Introduction
The National Institute for Health and Care Excellence (NICE) recently issued guidance for the use of faecal calprotectin (FC) as ‘an option to support clinicians with the differential diagnosis of inflammatory bowel disease (IBD) or irritable bowel syndrome (IBS) in adults with recent onset lower gastrointestinal symptoms for whom specialist assessment is being considered, if cancer is not suspected, and appropriate quality assurance processes and locally agreed care pathways are in place for the testing’ (NICE, 2013).
Currently, there is little primary-care-based evidence to support this guidance (Waugh et al., Reference Waugh, Cummins, Royle, Kandala, Shyangdan, Arasaradnam, Clar and Johnston2013).
The Department of Gastroenterology at York Teaching Hospital NHS Foundation Trust has experience of FC testing within secondary care but has restricted its use in primary care. It has now developed an evidence-based care pathway for FC as a triage tool to facilitate the initial clinical assessment of patients presenting with lower gastrointestinal symptoms (Turvill, Reference Turvill2012; Reference Turvill2014). The care pathway is designed to optimize the negative and positive predictive values (NPV and PPV) of FC. It does so by raising the cut-off threshold and using a ‘traffic light’ system for intervention.
With the publication of NICE guidance 11, the Vale of York Clinical Commissioning Group was keen to evaluate the care pathway and recruited five primary care practices to conduct an implementation assessment. This was supported by the NICE Health Technology Adoption Programme.
Methods
Guidelines design (Figure 1)
All patients presenting to five primary care practices with new lower gastrointestinal symptoms were eligible for entry into the evaluation when:
-
(i) Aged 18–60 years.
-
(ii) Cancer was not suspected (NICE, 2005). Those in whom cancer was suspected were referred urgently. Patients in whom the GP suspected cancer, despite symptoms not strictly fulfilling fast-track criteria, were also excluded.
-
(iii) The likely diagnosis was irritable bowel syndrome (IBS) or inflammatory bowel disease (IBD), but where there was clinical uncertainty in that diagnosis (NICE, 2015a).
-
(iv) There were normal or negative initial investigations as judged appropriate by the GP. These would normally have been expected to include a full blood count, renal function tests, C-reactive protein (CRP) with stool culture (and Clostridium difficile screen), coeliac screen and thyroid function tests as indicated.
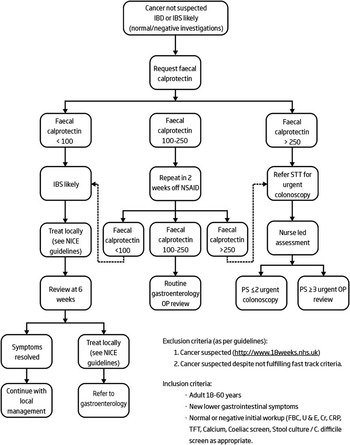
Figure 1 Local care pathway for the use of faecal calprotectin in primary care. IBD=inflammatory bowel disease; IBS=irritable bowel syndrome; NSAID=non-steroidal anti-inflammatory drugs; STT = straight to test; NICE=National Institute for Health and Care Excellence; PS = performance status; OP = outpatient; CRP=C-reactive protein; FBC = full blood count; U&E = urea and electrolytes; TFT = thyroid function tests.
Presenting patients were asked to provide a stool sample for FC testing, returning it within 24 h. The stool sample was processed using a monoclonal enzyme-linked immunosorbent assay (EK-CAL Calprotectin ELISA, Buhlmann; Alpha Laboratories Ltd, Eastleigh, Hants, UK) to determine the FC level. The normal cut-off is taken to be <50 mcg/g in line with the manufacturer’s guidance. A quality control sample set at a level of 150 was present in every test batch, the coefficient of variation being 5%. The upper limit of the assay is >600 mcg/g. Further dilutions were performed to complete the quantitation. Currently each test costs £40.00. The result turnaround was less than one week and the printed report included management guidance for the GP.
The care pathway directed that those with a low FC (<100 mcg/g) be treated on the presumption that IBS was likely (Figure 1). Patients were to be managed by:
-
(i) positive reassurance
-
(ii) local treatment based on NICE guidance for the management of IBS (http://pathways.nice.org.uk/pathways/irritable-bowel-syndrome-in-adults/managing-irritable-bowel-syndrome)
-
(iii) review at six weeks with routine referral to the Department of Gastroenterology at York Hospital if still symptomatic.
In those patients with an intermediate FC result (100–250 mcg/g) the test was repeated two weeks later and action thereafter was as directed by that result. Non-steroidal anti-inflammatory drugs (NSAID) and aspirin were asked to be avoided if clinically safe or reasonable to do so. A repeat result <100 mcg/g prompted expectant, positive, local management as outlined above; a repeat of 100–250 mcg/g prompted routine referral to the Department of Gastroenterology at York Hospital. Here the Gastroenterologist would investigate and manage as judged clinically appropriate. A high-risk FC result of >250 mcg/g directed to a ‘straight to test’ urgent colonoscopy at York Hospital or an urgent outpatient review if the patient was of a poor performance status.
Evaluation design
The evaluation was registered by the Clinical Effectiveness and Improvement Unit at York Hospital, no. 3232. It ran for six months. GPs were asked to record patient demographics, presenting symptoms, the provisional clinical diagnosis, use of therapies taken that might elevate FC levels (aspirin, anti-platelet therapy, NSAID and anticoagulants), the nature of the local intervention as appropriate, clinical outcome, the number of consultations the patient had and any additional comments.
Four clinical outcomes were recorded; IBS, non-enteric other, organic intestinal disease and incidental intestinal disease. IBS incorporated all functional intestinal disease diagnoses. On a number of occasions a normal FC assisted the GP in excluding a lower gastrointestinal cause from the differential of a patient’s symptoms. Thereafter, a gynaecological, urological or upper gastrointestinal referral was made. Here the outcome was recorded as non-enteric other. For the purposes of statistical analysis, these were included within the IBS group. Organic intestinal diseases are presented in the Results section. Findings not responsible for the patient’s symptomatic presentation are recorded as incidental intestinal disease.
Clinical outcomes were primarily recorded by the GP at six-week review with either the resolution of locally managed symptoms or the referral onto secondary care. From this point the diagnosis was made by the responsible Gastroenterologist and was obtained by accessing the hospital core patient database. A number of patients, however, did not return for six-week review, despite being sent a letter or telephoned to make contact with the practice. Those patients did not then re-consult with their symptoms in the subsequent six months and so a presumed diagnosis of IBS was made.
A web-based feedback questionnaire to assess the utility of the care pathway was sent to all contributing GPs.
Comparator data
In parallel, comparator data were provided by the Department of Gastroenterology, The Leeds Teaching Hospitals NHS Trust. This neighbouring trust recorded the care pathway of those newly referred with lower gastrointestinal symptoms into what was a conventional service and with no recourse to FC testing. Demographic data, presenting symptomatology, investigations and end diagnosis were obtained. The diagnostic yield for organic intestinal disease was compared with that of the care pathway.
Statistical analysis
Data are presented as means and standard deviations. Descriptive statistics are used to calculate predictive power (NPV, PPV with their corresponding 95% confidence intervals) and where appropriate analysis of variance is applied.
The likelihood ratio for FC to predict for organic intestinal disease was calculated using receiver–operator characteristics (ROC) curve analysis.
Results
Demographics
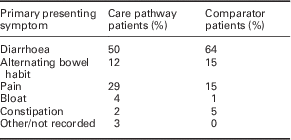
A total of 262 patients were evaluated [mean age of 36.8 years (SD 10.9) and 70% female]. Only 6% were regularly taking therapies that might elevate FC and their range was similar to that of the entire evaluated population. The primary presenting symptoms are presented in Table 1. The care pathway prompted approximately four referrals into secondary care per week and resulted in the diagnosis of organic intestinal disease in 26 patients, nine of whom had IBD. Other diagnoses were non-specific inflammation, microscopic colitis, diverticular disease, gastroenteritis, coeliac disease, pancreatic insufficiency and a 30 mm low-grade tubulovillous adenoma. The mean time from referral to diagnosis of IBD was 20 days. Ultimately, 25% of patients in the evaluation underwent colonoscopy.
Table 1 The primary presenting symptom in the evaluation and comparator sample
FC<100 mcg/g
The FC was <100 mcg/g in 82% of patients (Table 2). At six-week review 70% of these had been successfully managed locally, attending a mean of 2.5 (SD 1.0) GP consultations; however, 30% were still symptomatic, had attended 3.5 (SD 2.4) GP consultations (P<0.01) and were referred to secondary care. Of this group, 29% were treated clinically leaving 47 patients who were investigated. Organic intestinal disease was found in 8% of patients with FC<100 mcg/g, all of whom were 50 years and older or had an FC>50 mcg/g. Of the patients initially managed successfully locally, 8% became symptomatic during the subsequent six months of follow-up and were referred to secondary care where a diagnosis of IBS was made in all cases.
Table 2 Clinical outcomes based on the faecal calprotectin (FC) and referral

IBS=irritable bowel syndrome.
IBS includes other functional intestinal diseases and other non-enteric disease.
FC⩾100 mcg/g
The FC was 100–250 mcg/g when tested twice in 6.5% of patients and had a diagnostic yield for organic intestinal disease of 23%. FC was >250 mcg/g in 11.5% of patients with a diagnostic yield of 36% for IBD and 53% for all organic intestinal disease. An FC>250 mcg/g identified 89% of patients who proved to have IBD. Colonoscopy was performed in 95% and computed tomography or magnetic resonance imaging in 24% of those with a repeat FC>100 mcg/g or an FC>250 mcg/g. Of those with IBS as the diagnostic outcome whose FC was initially >250 mcg/g, 83% subsequently had a normal test.
In all, 15 diagnoses each of non-enteric other (a combination of gynaecological, urological and upper gastrointestinal) and incidental disease (predominantly sub-centimetre low-grade tubular adenomata and haemorrhoids) were made.
Statistical analysis
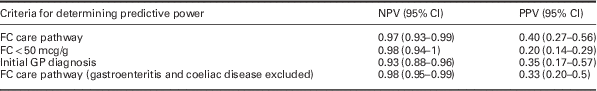
The NPV and PPV of the FC care pathway are presented in Table 3. It doubles the PPV when compared with FC when set at the normal standard <50 mcg/g. The NPV and PPV of the initial GP clinical diagnoses are also presented, as is the care pathway NPV and PPV when gastroenteritis and coeliac diseases are excluded. These would normally be expected to be screened out on initial work-up.
Table 3 Negative predictive value (NPV) and positive predictive value (PPV) of faecal calprotectin (FC)<100 mcg/g for irritable bowel syndrome (and other non-enteric disease) within the care pathway, <50 mcg/g, GP’s provisional diagnosis and the care pathway excluding gastroenteritis and coeliac disease
The positive and negative likelihood ratios for the care pathway were 6.16 (95% confidence intervals 4.05–9.36) and 0.31 (95% confidence intervals 0.16–0.58), respectively. An ROC curve demonstrates the area under the curve of 0.86 (95% confidence intervals 0.77–0.95) (Figure 2).
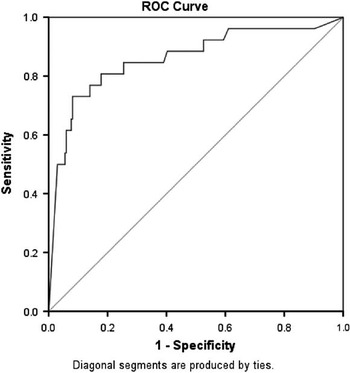
Figure 2 Receiver–operator characteristics (ROC) curve: case processing summary; faecal calprotectin: organic intestinal disease versus irritable bowel syndrome
Comparator data
Comparator data from the unselected cohort of 103 patients with new lower gastrointestinal symptoms who were referred to the Department of Gastroenterology at The Leeds Teaching Hospitals NHS Trust was obtained. The mean patient age was 36.5 years (SD 12.1) and 72% were female (ns). Despite the primary presenting symptoms being similar to those of the evaluated population, 53% underwent colonoscopy (Table 1). Organic intestinal disease was diagnosed in only six patients (two cases of IBD), giving a diagnostic yield of 5% compared with 21% for those patients referred to secondary care within the evaluated care pathway.
GP survey
In total, 90% of survey monkey responding GPs agreed or strongly agreed that the FC test had been useful in their clinical decision making and 71% indicated that a result <100 mcg/g would prevent the need for a referral, all scoring their trust in the test between 3 and 5 out of 5; 86% of GPs both trusted a raised FC result and indicated that they would choose to continue to use testing after the evaluation; 95% thought the test should sit in the early to middle stages of the diagnostic pathway.
Discussion
Background
An ideal triage tool to fulfil NICE guidance dg11 should:
-
∙ identify those patients likely to have IBS so that they can benefit from the NICE patient pathway for the management of IBS in primary care;
-
∙ identify those patients likely to have IBD so that they can benefit from timely referral to secondary care;
-
∙ improve patient experience through safer and quicker diagnosis and treatment decisions;
-
∙ realize potential cost savings in commissioning endoscopy services for suspected IBD;
-
∙ avoid inappropriate referral and investigation of patients with IBS to secondary care.
The Department of Gastroenterology at York Hospital had four major concerns about the introduction of FC testing into primary care as a triage tool:
-
∙ that the high NPV of the test applied largely to a clinical population in which the current means of assessment by GPs was good;
-
∙ that the high rate of false positives would result in an unmanageable increase in unnecessary referrals into secondary care;
-
∙ that the undefined number of false negatives in older patients would place them at risk;
-
∙ that the care of patients with difficult functional intestinal disease would be degraded.
To date there has been little assessment of the role of FC in primary care. Kok et al. (Reference Kok, Elias, Witteman, Goedhard, Muris, Moons and de Witt2012) recently determined the NPV and PPV of FC in patients referred for colonoscopy (or flexible sigmoidoscopy) by their GP. However, the population that they studied was at high risk of ‘organic bowel disease’ with a median age of 60 years (range 18–91). They found a 25.9% prevalence of organic bowel disease with an NPV 0.93 and a PPV of 0.23. Adenocarcinoma or an adenoma >10 mm was found in 35% of these patients. Earlier, a meta-analysis involving adults and children had found FC to be a useful screening tool for identifying patients likely to need endoscopy for suspected IBD (van Rheenen et al., Reference van Rheenen, Van de Vijver and Fidler2010). The authors however expressed reservations about its utility in primary care, noting that most studies were from secondary care. A Fagan plot was used to estimate for a population more representative of that seen in primary care (using a prevalence for IBD of 5%), and from this an NPV of 0.99 and PPV of 0.55 were calculated. By contrast, Jellema et al. (Reference Jellema, van Tulder, van der Horst, Florie, Mulder and van der Windt2011) performed a systematic review on the diagnosis of adult IBD presenting to primary care. Only three of the 24 studies included were actually carried out within primary care. They concluded that ‘in a setting with low disease prevalence, the same combination of sensitivity and specificity will lead to much lower PPV compared with a setting with a high disease prevalence’.
Local experience
We developed a care pathway based on our experience of FC in order to address these primary care challenges (Turvill, Reference Turvill2012; Reference Turvill2014). It incorporated:
-
∙ a higher FC cut-off value (100 mcg/g) than the standard <50 mcg/g. We had previously demonstrated, in a secondary care study, that this improved the PPV from 0.77 to 0.91 with only a 5% reduction in NPV;
-
∙ a ‘traffic light’ system for risk and so patient management (100–250 mcg/g; intermediate risk and >250 mcg/g; high risk);
-
∙ a repeat FC test for patients with intermediate risk, rather than immediate referral;
-
∙ a simple algorithm, the initiation of which was based on clinical suspicion;
-
∙ use early in the patient assessment;
-
∙ patients up to 60 years.
This has been an evaluation of that pathway of care rather than of the NPV and PPV of FC taken in isolation. We have demonstrated that the care pathway is safe and better than current primary care practice. It was readily introduced and embedded into GP practice. GPs believed the care pathway to be appropriate, trusted it and rapidly felt empowered by it. We believe that the isolated use of the normal standard for FC<50 mcg/g to triage for referral is inappropriate. It would overwhelm secondary care services. Over 60% of patients tested had an FC⩾50 mcg/g. A cut-off of FC<100 mcg/g is a safe initial threshold having a better NPV and PPV than clinical judgement alone and, in those referred into secondary care, gives a higher diagnostic yield than current comparator practice (von Roon et al., Reference von Roon, Karamountzos, Purkayastha, Reese, Darzi and Teare2007). By repeating the test when the FC⩾100 mcg/g, the number of false negatives is halved. An urgent colonoscopy is appropriate if the FC>250 mcg/g. Most patients with IBD will be identified by this means. CRP cannot be relied upon (Turvill, Reference Turvill2014). During this evaluation, the mean time to colonoscopy was three weeks, fulfilling the NICE quality standard 81 quality statement 1 for people with suspected IBD (NICE, 2015b). Diarrhoea as a primary symptom and age >50 years are most likely to be associated with a false negative FC. This reflects the limitations of using a marker of intestinal inflammation for diseases such as diverticulosis, pancreatic insufficiency, microscopic colitis and polyps. False positive results do also occur. There is the risk of over investigation and in future a new population of patients may be created who have IBS, are extensively investigated and have an unexplained persistently raised FC.
Conclusion
It is envisaged that a care pathway such as this will increase GP confidence in making a positive, local diagnosis of IBS and will of itself enhance the local service provision. This will need to be resourced. IBS can be a very challenging disease and often, secondary care referral is appropriate. This systematic care pathway, however, enhances rather than degrades the care of these patients. Nonetheless, approximately one-third of the patients referred to secondary care in this evaluation were discharged immediately after reassuringly normal investigations (Ford and Talley, Reference Ford and Talley2012).
To conclude, this evaluation demonstrates both the need for and the challenges of using a triage tool within a primary care population where the likelihood of organic intestinal disease is low. FC testing must be incorporated into a simple, useable care pathway that recognizes the intrinsic risk of organic intestinal disease within the population. It needs to identify that disease rapidly, retain clinical decision making and carefully offset NPV and PPV by identifying an appropriate cut-off level and incorporating a traffic light system that includes repeat testing. It should support an improvement in local management, be safe and not overwhelm secondary care. In this way, FC testing will become clinically cost-effective. A revised care pathway is proposed that repeats all FC>100 mcg/g and limits the initial secondary care referral if still symptomatic at six weeks to those aged ⩾50 years or with an FC>50 mcg/g. We estimate an NPV of 1 (0.98–1) and PPV of 0.33 (0.21–0.47). The care pathway would appear as in Figure 3 with the risk of organic intestinal disease in each group to inform GP decision making.
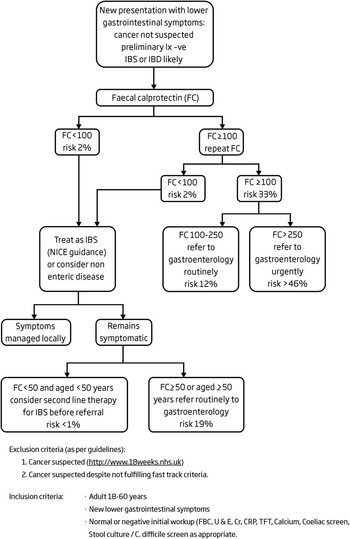
Figure 3 Proposed guidelines for the use of faecal calprotectin (FC) in the management of patients presenting with lower gastrointestinal symptoms. IBS=irritable bowel syndrome; IBD=inflammatory bowel disease; NICE=National Institute for Health and Care Excellence; CRP=C-reactive protein.
Acknowledgements
The authors are indebted to the NICE Health Technology Adoption Programme for their support of this project. The authors are grateful to Victoria Allgar, statistician, Hull York Medical School for her advice.
Financial Support
The NICE Health Technology Adoption Programme has provided £2000.00 to support the administration of this audit.
Conflicts of Interest
None.









