The introduction of atypical antipsychotics has brought with it systematic programmes of valid research and a corresponding increase in demand for evidence-based prescribing. Once, arcane combinations of antipsychotics were standard practice, but now prescribers are expected to be guided by robust trials of single agents in clearly defined illnesses.
In our own unit, evidence-based prescribing protocols had been in force since October 1994. Nevertheless, two published studies which incorporated some of our patients (Reference Taylor, Holmes and HiltonTaylor et al, 1997; Reference Taylor, Drummond and PendleburyTaylor et al, 1998) have shown clear deficits in prescribing practice. In particular, the co-prescription of atypical and typical antipsychotics seemed disturbingly prevalent. This observation was largely confirmed by our own nationwide survey of atypical antipsychotic prescribing (Reference Taylor, Mir and MaceTaylor et al, 2000) which revealed rates of co-prescription of regular atypical and typical antipsychotics to average as much as 40%. In this last study, the prescribing of regular anticholinergic medication was significantly more likely in patients receiving dual therapy.
As part of an agreed protocol development programme with our health authority, we undertook an evaluation of prescribing practices before and after the introduction of a new prescribing protocol and examined rates of co-prescription and the relative need for anticholinergics in patients treated with different drug combinations.
The study
An initial audit of prescribing (Audit 1) was carried out in one predetermined week in January 1998. Pharmacists of the trust collected data from all prescriptions for antipsychotics presented to any of the trust's pharmacies. This included in-patients and patients in community or outreach centres, but excluded out-patients. Data were collated and analysed using the SPSS software system. The audit separated drugs into atypical (amisulpride, clozapine, olanzapine, quetiapine, risperidone and sertindole) and typical groups (all other antipsychotics). Anticholinergic drugs included benzhexol, benztropine, orphenadrine and procyclidine, but excluded others not used to treat movement disorders (hyoscine, pirenzepine). Prescribing was classified as regular if patients were prescribed drugs to be given at least once daily, every day. P.r.n. prescribing was considered to be that which was irregular, with medication administered or taken according to need. Prescribing was classified as p.r.n. even if a patient had not received the drug prescribed.
In March 1998, a prescribing protocol was drawn up by members of the trust's drug and therapeutics committee and circulated to all consultant psychiatrists for comment. An amended protocol was then presented to the trust medical committee and further comments received and noted. A final protocol with expanded explanatory notes was eventually agreed in September 1998 and circulated to all medical staff in the trust. The protocol was also announced and explained in the trust drug information bulletin (this final version of the protocol appears in the Maudsley Prescribing Guidelines (Reference Taylor, McConnell and McconnellTaylor et al, 1999)).
A second prescribing audit (Audit 2) was conducted in one week in February 1999, using the method described above.
Findings
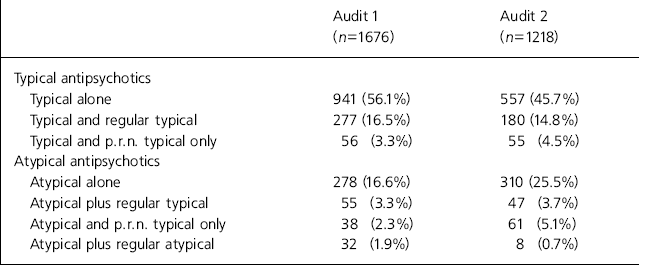
In Audit 1, prescribing data were collected for 1676 patients prescribed antipsychotics. Audit 2 reviewed 1218 prescriptions. A break down is shown in Table 1.
Table 1 Overview of prescribing
| Audit 1 (n = 1676) | Audit 2 (n = 1218) | |||
|---|---|---|---|---|
| Typical antipsychotics | ||||
| Typical alone | 941 (56.1%) | 557 (45.7%) | ||
| Typical and regular typical | 277 (16.5%) | 180 (14.8%) | ||
| Typical and p.r.n. typical only | 56 (3.3%) | 55 (4.5%) | ||
| Atypical antipsychotics | ||||
| Atypical alone | 278 (16.6%) | 310 (25.5%) | ||
| Atypical plus regular typical | 55 (3.3%) | 47 (3.7%) | ||
| Atypical and p.r.n. typical only | 38 (2.3%) | 61 (5.1%) | ||
| Atypical plus regular atypical | 32 (1.9%) | 8 (0.7%) | ||
Atypical prescribing was also analysed separately and the results presented in Table 2.
Table 2 Atypical antipsychotic prescribing

| Audit 1 | Audit 2 | |
|---|---|---|
| Total | 403 (100%) | 426 (100%) |
| Alone | 278 (69.0%) | 310 (72.8%) |
| Plus regular typical | 55 (13.7%) | 47 (11.0%) |
| Plus p.r.n. typical | 38 (9.4%) | 61 (14.3%) |
| Plus regular atypical | 32 (7.9%) | 8 (1.9%) |
In Audits 1 and 2 regular anticholinergic use was significantly more common in patients prescribed dual therapy. For Audit 1, P<0.0001 (χ2 test, d.f.=1) and for Audit 2, P=0.0008 (χ2 test, d.f.=1). Details are shown in Table 3.
Table 3 Prescribing of anticholinergic medication

| Audit 1 | Audit 2 | |||||
|---|---|---|---|---|---|---|
| Regular anticholinergic | No regular anticholinergic | Regular anticholinergic | No regular anticholinergic | |||
| Atypical alone | 30 (10.8%) | 248 (89.2%) | 36 (11.6%) | 274 (88.4%) | ||
| Atypical plus regular typical | 16 (29.1%) | 39 (70.9%) | 12 (25.5%) | 35 (74.5%) | ||
Comment
Three important observations can be made about our findings. The first is that prescribing practices changed little during the study period, with the notable exception that the proportion of patients prescribed atypical drugs increased substantially from 16.6% of the total of 25.5%. Second, co-prescription of atypical and typical antipsychotics was uncommon and did not change markedly. Third, co-prescription of regular typical antipsychotics alongside atypical agents was clearly and significantly associated with prescribing of regular anticholinergic medication.
The increased use of atypical antipsychotics in our study cannot definitively be linked to the publication of our prescribing protocol. Common sense would predict that new drugs with a reputation for improved efficacy and tolerability would, over time, be used more often. However, our new protocol was the first trust-produced document to recommend the use of atypicals as first line and our impression was that this had an important impact on prescribing practice. It is also noteworthy that during the period of the study, our trust suffered a severe financial crisis, during which prescribers were made aware of the need to limit prescribing costs. Clearly, under any other circumstances, this would tend to decrease the use of more expensive atypical drugs. This last point neatly encapsulates the dilemma faced by many trusts: atypicals are felt to be better tolerated and perhaps more effective, and may well prove cost-effective, but short-termism and parochial financial considerations militate against more widespread use.
Our observed rates of regular atypical-typical co-prescription were relatively low in comparison with rates cited in the literature. In a 1996 survey, the co-prescription rate for risperidone (with regular and/or p.r.n. typicals) was found to be 53% (Reference Taylor, Holmes and HiltonTaylor et al, 1997) and was 17% for olanzapine (regular antipsychotics only) in a 1997 survey (Reference Taylor, Drummond and PendleburyTaylor et al, 1998). In our UK survey conducted in 1998, co-prescription rates averaged more than 30% for regular atypicals with regular typicals (Reference Taylor, Mir and MaceTaylor et al, 2000). Co-prescription may reduce or abolish the perceived advantages of atypical drugs. In particular, adding a regular typical drug is likely to induce extrapyramidal symptoms. This effect is clearly shown in both audits where the use of regular anticholinergic drugs was significantly more common in those patients receiving regular atypical and typical drugs. We assume that regular anticholinergic medication is only needed when extrapyramidal side-effects occur and so regular prescription is a useful surrogate marker for this effect. Our findings in this respect are as expected and confirm our observations in previous surveys.
Prescription surveys such as this provide only a snapshot view of practices at a given time and cannot clearly associate changes in practice with supposed influences. Nevertheless, we have shown that prescribing patterns changed after the introduction of an agreed protocol, in line with recommendations made. The quality of our prescribing was relatively good in comparison with other findings and, as expected, the co-prescription of typical drugs with atypicals unequivocally made the use of anticholinergic drugs more prevalent. Widely-agreed prescribing protocols are recommended for assuring quality in the drug treatment of schizophrenia.






eLetters
No eLetters have been published for this article.