Schizophrenia can occur in children as young as 7 years old, but is very uncommon until after puberty (Reference Jacobsen and RapoportJacobsen & Rapoport, 1998; Reference Nicolson and RapoportNicolson & Rapoport, 1999; National Institute for Mental Health, 2003). Among young people the clinical features, particularly likely to be preceded by premorbid abnormalities in development and social adjustment, include more commonly disorganisation and affective symptoms (Reference Nicolson, Lenane and SingaracharluNicolson et al, 2000; Reference Schaeffer and RossSchaeffer & Ross, 2002). Reduction of environmental stress, psychoeducation (of the patient, the family and the school), psychotherapy (family work and cognitive-behavioural psychotherapy), promoting continued education, and social support are all considered important elements of treatment (Reference Briess and ReveleyBriess & Reveley, 1997). Nevertheless, pharmacotherapy remains the cornerstone of therapy, although side-effects and treatment resistance to antipsychotics are more common than in adults (Reference Clark and LewisClark & Lewis, 1998; Reference Malhotra, Gupta and SinghMalhotra et al, 2000; Reference Asarnow, Tompson and McgrathAsarnow et al, 2004).
Delay in treatment is associated with poorer results, whereas early intervention appears beneficial: the longer the duration of untreated psychosis the worse the condition becomes, with functional outcome appearing to decline sharply (Reference Joyce, Hutton and MutsatsaJoyce et al, 2002; Reference Harrigan, Mcgorry and KrstevHarrigan et al, 2003). Early treatment thus has the potential to reduce the secondary impacts of this serious mental illness such as suicide, stigma, isolation and reduction in social status (Reference Duggan, Warner and KnappDuggan et al, 2003; Reference Meltzer and BaldessariniMeltzer & Baldessarini, 2003).
Clozapine is the only drug licensed for the treatment of schizophrenia in individuals as young as 16 years who are unresponsive to or intolerant of conventional medication. In the summary of product characteristics by Novartis non-responsiveness is defined as ‘a lack of satisfactory clinical improvement despite the use of adequate doses of at least two marked neuroleptics prescribed for adequate duration’. Intolerance is defined as ‘the impossibility to achieve adequate benefits with conventional neuroleptic drugs because of severe and untreatable neurological adverse reactions (extrapyramidal symptoms or tardive dyskinesia)’.
The use of clozapine is affected by several possible complications. Fits, excess salivation, drowsiness, weight gain and autonomic side-effects (blurring of vision, increased intra-ocular pressure, constipation and urinary retention) are the most common (Reference Frazier, Cohen and JacobsenFrazier et al, 2003; British Medical Association & Royal Pharmaceutical Society of Great Britain, 2004). Patients taking clozapine also need regular blood tests, supervised at the time of starting this study only by the Clozaril Patient Monitoring Service. In the UK this system, available 24 h a day, was originally developed in 1990 to manage the risk of agranulocytosis associated with clozapine. It requires the patients, the responsible physicians and the supplying pharmacies to be registered with the service. This ensures that people who develop severe complications are identified early and that they are not exposed to the drug again in the future, if it has to be discontinued (Reference Atkin and O'SullivanAtkin & O’Sullivan, 1995). In addition, for patients under 18 years old taking clozapine, the advice from the original manufacturer (Novartis) is that they should have an electroencephalogram (EEG) prior to starting treatment and at regular intervals during the course of treatment owing to the increased incidence of absences and seizures (Reference Freedman, Wirshing and RussellFreedman et al, 1994; Reference FinkFink, 2002).
According to the Clozaril Patient Monitoring Service, in April 2004, out of more than 21 000 patients on the register, there were just 88 patients under the age of 18 years in the UK. This is such a small number that it is possible that young people are not being offered treatment with clozapine. This would not only contravene the National Institute for Clinical Excellence guidance on atypical antipsychotics, which states that ‘in individuals with evidence of treatment resistant schizophrenia clozapine should be introduced at the earliest opportunity’ (National Institute for Clinical Excellence, 2002), but it would also deprive patients of a potentially unique and effective treatment. Some studies in fact suggest that its use might be worthwhile and beneficial in children and young people (Reference Kumra, Frazier and JacobsenKumra et al, 1996; Reference Macewan and MortonMacEwan & Morton, 1996; Reference Turetz, Mozes and TorenTuretz et al, 1997).
Thus there is accumulating evidence to advocate the early use of clozapine, although this medication has the potential to cause severe side-effects. This paper reports the results of a survey of the attitudes and prescribing practices related to clozapine in a sample of consultants in adolescent psychiatry.
Method
The study employed a cross-sectional survey design using a questionnaire developed specifically for this project (Box 1). Questionnaire items were generated following an examination of the evidence available regarding clozapine use and through discussion with colleagues. The questionnaire was piloted with two consultants in child and adolescent psychiatry working in in-patient settings. Items covered four broad areas: usage, beliefs about effectiveness, prescribing practices, and side-effects and other problems encountered.
Box 1. Study questionnaire: respondents were asked to tick statements that reflected what happened in their practice
-
• I do not use clozapine because of:
-
• my unfamiliarity with it
-
• the need for intensive monitoring
-
• the patient’s age
-
• the side-effects
-
• the cost
-
-
• I use clozapine in my practice
-
• I use clozapine only in those aged 16 years or above
-
• Before using clozapine I try two or more antipsychotics
-
• One of them will have been an atypical
-
• One of them will have been a depot
-
• I try to use clozapine at the earliest opportunity
-
• I believe clozapine is effective in the presence of negative symptoms
-
• I use clozapine in patients who are intolerant of other antipsychotics
-
• The patients receive a routine ECG and an EEG. Other EEGs are performed during the course of treatment
-
• I believe clozapine reduces mortality due to effects on suicidality
-
• I believe clozapine reduces aggression
-
• Before starting clozapine benefits and side-effects are discussed with the patient and the family
-
• I use doses of up to 200, 450 mg or up to 900 mg maximum
-
• I routinely measure plasma concentration levels
-
• Most common side-effects I encounter in my practice are:
-
• drowsiness
-
• hypotension
-
• absences/seizures
-
• hypersalivation
-
• constipation
-
• urinary problems
-
• other problems
-
-
• I have had to stop clozapine because of severe side-effects:
-
• reduction of white cell count
-
• other problems (please state)
-
-
• I find the Clozaril Patient Monitoring Service useful
-
• Any other comments
ECG, electrocardiogram; EEG, electroencephalogram.
The population sampled comprised all consultant psychiatrists working in adolescent units in the UK. Contact details were obtained from the unit directory of the Royal College of Psychiatrists. All of the 83 practitioners eligible to take part were contacted. The questionnaire was anonymous; no attempt was made to identify the respondent consultants and there was no request for information regarding individual patients. The project was submitted to and approved by the South Sheffield research ethics committee.
Results
Of the 83 consultants contacted, 59 (71%) responded, but one person returned a blank questionnaire. Of the 58 respondents who provided data, 7 (12%) reported that they did not prescribe clozapine because of a lack of suitable cases and 17 (29%) reported that they just did not use it. Reasons given are summarised in Table 1. The prescribing practices and beliefs about clozapine in the 34 (58%) respondents who reported using it are shown in Table 2.
Table 1. Reasons for not using clozapine (n=17)
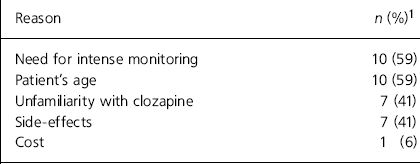
| Reason | n (%)1 |
|---|---|
| Need for intense monitoring | 10 (59) |
| Patient’s age | 10 (59) |
| Unfamiliarity with clozapine | 7 (41) |
| Side-effects | 7 (41) |
| Cost | 1 (6) |
Table 2. Respondents’ use of clozapine, beliefs and prescribing practice (n=34)
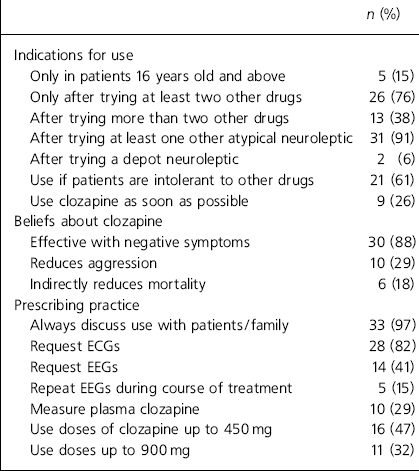
| n (%) | |
|---|---|
| Indications for use | |
| Only in patients 16 years old and above | 5 (15) |
| Only after trying at least two other drugs | 26 (76) |
| After trying more than two other drugs | 13 (38) |
| After trying at least one other atypical neuroleptic | 31 (91) |
| After trying a depot neuroleptic | 2 (6) |
| Use if patients are intolerant to other drugs | 21 (61) |
| Use clozapine as soon as possible | 9 (26) |
| Beliefs about clozapine | |
| Effective with negative symptoms | 30 (88) |
| Reduces aggression | 10 (29) |
| Indirectly reduces mortality | 6 (18) |
| Prescribing practice | |
| Always discuss use with patients/family | 33 (97) |
| Request ECGs | 28 (82) |
| Request EEGs | 14 (41) |
| Repeat EEGs during course of treatment | 5 (15) |
| Measure plasma clozapine | 10 (29) |
| Use doses of clozapine up to 450 mg | 16 (47) |
| Use doses up to 900 mg | 11 (32) |
Participants were asked about side-effects and other problems encountered when using clozapine. The most common side-effects reported were drowsiness, reported by 25 (73%) of respondents using this drug; hypersalivation, 23 (68%); hypotension, 11 (32%); constipation, 11 (32%); and others, including urinary problems, weight gain, tachycardia, diabetes and absences/seizures, reported by 7 (21%). A number of these doctors described difficulties with prescribing this drug for young people, citing poor adherence to treatment, particularly because of the risk of weight gain. Reductions in white cell count had been experienced by 18 of the 34 respondents (53%); 7 (21%) reported having to stop clozapine because of other serious problems such as myocarditis, arrhythmias, excessive sedation, untreatable hypersalivation, overdose and lack of adherence. Twenty-six (76%) found the patient monitoring service useful in managing their prescribing.
Discussion
Clozapine seems effective when used in children and adolescents, the National Institute for Clinical Excellence advocates this treatment and there is guidance available from the Clozaril Patient Monitoring Service on its use in those under 18 years old. However, this is countered by possible serious complications, and clinicians may be understandably wary of using it. As pointed out by some of the participants in the study, young patients themselves may not be prepared to adhere to the monitoring regimen and put up with the likely complications at an age when physical appearance and well-being are particularly important. Although this was a questionnaire survey, the response rate (at 71%) was respectable. Caution needs to be exercised about extrapolating from these findings to all consultant adolescent psychiatrists, but it is clear that within this group of respondents there is a need for further debate about clozapine usage, if young people with schizophrenia are to benefit from this treatment. This is particularly topical now that clozapine is available in generic form, produced by two additional manufacturers.
The data from this survey suggest that a considerable number of specialists in adolescent psychiatry working in in-patient units do not prescribe clozapine. Unfamiliarity with the drug and the need for monitoring of side-effects seem to influence its use, and it might be that clearer information about effectiveness and prescribing practices would result in increased usage. The need for information and training is emphasised by the fact that those who do use clozapine may not comply with nationally agreed recommendations about prescribing: for example, many do not seem to routinely request EEGs before or during the course of treatment.
Acknowledgements
I thank Drs A. Livesey and S. Spence from Sheffield for the comments given during the initial part of the project. I thank Professor D. Cottrell in Leeds for his tremendous support.





eLetters
No eLetters have been published for this article.