Medication errors are a major cause of injury and occur in 2-15% of hospital admissions (Reference LeapeLeape, 1994; Reference Bates, Cullen and LairdBates et al, 1995). Annual costs include £400 million in claims and £ 2 billion in hospital stays (Department of Health, 2000, 2001). The National Patient Safety Agency was established by the Department of Health; one of the roles of this agency is to collect and analyse medication errors and ensure that practice is modified (Department of Health, 2001). One target is to reduce medication errors by 40%; however, the current rate is unknown, so reliable baseline data must first be produced (Department of Health, 2001; Anon, 2003).
Traditionally within the East Kent National Health Service (NHS) and Social Care Partnership Trust, medication incidents have been reported on trust incident reporting forms. The forms were complex to complete, not designed for medication errors and practices were not amended in response to reports, as there was no link with clinical governance mechanisms. Furthermore, staff were reluctant to report errors owing to fear of disciplinary action.
Method
A Medline literature search (1966 to September 2004) using the search terms MENTAL HEALTH, PSYCHIATRY, MEDICATION ERROR REPORTING and MEDICATION ERROR was conducted. Although a substantial number of publications on medication errors in general (Reference Bates, Leape and PetryckiBates et al, 1993; Reference Dean and BarberDean & Barber, 2001; Reference Dean, Schachter and VincentDean et al, 2002; Reference KorenKoren, 2002; Reference Bumpus and Al-AssafBumpus & al-Assaf, 2003; Reference France, Miles and CartwrightFrance et al, 2003; Reference Howard, Avery and HowardHoward et al, 2003), including systems for primary care (Reference Dodds and LeaverDodds & Leaver, 2003) and secondary care (Reference Furukawa, Bunko and TsuchiyaFurukawa et al, 2003; Reference SmithSmith, 2003), were identified there was little research relating to mental health. An American study on error rates for 31 patients during a 2-month stay in an inpatient psychiatric unit (Reference Grasso, Genest and JordanGrasso et al, 2003) and a Japanese study on long-stay psychiatric wards (Reference Ito and YamazumiIto & Yamazumi, 2003) were identified. The search failed to identify any research relating to medication error reporting within mental health units in the UK.
The East Kent NHS and Social Care Partnership Trust introduced a medication error reporting system, entitled ‘Safemed’, based on a system developed by a community services pharmacist (Reference Dodds and LeaverDodds & Leaver, 2003). It was designed as a ‘no blame’ system to encourage reporting and obtain reliable baseline data, with links to the National Patient Safety Agency and clinical governance mechanisms within the trust via the Drugs and Therapeutics Committee. The Safemed pro forma aimed to capture the details of the staff and patients involved in each incident (details available from the authors on request). Anonymity, which might have encouraged reporting, was rejected, because it would have made individual feedback impossible (Reference Furukawa, Bunko and TsuchiyaFurukawa et al, 2003). Additional details to be listed on the form included any injury, action taken, contributory factors and suggestions for preventing a repeat incident.
The senior manager at each locality implemented the Safemed system. Awareness was raised in a number of ways. Safemed was regularly featured in a quarterly pharmacy newsletter and in the trust magazine (details available from authors on request). The chief pharmacist gave regular feedback, both on individual issues and any systems problems, via the Drugs and Therapeutics Committee.
Analysis of reports
We analysed all reports according to the type of error and the site. The prescribing support pharmacy technician (A.T.) set up a database in Access to enable the fields on the form to be recorded electronically and allow advanced data interrogation in Excel.
Results
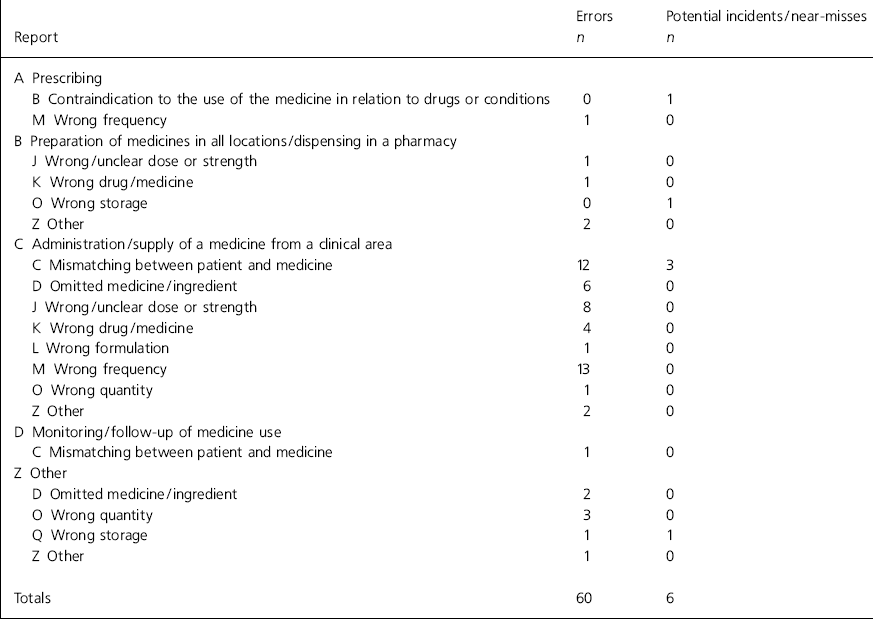
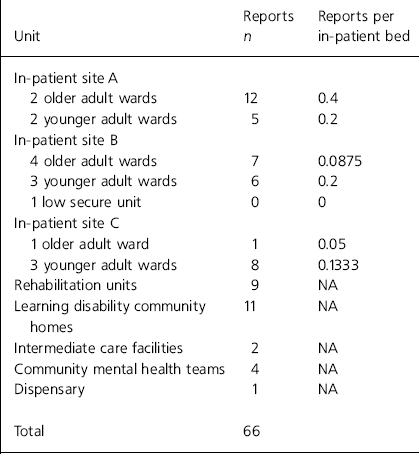
The results of the analyses of the error reports are shown in Table 1 for type of error and Table 2 for site. Trend analysis identified the following common threads: factors causing distraction during the administration round (n=18), unclear prescriptions (n=8), poor communication between teams (n=7), similar patient names (n=6) and use of support workers as runners (n=5).
Table 1. Analysis of reports according to National Patient Safety Agency categories
| Report | Errors n | Potential incidents/near-misses n |
|---|---|---|
| A Prescribing | ||
| B Contraindication to the use of the medicine in relation to drugs or conditions | 0 | 1 |
| M Wrong frequency | 1 | 0 |
| B Preparation of medicines in all locations/dispensing in a pharmacy | ||
| J Wrong/unclear dose or strength | 1 | 0 |
| K Wrong drug/medicine | 1 | 0 |
| O Wrong storage | 0 | 1 |
| Z Other | 2 | 0 |
| C Administration/supply of a medicine from a clinical area | ||
| C Mismatching between patient and medicine | 12 | 3 |
| D Omitted medicine/ingredient | 6 | 0 |
| J Wrong/unclear dose or strength | 8 | 0 |
| K Wrong drug/medicine | 4 | 0 |
| L Wrong formulation | 1 | 0 |
| M Wrong frequency | 13 | 0 |
| O Wrong quantity | 1 | 0 |
| Z Other | 2 | 0 |
| D Monitoring/follow-up of medicine use | ||
| C Mismatching between patient and medicine | 1 | 0 |
| Z Other | ||
| D Omitted medicine/ingredient | 2 | 0 |
| O Wrong quantity | 3 | 0 |
| Q Wrong storage | 1 | 1 |
| Z Other | 1 | 0 |
| Totals | 60 | 6 |
Table 2. Analysis of reported incidents according to site
| Unit | Reports n | Reports per in-patient bed |
|---|---|---|
| In-patient site A | ||
| 2 older adult wards | 12 | 0.4 |
| 2 younger adult wards | 5 | 0.2 |
| In-patient site B | ||
| 4 older adult wards | 7 | 0.0875 |
| 3 younger adult wards | 6 | 0.2 |
| 1 low secure unit | 0 | 0 |
| In-patient site C | ||
| 1 older adult ward | 1 | 0.05 |
| 3 younger adult wards | 8 | 0.1333 |
| Rehabilitation units | 9 | NA |
| Learning disability community homes | 11 | NA |
| Intermediate care facilities | 2 | NA |
| Community mental health teams | 4 | NA |
| Dispensary | 1 | NA |
| Total | 66 |
The chief pharmacist (I.D.M.) rated both the seriousness and likelihood of recurrence on a Likert scale of 1-5 (1 indicating minor significance or very low likelihood of recurrence, and 5 indicating catastrophic significance or very high likelihood of recurrence). Each incident was given a severity score obtained by multiplying the seriousness score by the likelihood of recurrence score. Using this formula, 40 incidents had a low severity score, 23 a moderate score and 3 a high score. The two incidents with the highest severity scores both involved pharmacy staff identifying potentially fatal prescribing errors. The first error involved a transcription error when the medicine card was rewritten and the lithium dosage was inadvertently increased from 400 mg to 800 mg daily. The second involved a clinically significant drug interaction - the additional prescription of fluvoxamine to a patient taking high-dose clozapine.
From 2002-3 to 2003-4 the mean number of reports per month increased from 4.6 to 5.5. The t-test found that the association between the introduction of Safemed and the increase in reporting had a very low significance (P=0.308).
The results were analysed statistically to see if there were any relationships between incident type, unit and severity. Moderate or severe incidents occurred disproportionately on older adult in-patient wards (χ2=6.1, d.f.=1, P=0.014). There were too few reports to establish any other relationships. The mean and median delays in the chief pharmacist receiving the report were 13.4 days and 11 days respectively. In over 27% of incidents the chief pharmacist did not receive the report until at least 16 days after the incident. There appears to have been a trend for reporting more severe incidents more quickly, as could be expected. However, the Kruskal-Wallis test found that the differences in the delay between low, moderate and high severity groups had very low significance (χ2=2.2, d.f.=2, P=0.333).
Discussion
Safemed allowed a number of medication errors to be identified and policies and procedures to be amended to prevent similar errors. In response to the most frequently identified factor, the trust administration policy (published on the East Kent NHS and Social Care Partnership Trust intranet, 2004) was amended to state that the medicine round should not be interrupted except in emergencies. Other changes included amending the pharmacy computing system to identify potentially fatal drug interactions, modifying the layout of a supplementary prescribing chart and establishing a procedure for cancelling prescriptions.
The observation that more severe incidents were more common on older people’s wards is of interest. A possible reason for this could be the complexity of treatment regimens, including both physical and psychotropic medications.
There are a number of problems with the system. First, although Safemed has been implemented within the mental health trust, it has not been implemented by the acute trust who provide the dispensing service under a service-level agreement. Dispensing errors are, therefore, significantly underreported. Second, the trend analysis relied upon nursing staff to identify causative factors on the open-format questionnaire. Third, the delay in the chief pharmacist receiving the report raises clinical governance concerns. An electronic system that listed common causes of errors in a tick box format could overcome both these problems. Fourth, a more robust severity rating system would have used a number of people to rate severity. Finally, the main flaw of the new system was the low level of reporting identified. Implementing Safemed had little effect on the level of reporting and it is likely that many errors are still going unreported. On five wards there was no report of any error, near-miss or potential incident over the 12-month period. There was a particularly low level of reporting near-misses and potential incidents (see Table 1).
Both administrative and cultural factors may create a barrier to adverse event reporting (Department of Health, 2003). Administrative barriers include a complex reporting system with which staff are unfamiliar, staff feeling too busy to report errors, and lack of feedback (Department of Health, 2003). Safemed minimised administrative barriers. The form was simple to complete, consisting of two sides of A4 paper, and placed no additional workload on staff because it replaced existing paperwork. Regular feedback was provided both individually and globally. Although staff might have been unfamiliar with the system initially, mainly because a training programme was not funded, awareness was raised over the 12-month period. This had no effect on reporting, suggesting that lack of familiarity was not a significant barrier.
Cultural barriers include failure to implement a ‘no blame’ system and an existing culture in which staff who admit mistakes fear losing the respect and esteem of colleagues (Department of Health, 2001). The NHS has a deeply ingrained culture; thus removing any barriers is likely to be challenging (Reference HannaganHannagan, 2002). Furthermore, mental health services appear to be particularly driven by the existing blame culture within the NHS (Reference LelliottLelliott, 2004). To remove cultural barriers, a clear and consistent message must be delivered to all staff (Reference HatchHatch, 1997; Department of Health, 2003). Although senior managers appeared to support the cultural change, evidence suggested that this message was not consistently communicated to front-line staff (published on the East Kent NHS and Social Care Partnership Trust intranet, 2003). This lack of consistency appears to be confirmed by a large variability in reporting between sites. For similar wards on different sites, there was up to an eight-fold variation in the number of reports per in-patient bed (see Table 2).
Conclusion
The implementation of Safemed was only partially successful. Safemed was successfully incorporated into the governance structure, allowing a number of practices to be modified in an attempt to prevent error repetition. However, the new system had no significant effect on the level of reporting. The number of reports received was very low, suggesting that underreporting is a huge issue. Evidence would suggest that this is a cultural problem and the trust has not fully moved to a ‘no blame’ culture. Until such a culture is fully implemented, error reporting will remain erratic and a systems approach to error prevention will not be developed. This failure to implement a ‘no blame’ culture could be confirmed by a future qualitative study involving focus groups.





eLetters
No eLetters have been published for this article.