Background
In the United Kingdom urinary tract infections (UTIs) are the commonest bacterial infection presented by women within the primary care setting (Butler et al., Reference Butler, Hillier, Roberts, Dunstan, Howard and Palmer2006; Little et al., Reference Little, Merriman, Turner, Rumsby, Warner, Lowes, Smith, Hawke, Leydon, Mullee and Moore2010), with ~40–50% of women experiencing one episode during their lives (Kunin, Reference Kunin1994). This accounts for between 1 and 3% of all consultations in general practice (Stapleton, Reference Stapleton1999). Recurrent urinary tract infections (RUTIs) are commonly defined in the literature as three episodes of UTI in the last 12 months or two episodes in the last six months (Albert et al., Reference Albert, Huertas, Pereiró, Sanfélix, Gosalbes and Perrota2004). Between 20 and 30% of women who have had one episode of UTI will have a RUTI (Sanford, Reference Sanford1975) and around 25% of these will develop subsequent recurrent episodes (Hooton et al., Reference Hooton, Scholes, Hughes, Winter, Roberts, Stapleton, Stergachis and Stamm1996).
Antibiotic prophylaxis, including long-term prophylaxis, post-coital prophylaxis, or patient-initiated therapy, is the main management strategy for RUTIs in primary care (Christofides et al., Reference Christofides, Swallow and Parkinson2013). Long-term prophylaxis has been shown to reduce the risk of recurrence of UTIs in comparison to placebo (Albert et al., Reference Albert, Huertas, Pereiró, Sanfélix, Gosalbes and Perrota2004) but when treatment is discontinued, risk of infection returns to that of a placebo controlled group. Antibiotic prophylaxis is associated with unpleasant, and occasionally serious adverse effects (Madani and Mann, Reference Madani and Mann2012). Furthermore, there are increasing concerns about microbial resistance (Kumarasamy et al., Reference Kumarasamy, Toleman, Walsh, Bagaria, Butt, Balakrishnan, Chaudhary, Doumith, Giske, Irfan, Krishnan, Kumar, Maharjan, Mushtaq, Noorie, Paterson, Pearson, Perry, Pike, Rao, Ray, Sarma, Sharma, Sheridan, Thirunarayan, Turton, Upadhyay, Warner, Welfare, Livermore and Woodford2010). Within the United Kingdom, in 2002 resistance to trimethoprim was estimated at 20% (Christiaens et al., Reference Christiaens, De Meyere, Verschraegen, Peersman, Heytens and De Maeseneer2002). By 2008 a study found resistance rates of 40% (Bean et al., Reference Bean, Krahe and Wareham2008). There is additional evidence of increasing resistance to other antibiotic treatments. Of particular concern is the emergence of multidrug resistant strains of Escherichia coli (Rogers et al., Reference Rogers, Sidjabat and Paterson2011) – the organisms responsible for around 80% of UTIs (Franco, Reference Franco2005). These factors add new challenges and complexities to how GPs manage RUTIs and contribute to what have been described as inconsistent (O’Brien et al., Reference O’Brien, Hillier, Simpson, Hood and Butler2007) and sub-optimal practices (Hummers-Pradier and Kochen, Reference Hummers-Pradier and Kochen2002). To our knowledge there has been little work on exploring GPs’ perspectives on this situation, which provides part of the rationale for this study.
The unsatisfactory nature of current RUTI management has led researchers to consider whether complementary and alternative medicine (CAM) and in particular herbal options such as Chinese herbal medicine (CHM) might be helpful. While cranberry products were once thought to help prevent RUTIs, the evidence is now less clear (Allan and Nicolle, Reference Allan and Nicolle2013). Other herbal preparations may be of use. For example, a herbal preparation containing nasturtium (Tropaeoli majoris) and horseradish root (Armoraciae rusticanae), has been shown to reduce recurrence in comparison with placebo (Albrecht et al., Reference Albrecht, Goos and Schneider2007). Rigorous randomised controlled trials are required to test specific herbal approaches in the management of RUTIs. However, it is important that, before these are carried out, GPs’ perceptions of RUTIs and herbal medicines are assessed. Previous studies have identified GP interest in CAM treatment options, including herbal medicines. Surveys of English general practices suggested that around 40% of GP partnerships provided access to some form of CAM for their National Health Service (NHS) patients (Thomas et al., Reference Thomas, Nicholl and Fall2001; Posadzki et al., Reference Posadzki, Watson and Ernst2012). There is more limited information available on GPs recommendation or endorsement of herbal medicines but one study suggests that this is relatively low at around 5% (Perry and Dowrick, Reference Perry and Dowrick2000). Interestingly, this is considerably lower than occurred in a survey of German GPs where referral rates to herbal medicine reached 28% (Schmidt et al., Reference Schmidt, Jacobs and Barton2002). As far as we are aware there has been no investigation into the reasons for this relatively reserved attitude towards herbal medicines above and beyond generic concerns expressed about CAM interventions relating to lack of evidence, possible harmful effects, interactions with pharmaceuticals, and inadequate professional regulation (Botting and Cook, Reference Botting and Cook2000; Schmidt et al., Reference Schmidt, Jacobs and Barton2002; van Haselan et al., Reference van Haselan, Reiber, Nickel, Jakob and Fisher2004; Poynton et al., Reference Poynton, Dowell, Dew and Egan2006).
In addition, we are not aware of any studies focusing on GP attitudes to herbal medicines including CHM in relation to the management of RUTIs. Therefore, we undertook a qualitative study in order to explore GPs’ experiences of managing RUTIs in primary care in general and their views on the possible role of herbal medicines. The primary aim was to better understand, from GPs’ perspectives, the challenges in managing RUTIs. The secondary aim was to identify possible opportunities and barriers for using herbal medicines, including CHM, in this context.
Methods
Design
An inductive qualitative design was chosen to allow in-depth exploration of GPs’ experiences and perceptions of primary care management of RUTIs including herbal medicines. Semi-structured interviews were carried out face-to-face and over the telephone and data were analysed thematically. A two-phase design was used. In phase 1, 12 interviews were conducted and analysed. In phase 2, three additional interviews were conducted to ensure themes were well-developed and to test the limits of the analysis.
Procedure
The South West primary care research network informed GPs about the study. Interested parties contacted the researchers, and were provided with a detailed written participant information sheet and a screening form before taking part in an interview. GPs reported age, gender, number of years in practice, type of practice (rural or urban), and number of patients seen per week. As the study progressed these data were used to inform recruitment and ensure that a varied purposive sample was obtained on these dimensions.
Interviews
An interview guide was developed with open-ended questions designed to encourage GPs to relate their experiences of recurrent UTIs and conventional and alternative treatment strategies. The topic guide was used flexibly and question-wording and order were adapted to allow interviews to flow freely. Reflective field notes were written after each interview and the interviews were regularly reviewed by the research team. Iterative improvements were made to the interview guide. For example, GPs were initially reluctant to give their own views on herbal medicines, citing a lack of knowledge, so the interview guide was revised to explicitly ask GPs about their personal views on herbal medicine. All interviews were digitally recorded and then transcribed verbatim.
Participants
A total of 15 GPs were interviewed, seven female and eight male. The mean age was 44 years old and the range 34 to 59 years. Seven worked full time while the remainder worked part time. Three GPs were from urban practices and three rural practices and the remainder were from practices described as mixed, semi-urban, semi-rural, or suburban. The GPs had been practising for between 3 and 31 years. A varied sample was thus obtained.
University ethical approval was obtained (ERGO code 3685) and all interviewees gave informed consent to take part in audio-recorded interviews and for verbatim quotes to be used in study reporting.
Data analysis
This study proceeded in two phases and both phases used the analytic approach described below. In phase 1, 12 interview transcripts were analysed primarily by D.W. (in discussion with co-authors) and analysis began before all interviews had been completed in order that emerging themes and ideas could inform future interviews. In phase 2, the final three interviews were integrated into the data corpus and A.F. repeated the analytic procedures applied in phase 1 (in discussion with co-authors). Phase 2 largely corroborated the categories identified in phase 1 and provided an expanded thematic account that went beyond the specifics of the medical treatment of recurrent UTIs and emphasised wider issues relating to patient management.
Inductive (data-driven) thematic analysis was used, to identify latent (implicit) and manifest (explicit) themes or patterns within the data (Braun and Clarke, Reference Braun and Clarke2006). While the analysis was led by the data itself, it was also shaped by the initial research questions. The analysis was managed using NVivo and proceeded as follows. Audio-recordings were listened to and transcripts were read repeatedly to achieve familiarisation with the data. Inductive open coding was applied, in which descriptive and/or interpretative labels (codes) were assigned to meaning units (discrete passages of a transcript that can be considered as a single idea) (Braun and Clarke, Reference Braun and Clarke2006). A list of codes and definitions was maintained and revised as more transcripts were coded. Meaning units associated with each code were reviewed: codes were grouped into conceptual categories and codes sharing very similar meanings were merged. Categories were then explored and themes were identified that captured the main patterns in the data.
Illustrative quotes were identified based on their ability to summarise or demonstrate contrasting views within the data set. Participant numbers were used for identification of quotes.
Results
The main themes to emerge from the analysis were as follows:
GPs appreciate that RUTIs severely impact on women’s quality of life
Participants had an appreciation of the wide-ranging impact that RUTIs and symptoms such as frequency and nocturia can have on women, and an understanding that women respond in different ways to the condition. A particularly vivid example is the description of RUTI symptoms as ‘quality of life wrecking’. Indeed, GPs appreciated that the symptoms associated with RUTIs can cause considerable distress to patients, have a destructive effect on personal and sexual relationships, and interfere with a women’s ability to work.
‘I think it has a huge impact on their lives, because … some of them … because they are experiencing such symptoms of frequency … um … they won’t actually go out of the house very often … the say they’re scared to leave the house … interrupts their sleep of course, they’re up numerous times through the night … um … they’re constantly on edge … they get very tired … um … and they can get quite depressed
‘… it can interfere with their sexual life and a lot of them feel dirty – and a lot of them are reluctant to embark on sexual relationships because of the risk of a recurrent UTI.’
(GP6)
Participants also commented on the psychological impact that RUTIs can have on women making them: ‘scared’, ‘constantly on edge’, ‘depressed’, ‘frustrated’, ‘fearful’, and prone to ‘low confidence’.
Causes and triggers as perceived by GPs
The causes and triggers that interviewees associated with RUTIs can be broadly divided into two categories: medical and lifestyle. Medical causes included the standard differential diagnoses for the symptoms frequency, urgency, abdominal pain and haematuria, and reflected GPs’ concerns about missing potentially more serious illness such as bladder cancer. Examples of lifestyle factors perceived relevant by GPs, of which sexual intercourse was highlighted as particularly important, are summarised in Figure 1.
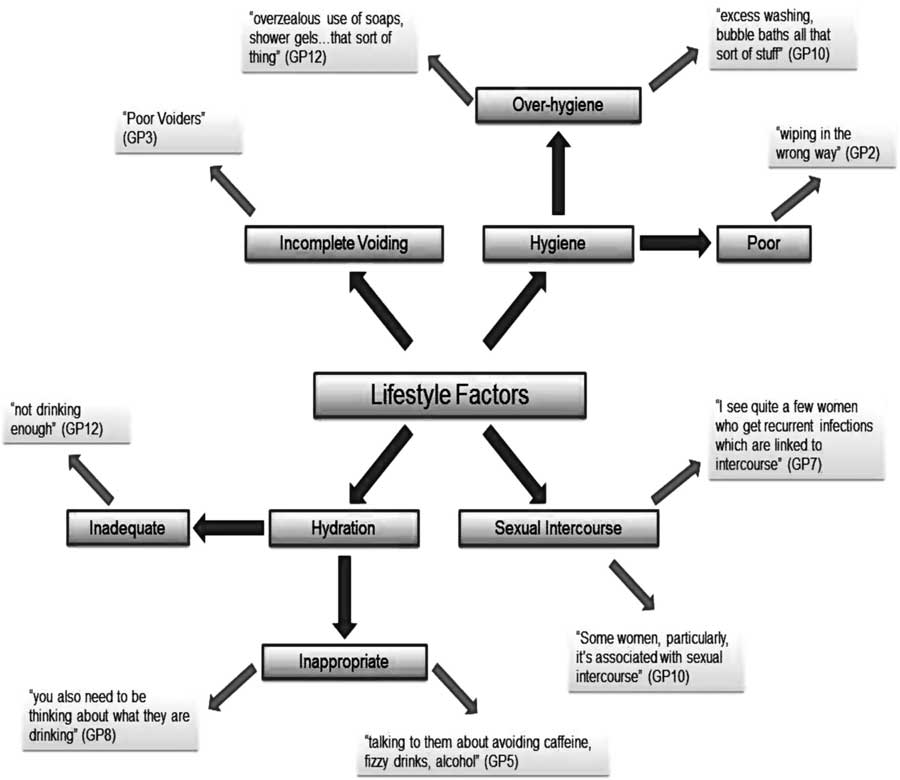
Figure 1 Diagram to show the lifestyle factors that participants perceived as causes or triggers of recurrent urinary tract infections
Managing recurrent UTIs: strategies, successes, and struggles
Participants described a wide range of expectations when treating UTIs. For some, treatment for acute infections was fairly ‘easy’ (GP12) and usually ‘symptomatically, it works really well, most women get better really quickly and they are usually very pleased’ (GP3). However these GPs were more circumspect about successful management of recurrent disease and some described on-going struggles in very negative terms.
‘a huge number of people who are very prone to urine infections who maybe have been investigated but still get their urine infections and it’s a little bit of a heart sink when they come and see me really.’
(GP8)
GPs described three main challenges to the management of recurrent UTIs.
The first set of challenges that GPs described related to making a diagnosis. GPs had to balance the various pros and cons relating to treating on the basis of a symptomatic diagnosis (usually in conjunction with a dipstick) or whether to send off a urine sample to be cultured. The perceived benefits and problems associated with sending a sample are summarised in Table 1.
Table 1 The benefits and limitations of sending a urine sample for culture in an acute infection as perceived by participants

In addition, several GPs commented on the challenge of being confronted by patients with RUTIs who may ‘sort of push for investigation, … when actually – you know – they are not warranted really’ (GP6) who become quite demanding when ‘it seems to become a focus and they become very distressed with the symptoms and – obviously then it takes a lot more time in intervention’ (GP3).
GPs also emphasised the importance of making the correct diagnosis for a UTI and not overlooking more serious pathology that can present with similar symptoms such as diabetes, bladder cancer, or pyelonephritis. There were diverse opinions regarding when to refer a patient for more specialist advice. Among those participants who were positive about referring, some were clear that if infections were not controlled then referral was appropriate, while others relied on a kind of tacit ‘GP gut feeling’ (GP10) to inform them of when things were sufficiently out of the ordinary to prompt a referal. By comparison, some GPs regarded referrals as a ‘waste of time’ and thought they resulted in unnecessary procedures such as cystoscopies that had little impact on management of the condition ‘because in the vast majority of them, they don’t find any significant abnormalities’ (GP13).
The second set of challenges that GPs described related to optimising management. Up until relatively recently, antibiotics have been routinely prescribed to treat UTIs. However, concerns now abound about bacterial resistance, adverse effects, and the short-lived relief that antibiotics provide for what is a chronic problem. This means that GPs have to balance the competing demands of patient expectations and their own previous experience with these newer concerns.
Our participants clearly experienced this dilemma with one GP expressing that antibiotics may cause ‘problems for them (the patient), side-effects, allergies and multi-resistance for the community’ (GP1) and another concerned about long-term antibiotic prophylaxis because of ‘resistance, the incidence of thrush and [potentially] the toxins and the nature of using antibiotics which are often only tested in short periods of times, for long periods of times’ (GP5). GPs described concerns about prescribing antibiotics (a) when no infections are actually present, (b) to appease patients during a busy clinic, and (c) when it might obfuscate a more serious diagnosis.
Once again GPs described feeling pressure from patient expectations that in this instance related to explicit requests for antibiotics. One GP described how ‘peoples’ perception is that if they have infection in the bladder it has to be treated with antibiotics so I suppose, you know, I prescribe the antibiotic because this is the expectation’ (GP2) while another had concerns over the amount of RUTI related antibiotics he has to prescribe because ‘people require – or demand – and need’ (GP14).
GPs were more willing to dispense antibiotics in cases of repeat infection. They were confident of the short-term benefits of the treatment and mindful of the impact of RUTIs on a woman’s quality of life:
‘I need to get on top of the infection for them, rather than give them a week’s worth of agony – because if you are having, you know, three or four days of severe discomfort every month, that’s rather different from having three or four days of discomfort and then getting better without treatment, once a year’ (GP14).
Participants also saw the provision of delayed prescriptions for antibiotics as a way of enabling women to ‘get on top of their symptoms and control the amount of impact it has on their lives’ (GP14) which can ‘empower’ them and restore their sense of ‘hope’ (GP7).
By contrast some GPs felt that the easy provision of antibiotics for what would probably be a self-limiting condition re-enforced the dependency of women on their GP and was a bit like ‘shooting ourselves in the foot’ (GP5). Others felt that infections were ‘over-treated’ (GP12) and that more information and ‘self-help advice’ (GP11) such as drinking more water, reducing caffeinated drinks (GP5) or using over-the-counter remedies such as cranberry (GP15) could improve self-management. However, one GP was more pragmatic about the limitations of self-help for women with RUTIs commenting that ‘it’s the recurrents that have turned round and said that I’d already been doing this or that for – so long – and it’s not getting better, Doc, therefore I need some antibiotics’ (GP10).
The third set of challenges that GPs described related to coping with unsuccessful outcomes. Several GPs reported feeling ‘rather helpless … and frustrated’ (GP13), ‘exasperated’ (GP1) and feeling ‘disappointed’ (GP2) that they cannot make their patients better. Others honestly admited to feeling ‘irritated’ and to using self-help measures such as fluids charts so that some of the responsibility for treatment failure falls on the patient: ‘it’s not necessarily just our fault that they are not getting better, it’s partly theirs.’ (GP5).
By contrast other participants claimed to have no emotional response to treatment failure, only ‘a clinical response to what we do next’ (GP3) or saw it as a challenge rather than a ‘heart sink’ situation (GP7). Others adopted a more ‘philosophical’ acceptance and ‘recognise that there are always going to be some patients like that’ (GP13).
Another option suggested by a GP involved referral to complementary medicine ‘I think if I had a real – what I would call a heart-sink recurrent UTI patient, then yes, I might explore something like that [CAM]’ (GP6).
GPs’ perceptions of herbal medicines and CHM
GPs expressed shared concerns and interests regarding CAM. Reviewing these, with a particular focus on herbal medicines, made it possible to identify the safeguards that GPs would require as a pre-requisite to testing and possibly providing an intervention such as CHM for RUTIs within the NHS.
Concerns about herbal medicines: knowledge, risk, and quality assurance
GPs were very forthcoming about their own lack of knowledge in relation to herbal medicines and how this would directly prohibit them from considering these options:
‘I would never tell a patient, you know, “if my approach didn’t help … go to the herbal or Chinese shop”. Because I don’t know enough about it’. (GP11).
Participants presented a wide range of beliefs of the risk of herbal medicines ranging from the ‘minimal’ (GP13) to ‘very worrying’ because of some ‘very toxic compounds’ (GP5) and several challenged the adage that because something is herbal or natural it is safe:
‘I think there is a common misconception with the public that it’s herbal so it’s safe and that worries me’.
(GP10)
In particular, concerns were expressed about the lack of quality assurance of herbal products, potential adulteration with pharmaceuticals such as dexamethasone, and possible interactions between herbs and drugs. The training, lack of regulation, or licensing, and the level of medical knowledge of herbal practitioners were additional sources of GP uncertainty. This is exemplified by one respondent: ‘But I don’t know the dose, I don’t know the name, I don’t know the shop, I don’t know what people or whom I can trust. And I don’t know how this herbal medicine would interact with maybe patient’s conditions or maybe they have side effects.’ (GP2).
The availability of herbal options such as CHM in rural Hampshire and the costs incurred for the patient in using this option were also causes for concern. Some participants worried about unproven remedies being a ‘waste of money’ (GP1) and that if they had suggested this approach then it would reflect badly on them: ‘if it has cost implications for the patient and it doesn’t work and I’ve said, yes, try it, then – they may lose faith in me’. If an actual referral was made then there was also a perceived legal risk: ‘I’m taking medico-legal responsibility, so I would be very wary about that’ (GP5).
Interest in herbal approaches
Despite expressing reservations and concerns, many participants were nonetheless open to the use of options such as herbal medicines when conventional treatment had failed – particularly if the patient had anecdotal evidence of effective treatment, or if they had a known predilection to use CAM options. In these instances GPs frequently adopted a pragmatic approach with one not wanting to ‘diss their (patient) beliefs in these things because quite often they can be quite useful’ (GP10) and another commenting on the hope that CAM could engender, probably because of its ‘huge placebo effect’ (GP7).
GPs also recognised that plant medicines had been used for ‘millions of years’ (GP11) and that some treatments were biologically plausible: ‘presumably a lot of the ingredients of Chinese medicines are therapeutic … over the aeons they have been found to be by trial and error’ (GP15).
Improving the acceptability of herbal medicines
It was clear from all the participants that establishing an evidence base for the effectiveness and safety of a herbal intervention was a pre-requisite for greater GP acceptance, recommendation, and use: ‘So as long as they had an evidence base, then I would back them up, yes’ (GP15) and ‘if it had gone double-blind trials, safety – and if they are proven to be efficacious, then I wouldn’t have any objection in recommending it’ (GP12). This might involve large randomised controlled trials, comparative effectiveness trials with antibiotics, or as one participant said even a number of ‘small trials would be helpful and – that would encourage me.’ (GP15).
Practitioner regulation and proper quality control procedures were also mentioned as key factors that would need to be in place before participants would be happy to recommend herbal treatment options.
Discussion
Summary
RUTIs can have profound and disabling consequences for the women unfortunate enough to suffer from them. Participants in this study were clearly aware of this.
There was considerable diversity in the way that GPs experience the process of diagnosing and treating women with RUTIs. Although some GPs thought they were effectively treated by antibiotics other participants were more acutely aware of the recalcitrant nature of this problem. GPs experienced significant challenges in their management of RUTIs with varying attitudes towards culturing urine, ensuring a correct diagnosis, and the value of referring to specialist urology care. Decisions about the provision of antibiotics were particularly complex with GPs having to balance patient preferences, their own experience and training, and the desire to facilitate symptom relief and a sense of being in control, with more recent recommendations to reduce antibiotic usage to prevent resistance, patient dependency, and to avoid unnecessary adverse effects. Finally, there was a wide range of responses to treatment failure with some processing it in a highly rational manner while others felt more emotionally upset that treatment may not have gone according to plan.
GPs did consider the importance of lifestyle changes and some were open to the idea of recommending complementary medicine in the event that a mainstream approach had not worked. Some GPs were relatively open to using herbal medicines and recognised the potential benefits to be gained from these approaches that may have specific benefits and also evoke a powerful ‘placebo’ response. However, GPs were unanimous in requiring more rigorous research into the safety and effectiveness of herbal medicines. They were also concerned about the quality control of herbal products, potential interactions between herbs and drugs, and of the training and regulation of herbal practitioners.
Strengths and limitations
This study allowed GPs to articulate their experiences of treating a common condition that is poorly described in the medical literature. It captures some of the complex management issues that GPs have to negotiate with a special focus on antibiotic treatment and on how GPs deal with unsuccessful treatment. It also gives a voice to GP concerns about CAM interventions in general and herbal medicines in particular and provides a developmental road map including rigorous research, practitioner regulation and adequate product quality assurance, that herbal proponents need to heed.
The study is limited by focusing on a relatively narrow field of investigation. The qualitative approach was not conducive to finding out more about how GPs’ perspectives might be affected by GP characteristics (age, sex, etc). Two issues in particular would be interesting to explore in more depth in future: how GPs deal with unsuccessful treatments and the mind maps (Gabbay and May, Reference Gabbay and May2004) that inform their treatment decisions.
Comparison with existing literature
We are not aware of any previous qualitative research exploring how GPs manage recurrent UTIs. However, there are definite parallels that can be drawn with qualitative work investigating decision-making processes in the treatment of acute upper respiratory tract infections (Kumar et al., Reference Kumar, Little and Britten2003; Tonkin-Crine et al., Reference Tonkin-Crine, Yardley and Little2011) where similar forces are at play that promote and restrain the provision of antibiotics (eg, patient expectation and the desire to meet these to preserve the doctor–patient relationship versus an awareness of the limited benefits of antibiotics and increasing risks of microbial resistance). Understanding this complex dynamic is essential to optimise the treatment of a range of acute, self-limiting infections within primary care. In the instance of RUTIs the recurrent nature and wide-ranging impact of the condition on women’s lives places additional pressure upon GPs to prescribe antibiotics.
In relation to herbal medicines, although previous studies describe a generally positive interest in CAM from primary care doctors they also identify common concerns that apply to herbal interventions such as CHM (Botting and Cook, Reference Botting and Cook2000; Schmidt et al., Reference Schmidt, Jacobs and Barton2002; Poynton et al., Reference Poynton, Dowell, Dew and Egan2006; Maha and Shaw, Reference Maha and Shaw2007). These include GPs lack of knowledge and experience, the absence of reliable evidence of effectiveness and safety, insufficient regulation, and the financial costs either to the patient or to the health care system. The focus of our investigation highlights specific attitudes in relation to herbal medicines where anxiety over adverse effects, the possibility of herb–drug interactions, and the lack of herbal quality control are of particular concern to GPs.
Conclusion
There is increasing pressure on GPs to avoid using antibiotics for a number of what are generally considered mild, self-limiting conditions. The treatment of RUTIs provides an excellent insight into the conflicting pragmatic pressures that GPs have to negotiate when treating these diseases. More in-depth research to explore GP decision-making and how they respond to poorly managed treatment is warranted from the provisional data supplied by this study. In addition, it is possible to formulate some practical recommendations that should encourage GP acceptance of research initiatives investigating herbal medicine treatments. Such research is a pre-requisite for the possible wider use of herbal medicines with NHS patients in primary care.
Acknowledgement
Andrew Flower is a NIHR funded post doctoral Fellow.
Financial Support
This work was supported by a grant from the National Institute of Health Research (grant number PDF-2011-04-027).





