prophylactic measures for addressing intestinal parasitic infections (IPIs) are well established, these infections continue to inhibit cognitive function and academic performance with an estimated disability-adjusted life years, or years of healthy life lost, of 560 840 in the Americas (Rey, Reference Rey2008; WHO, 2014). Despite universal coverage in the CM, a 29.4% prevalence of IPIs was identified in this territory (Ignacio et al., Reference Ignacio, Silva, Handam, Alencar, Sotero-Martins, Barata and Moraes Neto2017).
The continued interference of intestinal parasites in human health highlights the limits of the management of IPIs in many settings. When specific interventions for controlling IPIs in endemic communities are utilized, they often focus on preventive chemotherapy with albendazole or mebendazole among school-age children; however, this form of control only addresses soil-transmitted helminths, ignoring the role of protozoa, and may lead to rapid reinfection (WHO, 2006; Strunz et al., Reference Strunz, Addiss, Stocks, Ogden, Utzinger and Freeman2014). Some programs directly targeting IPIs utilize more complex combinations of preventive chemotherapy, hygiene and changes in sanitation; yet, in many areas, specific programs are not implemented and IPIs are predominantly managed through PHC (Strunz et al., Reference Strunz, Addiss, Stocks, Ogden, Utzinger and Freeman2014). Considering the role of PHC in providing territorialized health promotion, disease prevention, treatment and health education, the control of neglected diseases of poverty takes place in the context of increasing complexity and diversity of health needs. Therefore, this study sought to analyze how IPIs are managed in the FHS covering this urban territory.
Methods
Studied area and population
The CM is an agglomeration of urban slums in northern Rio de Janeiro, RJ, Brazil of 2.62 km2. Like most urban slums, or favelas, the CM developed as a sacrifice zone where low-income workers displaced from the city center and migrants looking for employment opportunities established their homes (Porto and Martinez-Alier, Reference Porto and Martinez-Alier2007; Porto et al., Reference Porto, Cunha, Pivetta, Zancan and Freitas2015). Currently this area is characterized by lack of enforcement of public policy, violence associated with drug trafficking, overcrowding in improvised homes, inadequate sanitary conditions, periodic flooding and continued disorganized growth (Lima and Barros, Reference Lima and Barros2010; Porto et al., Reference Porto, Cunha, Pivetta, Zancan and Freitas2015; Teais-Escola Manguinhos, n.d.).
A total of 13 FH teams from two PHC clinics care for the 38 461 residents registered with the FHS (Teais-Escola Manguinhos, n.d.). With all planned vacancies of the basic FH team filled in the CM, there are 117 FH basic team members, of which 13 are physicians, resulting in an average of one FH doctor for every 2960 registered inhabitants, or ~0.34 physician density per 1000 population. The managers of the clinics group the slums of the CM into five pre-existing major areas (MA) based on territory characteristics for research and planning. A description of the characteristics of the MAs is available in Ignacio et al. (Reference Ignacio, Silva, Handam, Alencar, Sotero-Martins, Barata and Moraes Neto2017).
Data collection
To understand the management of IPIs, data collection included PHC providers and patients during two simultaneous, major phases: at the clinics with the FH team members and in the homes with residents of the CM. Both of the clinics in the territory were included in the study.
The knowledge, attitude and practices (KAP) surveys applied were adapted from Mello et al. (Reference Mello, Pripas, Fucci, Santoro and Pedrazzani1988) and Moraes Neto et al. (Reference Moraes Neto, Pereira, Alencar, Souza-Júnior, Dias, Fonseca, Santos and Almeida2010). Health providers answered a KAP survey that included four parts: (1) professional characteristics; (2) IPIs; (3) clinical approach and (4) socioenvironmental approach. Both open and closed-ended questions were utilized. Additional health providers (ie, not essential members of the basic FH team) and FH providers without a fixed territory (ie, those with mobile units responsible for accessing the homeless population) were not included in this study; yet, they assisted in the pre-test and development of the KAP surveys. All providers of the basic FH teams were invited to participate in the study. Those professionals who were on vacation or leave of absence during at least five visits for questionnaire application were also excluded from the study. Participating health professionals received the survey and answered the questions independently (self-application).
Residents answered a shortened KAP survey which included questions on intestinal parasites, transmission and prevention. The shortened KAP was also pre-tested. To obtain the KAP of the residents of the CM and the language used by these residents regarding IPIs, the reduced KAP survey was applied to household representatives during home visits as part of a larger study regarding prevalence of IPIs in the CM where sample size was determined considering an estimated prevalence of 20%, a sample guarantee of 5%, a design effect of 1, and a 95% confidence interval. Systematic sampling was utilized respecting the proportions of the distribution of the MA. To be eligible to answer the KAP survey, at least one adult (⩾18 years) capable of understanding the terms of the study and providing consent by signing or thumb printing the Term of Free and Informed Consent had to be home during the visits. With residents, the survey was applied verbally with a trained interviewer who wrote down the answers in their entirety.
Data analysis
Answer categories were established based on the appearance of themes in answers provided by both the providers and the residents in order to quantify the answers (Minayo, Reference Minayo2010). Coding into these categories was done by the first author, who also participated in development and application of the KAP surveys, to guarantee coding consistency. An answer guide (Figure 1) was developed from the literature for classifying the answers as correct, partially correct or incorrect (Rey, Reference Rey2008; Gonçalves and Gonçalves, Reference Gonçalves and Gonçalves2013). Relevant field observations were also taken during and/or promptly after questionnaire application to provide a better understanding of the context of the answers.
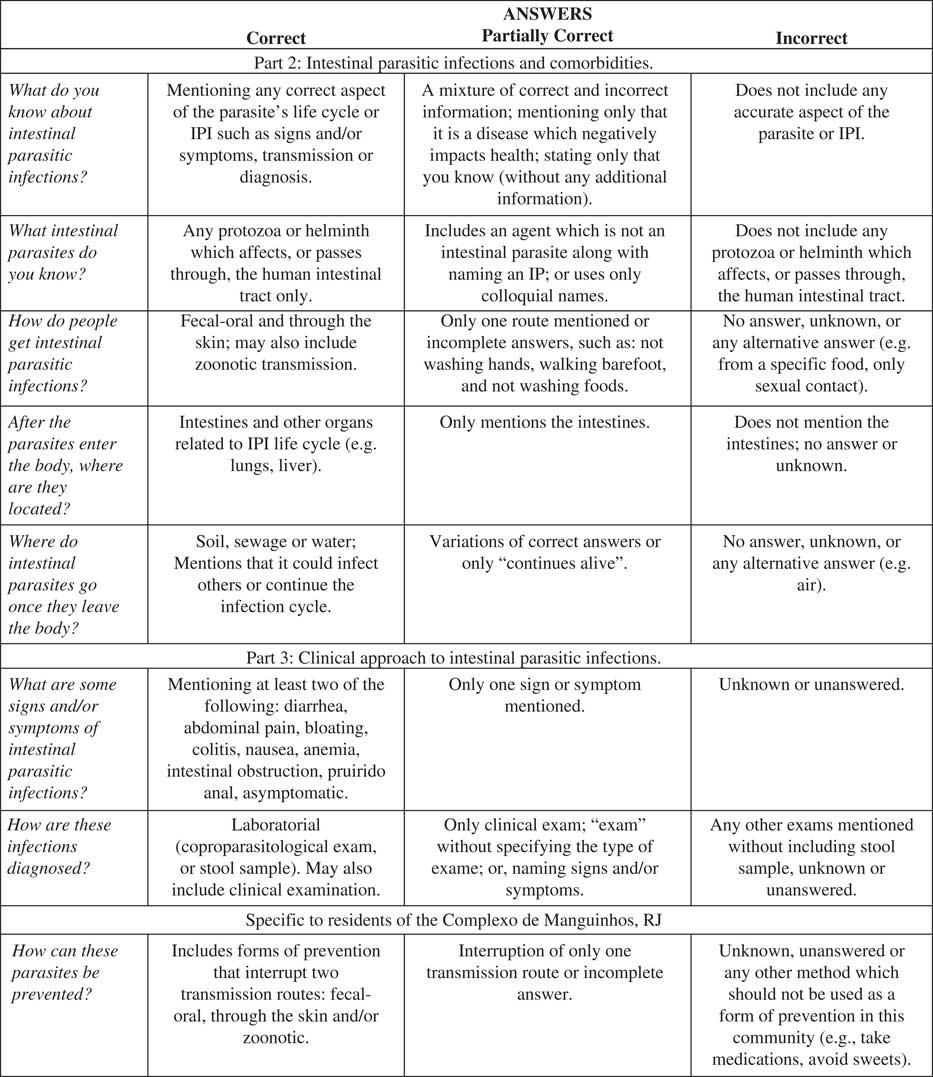
Figure 1 Answer guide developed for categorizing answers to the knowledge, attitudes and practices survey as correct, partially correct or incorrect.
Results
A total of 571 households, distributed proportionally throughout the five MA and 58 (49.6%) of the essential FH team members of the CM participated in the study. Three of the essential positions on the health teams (2.6%) were vacant, 11 (9.4%) of the professionals were on vacation or leave of absence, and 45 (38.5%) refused to participate. Participation of the providers was statistically associated with the MA (P=0.008), with MA1 having the greatest participation and MA5 the least. MA1 distinguishes itself from the rest of the CM due to a higher socioeconomic level, better living conditions and a self-perceived distance from the other communities more stereotypical of Brazilian favelas. The chance of providers participating in the study was also associated with the clinic where the provider was employed (OR, 2.87; 95% CI, 1.36–6.09; P=0.005). The professionals presented the following characteristics: 53.4% were residents of the territory, 72.4% were female, and an average age of 36.8 years (standard deviation±10.0 years). Among the providers, residence in the CM was statistically associated with profession (P=0.000), with only CHW and one nurse technician residing in the CM; however, some CHW did not want to answer (18.6%) or did not reside (11.6%) in the territory. Every essential professional category participated: 30.8% of the physicians, 46.2% of nurses, 38.5% of nurse technicians and 55.1% of CHW. Although 86.2% reported knowing about IPIs, only 12.1% claimed to have received any training or job preparation at any moment which included IPIs (P=0.02). All who reported receiving information regarding IPIs, except the physician, participated in previous editions of a community training program organized by the research team.
Of the household representatives, 82.7% were female, 64.5% were employed and the average age was 49 years (standard deviation±17.3 years) with 16.3% of the representatives being 65 years or older. The educational attainment of the representatives was as follows: 5.0% were illiterate, 54.9% had an incomplete elementary education, 10.2% only completed elementary school, 8.9% had some high school education, 17.9% had a high school diploma, 2.0% had attended but not graduated from a college/university, and 1.1% held a college/university degree.
Table 1 shows the answer categories and their frequencies regarding knowledge of the residents and providers. When residents were asked to provide any information known about intestinal parasites or IPIs (general knowledge), the most commonly repeated answer was ‘worms.’ Some of these answers were accompanied by pauses or tag questions, suggesting insecurity; yet, others were accompanied by descriptions of helminths’ appearances. Signs and/or symptoms of infection or transmission source also appeared. The answers suggested a familiarity with Enterobius vermicularis and Ascaris lumbricoides.
Table 1 Categorization of the family health team primary health care providers’ and resident’s knowledge regarding intestinal parasitic infections
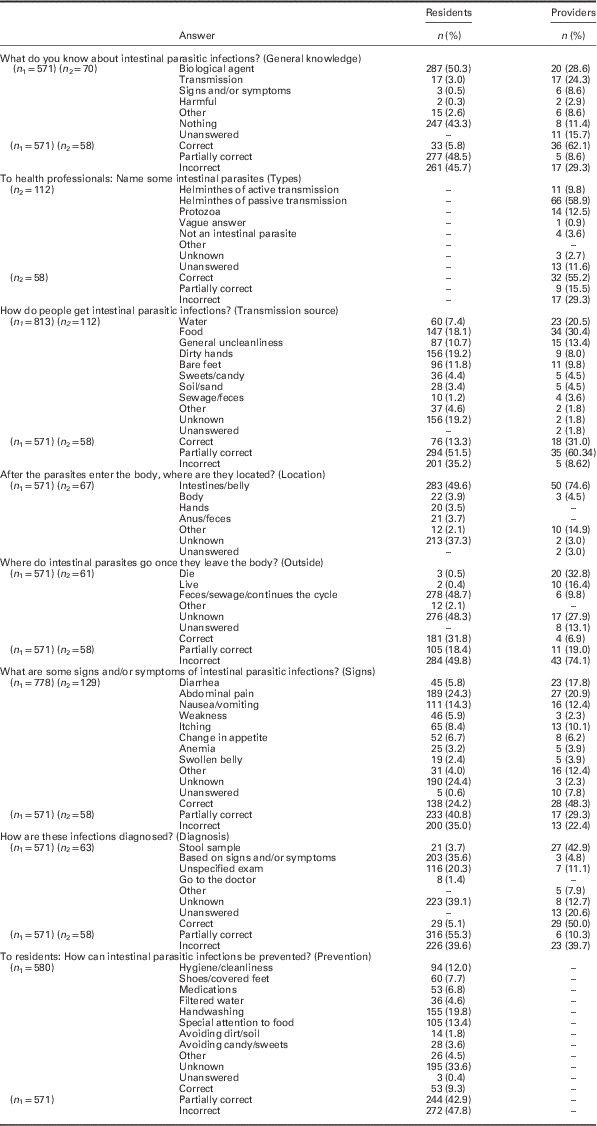
n 1 – Number of answers provided by the Complexo de Manguinhos residents.
n 2 – Number of answers provided by the family health team providers.
In comparison with the 43.3% of the residents who reported not knowing about IPIs, 11.4% of the professionals, of which all were CHW, claimed to not know. The CHW utilized similar language to the residents; however, the CHW answers were generally more elaborate. The other professionals mostly provided vague or incomplete answers.
The sources of IPIs reported by the health professionals focused on water (20.5%) and food (30.4%), but also included candy/ sweets (4.5% of total mentions) in the answers of five CHW (8.6% of the total professionals). The residents more frequently associated candy (4.4%) as a source of IPIs than contact with sewage or feces (1.2%).
Regarding the fate of intestinal parasites outside of the human body, the providers mostly either did not know (27.9%) or reported that the parasite died (32.8%). In comparison, the residents did not know (48.3%) or mentioned an aspect of a continued cycle (48.7%), such as: ‘they get out in the feces and go to the sewage,’ and, ‘they come in again.’ Health provider answers in the feces/sewage/continues the cycle category (9.8%) (eg, ‘lodge themselves in a host,’ ‘in contact with soil or water, they can reproduce,’ and ‘wait to enter the body’) were all from the CHW.
In terms of prevention, only two residents mentioned sewage or sanitation. References to hygiene as a form of prevention (12.0%) were typically vague (eg, ‘have hygiene’ and ‘diet and hygiene’); and, when some details were provided, they were not always relevant to IPI prevention with some including preventive measures for other endemic diseases. References to medications as a form of prevention (6.8%) sometimes included visits to the health center but mostly referred to self-medication or home remedies.
Table 1 also shows the distribution of incorrect, partially correct and correct answers. Both professionals and residents had the same proportion of incorrect answers regarding diagnosis (39.7% and 39.6%, respectively). For residents, knowledge of diagnosis and prevention was associated with the MA (P=0.01 and 0.00, respectively).
Discourse analysis showed that although some participants expressed great concern for IPIs and considered them of great severity (ie, ‘animal that finishes us, a disgusting thing which many times is our own fault…’ (resident) and ‘I’m aware that if you don’t take care of it, it can kill you and you get them out there [referencing the territory]’ (CHW)), generally IPIs were treated as part of life.
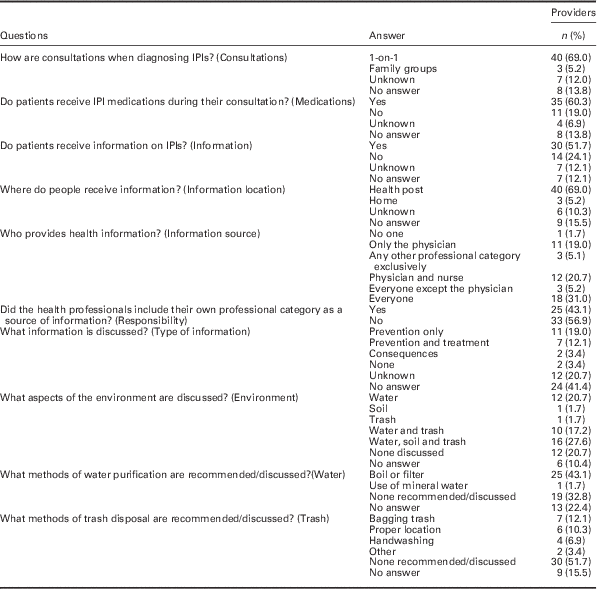
Health care providers’ practices regarding intestinal parasites and health education can be seen in Table 2. It should be noted that answers to ‘What information is discussed?,’ despite being open-ended, were vague.
Table 2 Questionnaire answers on practices of the family health team primary care providers regarding intestinal parasitic infections and health education (n=58)
Discussion
Despite awareness of IPIs, the knowledge necessary for managing these infections did not include appropriate reflections of the parasites or relevant environmental and social characteristics of the territory, as such, exhibiting limited management of IPIs.
Differential participation from the professionals responsible for MA1, supports the theory that in areas of greater need, activities not directly related to clinical practice remain secondary (Alves and Aerts, Reference Alves and Aerts2011; Oliveira and Wendhausen, Reference Oliveira and Wendhausen2014; Alicea-Planas et al., Reference Alicea-Planas, Pose and Smith2016). Alicea-Planas et al. (Reference Alicea-Planas, Pose and Smith2016) identified time constraints as a major barrier, especially to health education in community health clinics serving populations with multiple needs. Furthermore, this region presented low physician density which can strain health care quality, especially regarding disease prevention and health promotion (Luo et al, Reference Luo, Beckles, Zhang, Sotnikov, Thompson and Bardenheier2014). Luo et al. (Reference Luo, Beckles, Zhang, Sotnikov, Thompson and Bardenheier2014) found that in areas with greater disease burdens, health education (referred to as self-management education) was less likely despite the positive effects of health education on self-care behaviors previously identified.
This study supports Oliveira and Wendhausen’s (Reference Oliveira and Wendhausen2014) findings that providers feel unprepared to address health education. Providers knew more regarding topics needed for diagnosing IPIs than topics related to the construction of self-care practices (ie, general knowledge, source of IPIs, and fate of intestinal parasites outside the human body). As health promotion is based on promoting autonomy and self-care, health professional insecurities in discussing and constructing these practices with patients limits health promotion (Hing et al., Reference Hing, Hooker and Ashman2011; WHO, 2016). In this study, most participating health professionals did not recognize their professional category as an appropriate source of information and health education was considered the responsibility of others, although all FH team members share this role. The current Flexner inspired model of educating health providers is focused on the biomedical aspects of disease, specifically diagnosis and treatment, allowing for greater ease working within this paradigm (Besen et al., Reference Besen, Souza Netto, da Ros, da Silva and Pires2007). In both community health clinics and office-based practices, physicians are less likely to engage in health education activities when compared with other health professionals.
Resident familiarity with IPIs, as seen by resident knowledge and the codification used, led to the banalization of IPIs as an expected part of life with self-medication normalized for both prevention and treatment. Among the residents, similar percentage of respondents who reported dirty hands as a source of IPIs reported handwashing as a means of prevention, showing a connection between source and prevention strategy. However, food played a greater role as a source of IPIs than as a means of prevention. Although the professionals mentioned water and food as the main sources of contamination, discussion of IPIs rarely included prevention and safe food handling. Only in a follow-up specifically asked about water and prevention did the professionals report tackling water security/purification during patient–provider encounters. No aspect of how water and food became contaminated was discussed. Residents, on the other hand, recognized the presence of these parasites in fecal matter and sewage systems, but did not associate these as an infection source. When compared with knowledge of residents from other communities, residents of the CM had a greater understanding of the role of sewage as the destination of intestinal parasites but not as the infection source (Moraes Neto et al., Reference Moraes Neto, Pereira, Alencar, Souza-Júnior, Dias, Fonseca, Santos and Almeida2010). Linking a contaminated environment and the acquisition of an IPI is fundamental in constructing preventive practices on individual (eg, personal hygiene and use of footwear) and collective (eg, pressuring government officials for improvements in sanitation) levels.
In vulnerable low-income communities without appropriate access to sanitation, the underlying processes resulting in IPIs are not resolved by only addressing hygiene as the main source of prevention. Inequalities in the social determinants of health are important factors in the management of IPIs and, to avoid placing an unjust burden on individual responsibility for health, effective health education must incorporate critical reflection regarding the social determinants of health (Cyrino and Toralles-Pereira, Reference Cyrino and Toralles-Pereira2004; Freire, Reference Freire2007; Ferreira and Castiel, Reference Ferreira and Castiel2009; Luo et al., Reference Luo, Beckles, Zhang, Sotnikov, Thompson and Bardenheier2014; Oliveira and Wendhausen, Reference Oliveira and Wendhausen2014). However, integral approaches to managing IPIs are complex and providers are better prepared to address patients with straightforward needs and agendas than complex ones that require going beyond a biomedical analysis (Barry et al., Reference Barry, Bradly, Britten, Stevenson and Barber2000; Cyrino and Toralles-Pereira, Reference Cyrino and Toralles-Pereira2004).
Limitations
Low participation of FH professionals limited quantitative analysis of the findings, yet this limitation led to important qualitative findings. Self-reporting could have provided more optimistic answers and resulted in the presence of unanswered and/or uncompleted questions on the providers’ surveys; however, allowing for self-application of the survey by the health providers was needed to increase their participation.
Conclusions
Primary health services are where knowledge, attitudes and practices of providers and community residents come together to develop and implement strategies for managing IPIs and promoting health. The FHS as a form of providing primary care is privileged in its inclusion of CHWs as fundamental members of the team and its focus on health promotion. However, analysis of knowledge, attitudes and practices showed that management of IPIs in this agglomeration of urban slums was limited with most efforts related to preventive treatment. Parasitological exams of stool were considered but not prioritized. Neither the social determinants of health nor health education activities were frequently considered. A disconnect between knowledge on forms of transmission and prevention was identified; as well as, a perceived complacency regarding IPIs as an expected part of life.
Residents and primary health providers lacked the knowledge necessary to engage in an integral approach to managing IPIs. Inadequate health education is not a phenomenon exclusive to this study area – or even low and middle-income countries – and health care providers have identified the need for increasing their own knowledge base to fulfill their responsibility for health education.
Implications for policy and practice
The social determinants of health and health education should be incorporated as essential aspects of PHC; however, providers of the FHS responsible for the residents of an agglomeration of urban slums (favelas) did not recognize their role as health educators and their reflections on the social determinants of health were limited. Multiple, competing demands promote a hierarquization of the aspects of care where curative, biomedical activities predominate over prevention and an integral approach to health. However, the complex processes involving the cycle of poverty and disease go beyond the biomedical, limiting the potential for health in urban slums. Health services should re-examine their processes to promote territorialized care and permanent education. The inclusion of health education based on communication, local realities and critical reflection within the management of IPIs is recommended.
Acknowledgments
The authors would like to humbly thank all participating Family Health Team health care providers and the residents of the Complexo de Manguinhos for their contributions. They are also grateful to the management and staff of the Centro de Saúde Escola Germano Sinval Faria, the Clínica da Família Victor Valla, and Teias-Escola Manguinhos.
Financial Support
This work was supported by: the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Grant E-26/010.001915/2014 Edital ExtPesq 16/2014; Financiadora de Estudos e Projetos (FINEP), Agreement FINEP/FIOCRUZ 01.11.0025.04, Rede Morar.Ts; Vice-Presidência de Ambiente, Atenção e Promoção da Saúde (VPAAPS)/ Vice-Presidência de Pesquisas e Laboratórios de Referência (VPPLR)/Fundação Oswaldo Cruz (FIOCRUZ); the Instituto Oswaldo Cruz (IOC/FIOCRUZ); and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) Agreement FIOCRUZ/Ministério da Saúde/Ministério do Desenvolvimento Social – Edital Brasil Sem Miséria. The funders had no role in the study design, analysis or writing of this manuscript and the authors have no conflicts of interest to report.
Conflicts of Interest
There are no conflicts of interest to declare.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The home visits and subsequent application of the KAP survey in the households was approved by the Instituto Oswaldo Cruz Committee for Ethics in Research with Humans through Protocol 548/10. Research conducted at the health center was approved by the Instituto Oswaldo Cruz Committee for Ethics in Research with Humans through CAAE 03706212.2.0000.5248. A Term of Free and Informed Consent was explained and signed by all participants. All FH team providers at the clinics involved were invited to participate in information sessions where IPIs and the findings of this study were discussed. IPIs were discussed with residents during the home visits after questionnaire completion and all participating residents were invited to enroll in a free health promotion educational program offered by IOC/FIOCRUZ.






