Introduction
Development of the chronic care model
The chronic care model (CCM), a conceptual model for improving chronic illness care, was first described in an article published in 1998 by Wagner (Reference Wagner1998). It was developed on the basis of an examination of literature which reported successful practice and system changes leading to improved chronic illness care, and on a consensus among experts (Wagner et al., Reference Wagner, Austin and Von Korff1996; Reference Wagner, Davis, Schaefer, Von Korff and Austin1999).
The CCM describes the interacting system components required for providing high-quality chronic illness care (see Figure 1). Within a health care system, there are four components at the practice level: self-management support, delivery system design, decision support and clinical information systems. A higher level component, organization of health care, plays an overarching role to guide practice-level development. A broader component, the community, provides necessary resources and policies linked to chronic illness care. The development and integration of these components is seen to foster productive interactions between prepared, proactive health providers and informed, activated patients. As a result, patients’ outcomes are expected to be improved.

Figure 1 The chronic care model (CCM) (Wagner, Reference Wagner1998). Reproduced with permission from the American College of Physicians.
The CCM has gained considerable attention and has been used in a variety of health care organizations. In the United States, more than 100 health care organizations, including hospital systems, academic health centres and community health systems, have completed the national chronic condition quality improvement programmes using the CCM framework (Wagner et al., Reference Wagner, Austin, Davis, Hindmarsh, Schaefer and Bonomi2001a). In Canada, the CCM has been applied in the Capital Health Region of Victoria, British Columbia, to develop and evaluate a region-wide initiative to improve the outcomes of people with diabetes and those at risk of diabetes (Fulton et al., Reference Fulton, Penney and Taft2001). It was suggested that the CCM could help the National Health Service in the UK to focus on wider policies for generic chronic illness care, rather than implementation of service frameworks for selected chronic conditions (Lewis and Dixon, Reference Lewis and Dixon2004). Furthermore, the CCM has been adopted and expanded by the WHO to develop an Innovative Care for Chronic Conditions framework, which serves as a basis for policy development and system redesign in global contexts (Epping-Jordan et al., Reference Epping-Jordan, Pruitt, Bengoa and Wagner2004).
Evidence base for the chronic care model
Several published observational studies have demonstrated the implementation of the CCM in clinical practices and observed improved quality of care (Wagner et al., Reference Wagner, Glasgow, Davis, Bonomi, Provost, McCulloch, Carver and Sixta2001b; Bonomi et al., Reference Bonomi, Wagner, Glasgow and VonKorff2002; Sperl-Hillen et al., Reference Sperl-Hillen, Solberg, Hroscikoski, Crain, Engebretson and O’Connor2004; Wu et al., Reference Wu, Pearson, Schaefer, Bonomi, Shortell, Mendel, Marsteller, Louis and Keeler2003). However, these evaluations employed uncontrolled before-and-after study designs, making it difficult to conclude whether the changes in patient care resulted from the interventions or from other un-measured factors. Two recently published experimental studies provide stronger evidence in support of CCM-oriented interventions (Piatt et al., Reference Piatt, Orchard, Emerson, Simmons, Songer, Brooks, Korytkowski, Siminerio, Ahmad and Zgibor2006; Landon et al., Reference Landon, Hicks, O’Malley, Lieu, Keegan, McNeil and Guadagnoli2007). However, the generalizability of these two US-based studies is not clear. In addition to this relatively limited evidence base for explicitly CCM-oriented interventions, the impact of primary care system-oriented interventions relating to specific components of the CCM has been examined by many studies across the world. An attempt to systematically review the impact of such interventions in the context of diabetes care has been made by Bodenheimer et al. (Reference Bodenheimer, Wagner and Grumbach2002). They re-analysed studies identified by a Cochrane review that assessed the effects of interventions targeting health professionals and the structure of care on diabetes management in primary health care (Renders et al., 2001). However, the scope designated in the CCM is broader than that covered by the Cochrane review, where some relevant studies might have been systematically excluded from the analysis by Bodenheimer. Another recent systematic review took a similar approach, but included impacts on a wide range of chronic disease outcomes (Zwar et al., Reference Zwar, Harris, Griffiths, Roland, Dennis, Powell Davies and Hasan2006). The effectiveness of interventions across the scope of the CCM requires further study.
Aims of this review
1) To systematically identify studies of diabetes care that assess effects of interventions featuring the CCM components;
2) To examine the extent to which interventions featuring the CCM components improve diabetes care and
3) To determine the relative effectiveness of different CCM components on diabetes care.
Methods
Criteria for selecting studies
Types of studies
Two types of studies were included: randomized controlled trials (RCTs) and controlled before-and-after studies (CBAs). Inclusion of non-randomized controlled studies such as CBAs in reviews has been recommended by the Cochrane Effective Practice and Organisation of Care Group (2006), as many organizational and professional interventions may not be feasible to be evaluated in a RCT.
Types of populations
Participants in studies were required to meet the following three criteria:
1) Diagnosis of type 1 or type 2 diabetes;
2) Aged 16 years or more;
3) Non-hospitalized patients, who received care in a primary care, outpatient or community setting.
Studies that focused on gestational diabetes were excluded.
Classification of interventions
Studies included are those with interventions targeting organizational systems, management, professionals or patients using systematically developed approaches for diabetes care (detailed in Table 1). Studies aimed exclusively at evaluations of single treatment methods (eg, psychotherapy or specific drugs) and drug compliance studies were excluded. In line with the concept of the CCM, intervention components were classified into six categories (Table 1). In general, the control groups in included studies received ‘usual care’ or ‘standard care’.
Table 1 Classification of interventions

a We use ‘organizational influence’ to replace the original term ‘ health care organizations’ in the chronic care model (CCM). As ‘health care organizations’ and ‘health care systems’ are usually used interchangeably, our modification would allow the six components in the CCM to be termed logically as system components.
Outcome measures
Intermediate patient outcomes:
• HbA1c control;
• Systolic and diastolic blood pressure control;
• Blood lipid control (including total cholesterol, HDL, LDL and triglycerides).
We only included patient outcome data obtained by chart reviews, direct clinical examinations or through clinical information systems. Data from patient or provider self-reporting were excluded.
Search strategy for identification of studies
a) The following electronic databases were searched:
Cochrane Effective Practice and Organisation of Care (EPOC) specialized register, the Cochrane Controlled Trials Register (CCTR) (Cochrane Library Issue 2, 2005) and MEDLINE (1966 to December 2004).
We adapted search strategies recommended by the Cochrane Metabolic and Endocrine Disorders Group (2003), Cochrane Effective Practice and Organisation of Care (2004), and other Cochrane reviews (Giuffrida et al., 1999; Renders et al., 2001; Griffiths et al., 2005; Roger, Reference Roger2004) (search strategy available on request from the authors).
b) References of published systematic reviews related to diabetes care.
We identified systematic reviews or meta-analyses related to diabetes care by searching two electronic databases in the Cochrane Library (keyword ‘diabetes mellitus’ was used): the Cochrane Database of Systematic Reviews (CDSR) and Database of Abstracts of Reviews of Effects (DARE). Systematic reviews (or meta-analyses) with their aims related to any areas defined in Table 1 were retrieved to obtain their reference lists.
c) Reference list of each retrieved article was scanned to identify further studies.
Review process and data abstraction
Initially, all abstracts of studies identified by electronic and hand search were screened by a single reviewer (DS) against the inclusion criteria, to identify potential studies that merited full-text reviews. The author repeated this process to ensure the reliability for selection into the review. The full articles of all studies for which abstracts were identified as possibly meeting the inclusion criteria at either screening were retrieved for further assessment.
At the full-text level, a standardized abstraction form was used by the single reviewer to extract information on study characteristics, interventions, outcomes and design quality. For each study, the reviewer repeated the processes of classifying interventions and appraising study quality. Any discrepancies between the initial and repeat process were reviewed and resolved.
When a single study led to multiple publications, all relevant papers were reviewed together and one single form was used to collect data.
When a single study involved several intervention arms (versus a control arm), each pair of intervention and control arms was considered as a separate comparison. For example, if a study composed of three intervention arms and one control arm, three data abstraction forms were used for each of three comparisons.
Outcomes in each study, where possible, were reported in the following ways:
• Patient outcomes (HbA1c, blood pressure, total cholesterol, HDL, LDL and triglycerides): mean and standard deviation for each of the intervention and control arms before and after the intervention.
Quality assessment
As part of data collection, quality assessment for each included study was conducted using a standardized assessment tool. The tool was developed by the Effective Public Health Practice Project (2006) and consists of six quality criteria:
• Selection bias
• Allocation bias
• Confounders
• Blinding of outcome assessors
• Data collection methods
• Withdrawals and dropouts
Each of these six criteria was rated as ‘strong’, ‘moderate’ or ‘weak’ in quality for a given study using predetermined standards. Each study also received an overall assessment of strong, moderate or weak quality by the following definition: (1) strong – at least four of six criteria were rated as strong, with no weak ratings; (2) moderate – one criterion was rated as weak; and (3) weak – two or more criteria were rated as weak.
Statistical analysis
Following the method employed in a recent systematic review of quality improvement strategies for diabetes care (Shojania et al., Reference Shojania, Ranji, McDonald, Grimshaw, Sundaram, Rushakoff and Owens2006), we calculated the effect size (and its standard error) for each single study using the following formulas:
• Effect size (mean difference) = difference in postintervention values between intervention and control groups for mean patient outcomes. In this article, we simply state effect sizes for different patient outcomes in the following way: reduction in HbA1c, blood pressure, total cholesterol, LDL, triglycerides, and increase in HDL, as those directions represent an improvement.
• Standard error of effect size =
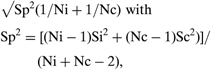
where Ni and Nc are the intervention and control group sample sizes, respectively, and Si and Sc are the intervention and control group sample standard errors (Normand, Reference Normand1999).
In some included studies interventions were allocated at the clinician or clinic level but data were collected at the patient level. This meant there was some clustering of patients at the clinician (or clinic) level, and adjustment for clustering was required to avoid overstating the significance of differences. In order to deal with the clustering, we calculated an effective sample size for each such study: N effective = (k × m)/[1+(m − 1) × ICC], where k is the number of clusters and m the number of patients per cluster; and ICC refers to the intracluster correlation coefficient (Shojania et al., Reference Shojania, Ranji, McDonald, Grimshaw, Sundaram, Rushakoff and Owens2006). We imputed the ICC value as 0.035 based on previous empirical estimations (Campbell et al., Reference Campbell, Mollison and Grimshaw2001).
We used a random-effects meta-analysis to estimate pooled mean effect sizes among included studies. A test for heterogeneity was performed and indicated evidence of statistical heterogeneity (for patient HbA1c results, Cochran’s Q = 4341, P = 0.001). The random-effects meta-analysis allows for statistical heterogeneity between studies by assuming that the true effects in the individual studies differ from each other (Normand, Reference Normand1999).
We used a random-effects meta-regression to formally test whether specific study features influenced the magnitude of the effect sizes across studies. Meta-regression is a useful technique to investigate sources of heterogeneity with respect to clinical diversity (eg, participants and interventions) and methodological diversity (eg, randomization and blinding) of studies. The specific features considered as potentially important sources of clinical and methodological heterogeneity for studies in this review included baseline levels of patient outcomes, study designs (RCTs versus CBAs), methodological quality and the number of intervention components.
Potential publication bias was explored using an inverted funnel plot for patient HbA1c outcome (Lau et al., Reference Lau, Ioannidis and Schmid1997). All data analyses were conducted using Stata (version 9.2, College Station, TX, USA).
Results
Search results
In all, 615 abstracts were initially identified by electronic and hand search (Figure 2). Of those abstracts, 90 met our explicit inclusion criteria and their full-texts were retrieved for data abstraction. During the full-text review, 25 papers failed to meet inclusion criteria and the remaining 65 papers were included for the current review. These 65 papers (listed at the end of this paper) reported 62 studies, with a total of 69 comparisons.

Figure 2 Search results
Characteristics of included studies
Studies conducted in 15 countries were included (Table 2). Nearly half were from the US, followed by the Netherlands and the UK. In all, 18% of included studies were conducted among selected patients with poor control of diabetes, and 10% among socioeconomically disadvantaged populations. A total of 39 studies (63% of total) were RCTs and 23 (37%) were CBAs. A detailed summary of each included study is available on request from the authors.
Table 2 Characteristics of included studies and comparisonsa

aFull references of included studies are list at the end of this paper.
RCTs: randomized controlled trials; CBAs: controlled before-and-after studies.
Types and numbers of components used in interventions
Around half of the included comparisons used decision support, self-management support, delivery system design or clinical information systems in their interventions (Table 3). In contrast, only three comparisons included organizational influence as part of their interventions and two employed community linkages.
Table 3 Types of chronic care model components used in interventions

RCTs: randomized controlled trials; CBAs: controlled before-and-after studies.
In all, 28% of included studies reported interventions on a single system component, 48% on two components, and 20% on three components. The maximum number of components targeted by an intervention was four (4% of all studies).
Methodological quality
Overall, the methodological quality of RCTs was more rigorous than that of CBAs. Of the 39 RCTs, 23 (59%) were rated as strong in quality, 11 (28%) as moderate and only 5 (13%) as weak. In contrast, 13% of CBAs were rated as strong in quality, 39% as moderate and 49% as weak in quality.
Impact of different intervention components on diabetes care
Impact on patient outcomes
Effects of different intervention components on HbA1c control are shown in Table 4. Forty-six comparisons had sufficient data to allow for calculation of effect sizes in terms of reduction in HbA1c. Overall, the mean reduction in HbA1c was 0.46% (95% CI 0.38, 0.54). RCTs tended to report smaller effect sizes than CBAs (0.38% versus 0.68%, P = 0.01).
Table 4 Effects of different intervention components on HbA1c (%) control

RCTs: randomized controlled trials; CBAs: controlled before-and-after studies.
Studies that included organizational influence reported the greatest reduction in HbA1c (0.69%). It was noted that effect sizes estimated for organizational influence and community linkages were based on only one or two CBAs. Therefore, these findings may be less robust than the estimated mean effect sizes for the other intervention components reflected in a larger number of studies. For this reason, we mainly compared effect sizes among the four practice-level intervention components.
For the four practice-level intervention components, studies involving delivery system design had the largest reduction in HbA1c (0.58%), followed by those with self-management support (0.46%), decision support (0.44%) and clinical information systems (0.42%).
As shown in Table 5, strong-quality RCTs and CBAs reported a similar reduction in HbA1c when compared with moderate/weak-quality RCTs and CBAs (0.48% versus 0.44%, P = 0.70).
Table 5 Effects of interventions on HbA1c (%) control by quality of studies

RCTs: randomized controlled trials; CBAs: controlled before-and-after studies.
Effects of different intervention components on blood pressure control are shown in Table 6 and Table 7. Twenty-six comparisons had sufficient data to allow for quantitative analysis (25 with both systolic and diastolic results and remaining 1 with systolic results only). Overall, studies reported a mean reduction of systolic (diastolic) blood pressure by 2.2 (1.3) mmHg. RCTs and CBAs had similar effect sizes (P > 0.7). Studies with intervention components of delivery system design or self-management support were likely to achieve greater reduction in blood pressure.
Table 6 Effects of different intervention components on systolic BP (mmHg) control

BP: blood pressure; RCTs: randomized controlled trials; CBAs: controlled before-and-after studies.
Table 7 Effects of different intervention components on diastolic BP (mmHg) control

BP: blood pressure; RCTs: randomized controlled trials; CBAs: controlled before-and-after studies.
Effects of interventions on total cholesterol control are presented in Table 8. A total of 17 comparisons had sufficient data to allow for quantitative analysis. Overall, studies reported a mean reduction of 0.24 mmol/L in total cholesterol. RCTs and CBAs reported similar effect sizes (0.21 versus 0.29, P = 0.68). Studies with self-management support components had the highest reduction in total cholesterol (0.32 mmol/L).
Table 8 Effects of different intervention components on total cholesterol (mmol/L) control

RCTs: randomized controlled trials; CBAs: controlled before-and-after studies.
A few studies reported the lipid profile in terms of HDL, LDL or triglycerides, and pooled mean effects of relevant parameters are summarized as follows: (1) pooled mean increase in HDL (95% CI) [number of studies]: 0.02 mmol/L (−0.01, 0.04) [7]; (2) pooled mean reduction in LDL: 0.12 (−0.10, 0.34) [7]; and (3) pooled mean reduction in triglycerides: 0.20 (0.07, 0.32) [8].
Associations between study features and effect sizes
Based on meta-regression analysis, effect sizes (including reduction in HbA1c, BP and total cholesterol) did not differ on the basis of baseline levels of patient outcomes, methodological quality (strong versus moderate/weak) or the number of intervention components (multiple versus single).
Publication bias
The reduction in HbA1c in each study is plotted against the standard error of reduction (Figure 3). The plot shows that, in relation to the estimated mean effect (the vertical line), the numbers of studies are fairly asymmetrical, indicating that some studies with small or negative effect sizes were likely not published.

Figure 3 An inverted funnel plot to detect publication bias
Discussion
This review found that the most common CCM components employed in trials were decision support, self-management support, delivery system design and clinical information systems. The least reported CCM components were organizational influence and community linkages. Most trials reported using multiple components in their interventions.
Overall, included studies suitable for quantitative analysis reported small-to-moderate, statistically significant improvements in a range of patient intermediate outcomes. For example, there was a mean reduction of 0.46% in HbA1c, mean reduction of 2.2 (1.3) mmHg in systolic (diastolic) blood pressure, and mean reduction of 0.24 mmol/L in total cholesterol due to interventions as compared to ‘usual care’.
For specific CCM components, interventions employing delivery system design reported the largest improvements in patient outcomes, followed by those employing a self-management support component. Interventions involving decision support or clinical information systems reported relatively smaller effect sizes.
Comparison with previous relevant reviews
Consistent with a previous review (Shojania et al., Reference Shojania, Ranji, Shaw, Charo, Lai, Rushakoff, McDonald and Owens2004), this review showed that studies with a randomized controlled design generally reported smaller effect sizes than did studies with a controlled before–after design. However, our data showed that 41% of the RCTs included in this reviews were not of high quality, and that 13% of CBA studies were of high quality. Therefore, it would be useful to report the findings based on high-quality studies, irrespective of RCTs or CBAs. Our further analysis showed that high-quality RCTs plus high-quality CBAs reported a similar reduction in HbA1c as compared with moderate/low-quality RCTs and CBAs (0.48% versus 0.44%). This mean effect size (0.48%) reported by high-quality studies tended to be greater than that reported by RCTs (0.38%), but was smaller than that reported by CBAs (0.68%). Effect sizes based on high-quality studies are likely to be closer to true effect sizes than those from low-quality studies.
Greater benefit of multiple interventions over single-facet interventions has been suggested by a meta-analysis of disease management programmes for patients with chronic illness (Weingarten et al., Reference Weingarten, Henning, Badamgarav, Knight, Hasselblad, Gano and Ofman2002). However, our meta-regression analysis showed that effect sizes did not differ between interventions targeting multiple CCM components and those involving a single component. Plausibly, intensity of the interventions (instead of the number of intervention components) may have an impact on the effect sizes. However, we were unable to explore this relationship due to the lack of explicit description of intervention intensity from the original studies.
Our review highlighted delivery system design as one of the most important intervention components in achieving improvements in diabetes care. Delivery system design included an emphasis on role definitions for different professionals, patient care planning and regular follow-up, and coordination between primary care and specialist services. In a Cochrane review of diabetes care (Renders et al., 2001), Renders and colleagues reported that multiple interventions in which the role of the nurse in the follow-up of patients was enhanced had favourable effects on patient outcomes. In a systematic review assessing the effectiveness of disease management (defined as organized, proactive and integrated health care delivery that focused on the entire spectrum of the disease and its complications, and consistent with our definition of delivery system design) on diabetes care, Norris et al. (Reference Norris, Nichols, Caspersen, Glasgow, Engelgau, Jack, Isham, Snyder and Carande-Kulis2002b) reported that disease management interventions achieved a net reduction of 0.5% in HbA1c (corresponding figure in our review is 0.58%).
The effectiveness of self-management training interventions on diabetes care has been reported in previously published reviews (Norris et al., Reference Norris, Engelgau and Venkat Narayan2001; Reference Norris, Lau, Smith, Schmid and Engelgau2002a). In their reviews, Norris et al. (Reference Norris, Lau, Smith, Schmid and Engelgau2002a) included interventions exclusively focusing on self-management training (education). With a median intervention duration of 6 months, included studies reported an average reduction of 0.76% in HbA1c during or immediately after the intervention. However, this effect size shrank to 0.26% four or more months after the intervention ceased. Furthermore, effects of self-management training on blood pressure and lipid control were inconsistent (Norris et al., Reference Norris, Engelgau and Venkat Narayan2001). Considering that, in our review, a reduction of 0.46% in HbA1c was reported by studies using self-management support as part of multi-component interventions (with a median duration of 1 year), it appears that incorporating self-management support into other intervention components is more likely to achieve sustained improvement in diabetes care.
The review by Zwar et al. (Reference Zwar, Harris, Griffiths, Roland, Dennis, Powell Davies and Hasan2006) discussing the impact of interventions using the CCM components on a range of chronic disease showed the evidence to be largely focused on diabetes care, with relatively few studies of the impact on other chronic diseases. Their findings of the impact of interventions that addressed delivery system design and self-management support are consistent with our review findings. However, their review did not incorporate the research evidence on organizational influence and community linkages as has been done in our review. This may reflect a different approach to defining these two CCM components, and highlights the need for clearer conceptualization and description of these components on further research.
Strengths and limitations of the present review
The impact of different interventions on diabetes care was assessed in terms of patient intermediate outcomes. We examined HbA1c, blood pressure and blood lipid control simultaneously, to reflect advances in scientific knowledge that control of blood pressure and blood lipids for diabetes patients is as important as, if not more important than, control of HbA1c in reducing micro- and macro-vascular complications (Williams et al., Reference Williams, Herman, Kinmonth and Wareham2002); most other reviews have evaluated only patients’ HbA1c control (Weingarten et al., Reference Weingarten, Henning, Badamgarav, Knight, Hasselblad, Gano and Ofman2002; Norris et al., Reference Norris, Lau, Smith, Schmid and Engelgau2002a; Reference Norris, Nichols, Caspersen, Glasgow, Engelgau, Jack, Isham, Snyder and Carande-Kulis2002b; Shojania et al., Reference Shojania, Ranji, Shaw, Charo, Lai, Rushakoff, McDonald and Owens2004).
This review has several limitations. First, only one reviewer screened search results and extracted data for this review, and the process was repeated to increase the reliability of data extraction. However, the ‘gold standard’ is to have two reviewers doing this independently. Second, due to complexity of the interventions and lack of detailed description in some studies, there was potential for misclassification of interventions in relation to the CCM components. Third, many studies provided insufficient detail in the method sections for us to understand the intensity of the interventions. For example, a study might report that patient self-management education was used, but provide insufficient information on the frequency of engagement with participants and the type of process and materials used. Fourth, this review only indicates the relative effectiveness of different intervention components, and the absolute effect attributable to a particular component remains unknown, as most included studies employed multi-component interventions. Last, this review found that interventions featuring the CCM components had little effect on blood pressure control. This finding may not reflect the true effect, because studies considered in this review were selected on the basis of an intervention primarily against diabetes rather than hypertension, and the blood pressure outcomes may have been secondary compared to the primary outcome of glycaemic control. It would be useful to perform a similar systematic review of the effectiveness of CCM-oriented interventions on hypertension control.
Reviewer’s conclusions
Implications for practice
Overall, the findings support the concept of the CCM in which the state of development of various aspects of primary care service systems defined in this model appears to be important factors in the quality of care provided to people with diabetes.
Implications for research
Further studies need to describe in sufficient detail the type and dose of interventions used, to enable others to understand and replicate them in different settings. Importantly, studies providing information on factors that facilitate or inhibit implementation of interventions are particularly useful, as such factors can be incorporated into future intervention designs.
There is also a need for more research to be conducted among diabetes patients from poorer socioeconomic backgrounds, who generally experience higher morbidity and mortality due to diabetes. Of the studies included in this review, only six were conducted in socioeconomically disadvantaged populations, and only one was conducted in an Australian Indigenous setting (McDermott et al., Reference McDermott, Schmidt, Sinha and Mills2001), highlighting the lack of studies with direct applicability to disadvantaged populations in specific settings.
The small number of studies of interventions addressing organizational influence and community linkages provides less certainty on the benefits associated with those components. However, given the fact that studies that included organizational influence in interventions reported superior reduction in HbA1c (0.69%), this system component may be of considerable importance to accelerate improvements in chronic illness care. There is a need for further research to determine the significance of this component of the CCM.
Acknowledgements
DS’s work is supported by an NHMRC Population Health Capacity Building Grant (#236235). RB’s work is supported by an NHMRC Senior Research Fellowship (#283303).













