Venlafaxine is an antidepressant that selectively inhibits the re-uptake of serotonin (5-HT) and noradrenaline (Reference Harvey, Rudolph and PreskornHarvey et al, 2000). It is licensed for the treatment of depressive illness at doses between 75-375 mg/day, or 75-225 mg/day for the XL formulation (Association of the British Pharmaceutical Industry, 2002). The XL formulation is the first antidepressant to be licensed for generalised anxiety disorder.
Venlafaxine is an effective antidepressant (Reference Khan, Upton and RudolphKhan et al, 1998; Reference Schweizer, Weise and ClarySchweizer et al, 1991) that may have some advantages over other antidepressants. Comparative studies against amitriptyline (Reference Gentil, Kerr-Correa and MorenoGentil et al, 2000) report no difference in efficacy in treating depressive symptoms, whereas comparisons with imipramine (Reference Shrivastava, Cohn and CrowderShrivastava et al, 1994) and fluoxetine (Reference Clerc, Ruimy and VerdeaupaillesClerc et al, 1994) have suggested venlafaxine to be significantly superior in certain populations. Dierick et al (Reference Dierick, Ravizza and Realini1996) reported venlafaxine at 75 mg daily to be comparable to fluoxetine, but at 150 mg daily it was shown to be superior to fluoxetine in treating out-patients with major depression.
Venlafaxine may be effective in treatment-resistant depression. Nierenberg et al (Reference Nierenberg, Feighner and Rudolph1994) found that venlafaxine (mean dose 245.2 mg daily) was effective in the treatment of patients considered to be treatment-resistant. In a comparative study with paroxetine, Poirier et al (1999) reported that venlafaxine (mean dose 269 mg) was superior to paroxetine in treating resistant depression. It should be noted that the doses used in these trials were at the higher end of the dose range. Treatment-resistance in these trials was defined as failure to respond to at least two (Poirier et al, 1999) or three (Reference Nierenberg, Feighner and RudolphNierenberg et al, 1994) antidepressants, given at therapeutic doses for more than 4 weeks.
In a local audit of prescribing to patients in our trust, venlafaxine accounted for 54% of the total cost of antidepressant prescriptions, despite only representing 15% of the total number. The mean dose of venlafaxine was 146 mg per day. The aim of this study was to discover if treatment-resistance was related to the dose of venlafaxine prescribed.
The study
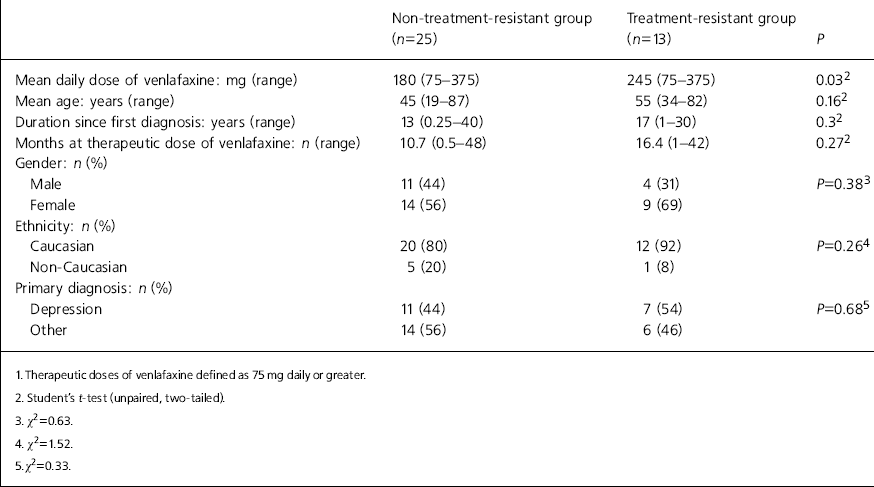
We identified all in-patients prescribed venlafaxine at Maudsley and Bethlem Hospitals during a 1-week period in October 2001. The patients' notes were then reviewed and data extracted (see Table 1).
Table 1. Summary of patient characteristics
| Non-treatment-resistant group (n=25) | Treatment-resistant group (n=13) | p | |
|---|---|---|---|
| Mean daily dose of venlafaxine: mg (range) | 180 (75-375) | 245 (75-375) | 0.032 |
| Mean age: years (range) | 45 (19-87) | 55 (34-82) | 0.162 |
| Duration since first diagnosis: years (range) | 13 (0.25-40) | 17 (1-30) | 0.32 |
| Months at therapeutic dose of venlafaxine: n (range) | 10.7 (0.5-48) | 16.4 (1-42) | 0.272 |
| Gender: n (%) | |||
| Male | 11 (44) | 4 (31) | P=0.383 |
| Female | 14 (56) | 9 (69) | |
| Ethnicity: n (%) | |||
| Caucasian | 20 (80) | 12 (92) | P=0.264 |
| Non-Caucasian | 5 (20) | 1 (8) | |
| Primary diagnosis: n (%) | |||
| Depression | 11 (44) | 7 (54) | P=0.685 |
| Other | 14 (56) | 6 (46) |
Patients were assigned to one of two groups. Those who had previously had no or one adequate trial of an antidepressant were assigned into the non-treatment-refractory group. Patients who had previously received two or more adequate trials of antidepressants were assigned to the treatment-refractory group. An adequate trial of antidepressant was defined as receiving a recognised therapeutic dose for a minimum of 6 weeks with no response.
The patient's ethnicity was grouped as Caucasian or non-Caucasian, with data taken from the admissions clerking form. Primary diagnosis was designated as either depression or ‘other’ and obtained from the admission notes. The patient's existing dose of venlafaxine was obtained from the current prescription chart.
Statistical analysis was performed using the statistics function on Excel 97.
Results
Between the two sites, 38 patients were being prescribed venlafaxine. Twenty-five were assigned to the non-treatment-refractory group and 13 were allocated to the treatment-refractory group.
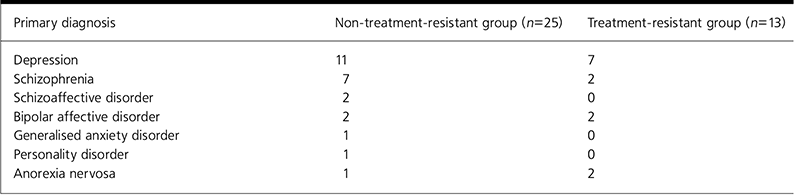
The recorded diagnoses for subjects are summarised in Table 2.
Table 2. Summary of primary diagnosis of patients
| Primary diagnosis | Non-treatment-resistant group (n=25) | Treatment-resistant group (n=13) |
|---|---|---|
| Depression | 11 | 7 |
| Schizophrenia | 7 | 2 |
| Schizoaffective disorder | 2 | 0 |
| Bipolar affective disorder | 2 | 2 |
| Generalised anxiety disorder | 1 | 0 |
| Personality disorder | 1 | 0 |
| Anorexia nervosa | 1 | 2 |
Comments
In this study, patients who were classified as treatment-resistant were prescribed signicantly higher daily doses of venlafaxine (245 mg v. 180 mg) than patients who were not classed as such. There were no other significant differences between the two groups (Table 1).
It may be inferred that treatment-resistant patients only respond to higher doses of venlafaxine, but further work is needed to determine exactly why clinicians prescribed higher doses in this patient group. It may have been that prescribers aimed for higher doses of venlafaxine from the outset because patients had previously failed to respond to other treatments. In this case, patients may not have been given an opportunity to respond to lower doses. In contrast, it may have been that lower doses were found to be ineffective so doses were titrated upwards in line with response, towards the top end of the therapeutic range. However, this would imply a dose—response relationship, one which Smith et al. (Reference Smith, Dempster and Glanville2002) failed to demonstrate in a meta-analysis of venlafaxine studies.
The mean daily dose seen in the non-treatment-resistant group (180 mg) was considerably higher than the accepted minimum effective dose (75 mg a day). This may have been because of the improved efficacy seen at higher doses, even in non-resistant illness, as reported by Dierick et al (Reference Dierick, Ravizza and Realini1996). It is also possible that patients hospitalised for depression require higher doses of antidepressants than might be used in the community.
The results of this study reflect findings of formal studies of treatment-resistant depression (Reference Nierenberg, Feighner and RudolphNierenberg et al, 1994; Poirier et al, 1999), where higher doses of venlafaxine were found to be effective. The mechanism of venlafaxine's apparent efficacy in treating resistant patients is unclear. It has been shown to inhibit serotonin re-uptake across its dose range, but at higher doses it also inhibits noradrenaline re-uptake (Reference Harvey, Rudolph and PreskornHarvey et al, 2000). Thus, higher doses of venlafaxine may be more effective because of this additional effect.
Of course, the observed difference in dose prescribed may have resulted from factors other than those assessed in the study. It is possible, for example, that exposure to interacting drugs differed between groups and that this affected the venlafaxine doses required. However, venlafaxine metabolism does not seem to be significantly affected by enzyme inhibitors or inducers (Association of the British Pharmaceutical Industry, 2002), so this seems unlikely. It is also possible that the use of potential augmenters of antidepressant therapy differed between groups (higher use of augmenting agents may allow lower doses of venlafaxine). Such drugs (lithium, mood stabilisers, antipsychotics and other antidepressants) were recorded as being prescribed in 14 out of 25 (56%) of the non-refractory group and nine out of 13 (69%) of the refractory group. Another factor that may have affected the observed differences in dose is patients' weight: heavier patients might be expected to require and receive higher doses of venlafaxine. Weights were not available for most subjects in this study. Moreover, the summary of product characteristics recommends that patients with renal failure should receive lower doses of venlafaxine. It may have been the case that a higher proportion of the non-refractory group had some degree of renal impairment that accounted for the lower doses seen in the study. However, this seems unlikely, given that patients in this group were relatively young.
In conclusion, prior failure to respond to two or more antidepressants was associated with the use of significantly higher doses of venlafaxine. Future studies should address the reasons for this prescribing practice.
Declaration of interest
A. T. — None. D. T. has previously received consultancy fees from Wyeth, whose interests may be affected by publication of this paper.





eLetters
No eLetters have been published for this article.